Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
2016-07-25YingKuangYingJieNieCentralLabGuizhouProvincialPeopleHospitalGuiyangCityGuizhouProvince550000China
Ying Kuang, Ying-Jie NieCentral Lab, Guizhou Provincial People’s Hospital, Guiyang City, Guizhou Province, 550000 China
ABSTRACT
Objective: To study the regulatory eff ects of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes. Methods: Breast cancer cell lines MCF-7 were cultured and transfected with miR-21 mimics and the corresponding negative control mimics (NC mimics), and then MTS kits were used to detect cell viability. Transwell experiment was used to detect cell invasion ability, and fl uorescence quantitative PCR was used to detect the expression of proliferation and invasion-related genes in cells. Results: 24h after transfection of miR-21 mimics and NC mimics, cell OD value and the number of invasive cells of miR-21 group were signifi cantly higher than those of NC group, and mRNA contents of PDCD-4, FasL, PTEN, RhoB, Maspin, TIMP3 and RECK in cells were significantly lower than those of NC group. Conclusion: miR-21 can promote the proliferation and invasion of breast cancer cell lines, and its downstream target genes include PDCD-4, FasL, PTEN, RhoB, Maspin, TIMP3 and RECK.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
Ying Kuang, Ying-Jie Nie*
Central Lab, Guizhou Provincial People’s Hospital, Guiyang City, Guizhou Province, 550000 China
ABSTRACT
Objective: To study the regulatory eff ects of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes. Methods: Breast cancer cell lines MCF-7 were cultured and transfected with miR-21 mimics and the corresponding negative control mimics (NC mimics), and then MTS kits were used to detect cell viability. Transwell experiment was used to detect cell invasion ability, and fl uorescence quantitative PCR was used to detect the expression of proliferation and invasion-related genes in cells. Results: 24h after transfection of miR-21 mimics and NC mimics, cell OD value and the number of invasive cells of miR-21 group were signifi cantly higher than those of NC group, and mRNA contents of PDCD-4, FasL, PTEN, RhoB, Maspin, TIMP3 and RECK in cells were significantly lower than those of NC group. Conclusion: miR-21 can promote the proliferation and invasion of breast cancer cell lines, and its downstream target genes include PDCD-4, FasL, PTEN, RhoB, Maspin, TIMP3 and RECK.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Keywords:
Breast cancer
Micro RNA
Proliferation
Invasion
1. Introduction
Breast cancer is malignant tumor with highest morbidity in women and also the main cause of cancer death in women. In recent years, both morbidity and mortality of breast cancer are showing a rising trend. Breast cancer is derived from the mammary epithelial cells, the prognosis of the disease is related to the abnormal expression of cell proliferation and invasion-related genes, and the stronger the cell proliferation and invasion ability, the worse the prognosis of tumor. At present, the upstream molecular mechanism regulating the expression of breast cancer cell proliferation and invasion-related genes is not yet clear [1-2] . Micro RNA (miRNA) is the singlestranded small RNA that has received more and more attention in recent years, and it can induce mRNA degradation or inhibit mRNA translation at posttranscriptional level, thus achieving the regulation of the expression of a variety of target genes. In a variety of miRNAs, miR-21 is confirmed to have antitumor effect and is associated with the pathogenesis of solid malignant tumors such as breast cancer, stomach cancer and colon cancer [3-5] . This study aims to clarify the relationship between miR-21 and the occurrence and progression of breast cancer and to identify its regulating eff ects on the biological behavior of breast cancer cells, breast cancer cell lines were used as research tools to specifically analyze the regulating eff ects of miR-21 on breast cancer cell line proliferation and invasion as well as the expression of downstream target genes.
2. Materials and methods
2.1. Experimental materials
Human breast cancer cell lines MCF-7 were purchased from the cell bank of Chinese Academy of Sciences in Shanghai, DMEM, fetal bovine serum and trypsin for cell culture were purchased from Gibco company. MiR-21 mimics and the corresponding negative control mimics (NC mimics) were synthesized byShanghai GenePharma Company; MTS cell viability detection kits were purchased from Promega company. Transfection reagents Lipofectamine 2000 and RNA extraction kits Trizol were purchased from Invitrogen company. Primers for fl uorescence quantitative PCR were synthesized by Shanghai Sangon Company, and PCR kits were purchased from Beijing Tiangen Company.
2.2. Cell culture and transfection
MCF-7 cells were recovered, cultured with DMEM containing 5% fetal bovine serum until cell density reached 70%-80%, and then digested and amplifi ed with 0.125% trypsin, amplifi ed cells were divided into two, one was inoculated in culture bottle, continued to be cultured and used for subsequent digestion and amplif ication, and the other was inoculated in culture plate, continued to be cultured and used for subsequent transfection. For cell transfection, miR-21 mimics or NC mimics were mixed with Lipofectamine 2000 according to the proportion of 1:1, let stand at room temperature for 20 min, then added to the culture wells of cell plate, continued to be cultured for 24h and used for subsequent detection.
2.3. Cell viability detection
For cell viability detection, cells were inoculated in 96-well cell culture plate and transfected for 24h, then 20 μL MTS detection solution was added to the culture system for 4h of continuous incubation, culture plate was placed in microplate reader and the absorbance (OD value) at 490 nm wave length was detected.
2.4. Cell invasion detection
For cell invasion detection, cells were inoculated in Transwell chambers and transfected for 24h, then cell membrane at the bottom of the chambers was taken out, stained with DAPI dye and were observed under fl uorescence microscope, and the number of cells within 3 high-power f ields was counted.
2.5. mRNA contents detection
For detection of mRNA contents in cells, cells were inoculated in 12-well cell culture plate and transfected for 24h, culture medium was discarded, cells were washed with PBS twice. Then Trizol lysate was added, total RNA was extracted, 1 μg RNA was collected for reverse transcription, obtained cDNA was amplif ied according to the following procedure: 95 ℃ and 10s, specifi c annealing temperature and 20s, 72 ℃ and 30s, repeating for 40 cycles, and after amplifi cation curves and take-off cycle number Ct were obtained, mRNA contents were calculated according to 2-ΔΔCt .
2.6 Statistical methods
Data were analyzed with SPSS20.0 software, and comparison between two groups was conducted by t test and diff erences were considered to be statistically signifi cant at a level of P<0.05.
3. Results
3.1. Cell viability
24h after transfection of miR-21 mimics and NC mimics, cell OD value of miR-21 group was 0.980±0.101 which was significantly higher than 0.561±0.077 of the NC group showing significant diff erences between two groups (P<0.05).
3.2. Cell invasion
24h after transfection of miR-21 mimics and NC mimics, the number of invasive cells of miR-21 group was (131.00±19.54) which was signifi cant higher than (71.25±8.34) of NC group, and diff erence between two groups was statistically signifi cant (P<0.05).
3.3. Proliferation-related gene expression
24h after transfection of miR-21 mimics and NC mimics, mRNA contents of PDCD-4, FasL, PTEN and RhoB in cells of miR-21 group were signifi cantly lower than those of NC group.
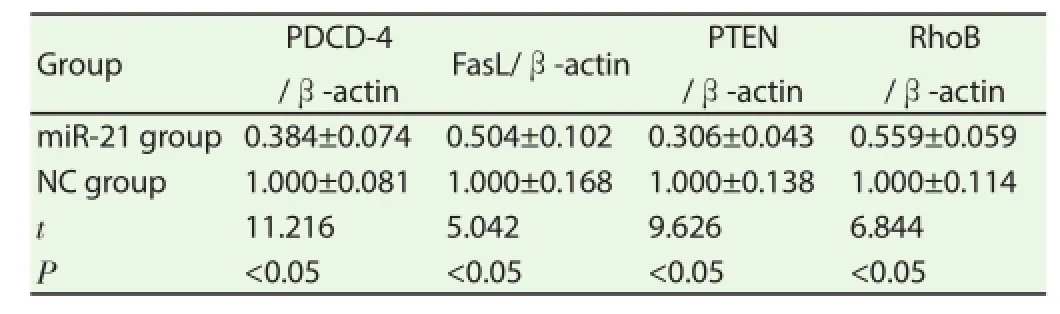
Table 1Effect of transfection of miR-21 mimics on proliferation-related gene expression.
3.4. Invasion-related gene expression
24h after transfection of miR-21 mimics and NC mimics, mRNA contents of Maspin, TIMP3 and RECK in cells of miR-21 group were signifi cantly lower than those of NC group.
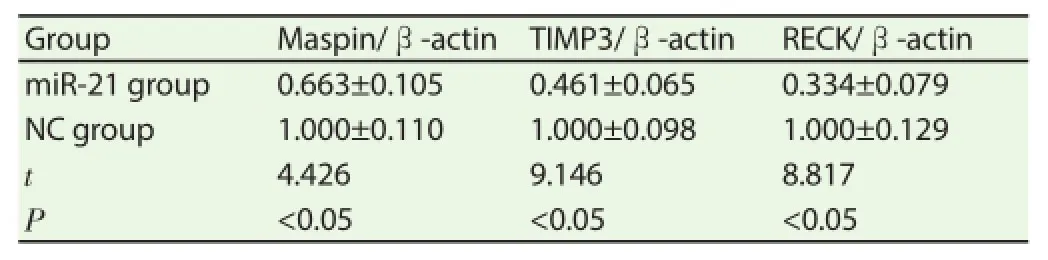
Table 2Eff ect of transfection of miR-21 mimics on invasion-related gene expression.
4. Discussion
MicroRNAs is a type of non-coding small RNA, and the length of mature miRNA is about 18-24bp. Primary transcript PrimiRNA becomes mature miRNA by the hydrolysis of Drosha, Dicer and other enzymes, then is assembled into RNA-induced silencing complex (RISC) and combined with mRNA 3' nontranslated region in the form of complementary base pairing to induce mRNA degradation or inhibit mRNA translation, thus achieving the posttranscriptional regulation of gene expression [6-7] . The pathogenesis of breast cancer is associated with the abnormal expression of multiple genes, but upstream molecules regulating gene expression are unclear. MicroRNA is involved in the regulation of more than 50% of genes in the body by means of posttranscriptional regulation, and miR-21 is confi rmed to be related to the occurrence, metastasis and poor prognosis of tumor. A number of studies have shown that miR-21 expression in breast cancer tissue is signifi cantly higher than that in normal breast tissue suggesting that miR-21 has the function of oncogene in the development process of cancer [8-10] .
In the occurrence and development process of breast cancer, the strong proliferation ability and invasive ability of cancer cells are the key links causing local tumor growth and distant metastasis. In this study, the in vitro cultured breast cancer cell lines were chosen as research tools, transfection of miR-21 mimics was adopted to simulate high miR-21 expression state in breast cancer tissue, and then cell proliferation and invasion ability were evaluated. Analysis of the detection results of cell vitality by MTS showed that OD value of the cells of miR-21 group was significantly higher than that of NC group; analysis of the detection results of cell invasion by transwell showed that the number of invasive cells of miR-21 group was signifi cantly higher than that of NC group suggesting that miR-21 played a promoting role in breast cancer cell proliferation and invasion, and high expression of miR-21 in breast cancer tissue could increase the cell vitality and promote invasive growth of cells.
The main way for miRNA to play its role is to be combined with mRNA 3' non-translated region, and its effect is to cause mRNA degradation or inhibit mRNA transcription. In the occurrence and development process of breast cancer, a variety of molecules are involved in regulating cell proliferation and invasion, and there are miR-21 binding sites in mRNA 3' non-translated region of a variety of proliferation and invasion-related molecules. PDCD-4, FasL, PTEN and RhoB gene-encoded molecules are closely related to the regulation of breast cancer cell proliferation, and Maspin, TIMP3 and RECK gene-encoded molecules are closely related to the regulation of breast cancer cell invasion. Studies have reported that miR-21 can target and regulate PDCD-4, FasL, PTEN, RhoB,
Maspin, TIMP3, RECK and other proliferation and invasion-related genes in bladder cancer, stomach cancer, esophageal cancer and other malignant tumor cells [11-15] . However, it has not been reported whether miR-21 regulates the above expression of proliferation and invasion-related genes in breast cancer cells.
PDCD-4, FasL, PTEN and RhoB are tumor suppressor genes, and their expression significantly reduces in breast cancer, lung cancer and other malignant tumors. Fatty acid synthase ligand (FasL) belongs to the tumor necrosis factor receptor super family, is cell apoptosis signal receptor, and can be combined with Fas to induce cell apoptosis and activate cellular immunity to kill target cells [16] ; programmed cell death receptor 4 (PDCD4) is a kind of tumor suppressor gene, and the protein expressed by it is located in the nucleus and can inhibit cell proliferation and promote cell apoptosis through Sp-transcription factors; PTEN-encoded protein contains 4 cyclic structures of amino acid residue insertion sequence, which can induce cell cycle arrest and apoptosis through PI3K/Akt pathway[17]. After transfection of miR-21 mimics, mRNA contents of proliferation-related molecules PDCD-4, FasL, PTEN and RhoB in breast cancer cell lines decreased signifi cantly. This meant that miR-21 could target and regulate proliferation-related molecules PDCD-4, FasL, PTEN and RhoB in breast cancer cells.
Maspin, TIMP3 and RECK are genes with invasion-inhibiting eff ect, and they show a trend of low expression in breast cancer and lung cancer, which will cause enhanced cancer cell invasion ability. Maspin belongs to the serpin super family, and is a kind of secretory protein that can block the combination of uPA with receptor uPAR to inhibit cell invasion[18]. RECK-encoded protein contains three serine hydrolase inhibitors regions, has inhibitory eff ect on the secretion and activity of several kinds of MMPs, and can prevent MMP2 and MMP9 degradation of extracellular matrix [19] ; tissue inhibitor of metalloproteinase TIMPs can directly combine with MMPs and suppress their function, blocking the degradation of extracellular matrix [20] . After transfection of miR-21 mimics, mRNA contents of invasion-related molecules Maspin, TIMP3 and RECK in breast cancer cell lines decreased significantly. This meant that miR-21 could target and regulate invasion-related molecules Maspin, TIMP3 and RECK in breast cancer cells.
To sum up, miR-21 can promote the proliferation and invasion of breast cancer cell lines, and its downstream target genes include PDCD-4, FasL, PTEN, RhoB, Maspin, TIMP3 and RECK.
Conflict of interest statement
We declare that we have no confl ict of interest.References
[1] Moon HG, Oh K, Lee J, Lee M, Kim JY, Yoo TK, et al. Prognostic and functional importance of the engraftment-associated genes in the patientderived xenograft models of triple-negative breast cancers. Breast Cancer Res Treat 2015; 154(1):13-22.
[2] Stock AM, Klee F, Edlund K, Grinberg M, Hammad S, Marchan R, et al. Gelsolin is associated with longer metastasis-free survival and reduced cell migration in estrogen receptor-positive breast cancer. Anticancer Res 2015; 35(10):5277-5285.
[3] Tian L, Shan W, Zhang Y, Lv X, Li X, Wei C. up-regulation of mir-21 expression predicate advanced clinicopathological features and poor prognosis in patients with non-small cell lung cancer. Pathol Oncol Res 2016; 22(1):161-167.
[4] Karimi Kurdistani Z, Saberi S, Tsai KW, Mohammadi M. MicroRNA-21: Mechanisms of oncogenesis and its application in diagnosis and prognosis of gastric cancer. Arch Iran Med 2015; 18(8):524-536.
[5] Tang Y, Zhou X, Ji J, Chen L, Cao J, Luo J, et al. High expression levels of miR-21 and miR-210 predict unfavorable survival in breast cancer: a systemic review and meta-analysis. Int J Biol Markers 2015; 30(4): 347-358.
[6] Moreno-Moya JM, Vilella F, Simón C. MicroRNA: key gene expression regulators. Fertil Steril 2014; 101(6):1516-1523.
[7] Blahna MT, Hata A. Regulation of miRNA biogenesis as an integrated component of growth factor signaling. Curr Opin Cell Biol 2013; 25(2):233-240.
[8] Yan LX, Liu YH, Xiang JW, Wu QN, Xu LB, Luo XL, et al. PIK3R1 targeting by miR-21 suppresses tumor cell migration and invasion by reducing PI3K/AKT signaling and reversing EMT, and predicts clinical outcome of breast cancer. Int J Oncol 2016; 48(2): 471-484.
[9] De Mattos-Arruda L, Bottai G, Nuciforo PG, Di Tommaso L, Giovannetti E, Peg V, et al. MicroRNA-21 links epithelial-to-mesenchymal transition and infl ammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget 2015; 6(35): 37269-37280.
[10] Abdel-Hamid NR, Mohammed EA, Abbas AH, Badr FM. microrna-21 expression in primary breast cancer tissue among egyptian female patients and its correlation with chromosome 17 aneusomy. Mol Diagn Ther 2015; 19(6):365-373.
[11] Fitzgerald JB, Chennathukuzhi V, Koohestani F, Nowak RA, Christenson LK. Role of microRNA-21 and programmed cell death 4 in the pathogenesis of human uterine leiomyomas. Fertil Steril 2012; 98(3):726-734.
[12] Wang N, Zhang CQ, He JH, Duan XF, Wang YY, Ji X, et al. MiR-21 down-regulation suppresses cell growth, invasion and induces cell apoptosis by targeting FASL, TIMP3, and RECK genes in esophageal carcinoma. Dig Dis Sci 2013; 58(7):1863-1870.
[13] Yazdani Y, Farazmandfar T, Azadeh H, Zekavatian Z. The prognostic eff ect of PTEN expression status in colorectal cancer development and evaluation of factors aff ecting it: miR-21 and promoter methylation. J Biomed Sci 2016; 23(1):9.
[14] Liu M, Tang Q, Qiu M, Lang N, Li M, Zheng Y, et al. miR-21 targets the tumor suppressor RhoB and regulates proliferation, invasion and apoptosis in colorectal cancer cells. FEBS Lett 2011; 585(19):2998-3005. [15] Zhang HH, Qi F, Cao YH, Zu XB, Chen MF. Expression and clinical signifi cance of microRNA-21, maspin and vascular endothelial growth factor-C in bladder cancer. Oncol Lett 2015; 10(4):2610-2616.
[16] Wang Z, Gu J, Nie W, Xu J, Huang G, Guan X. Quantitative assessment of the association between three polymorphisms in FAS and FASL gene and breast cancer risk. Tumour Biol 2014; 35(4):3035-3039.
[17] Lebok P, Kopperschmidt V, Kluth M, Hube-Magg C, Özden C, B T, et al. Partial PTEN deletion is linked to poor prognosis in breast cancer. BMC Cancer 2015; 15(1):963.
[18] Panou M, Kavantzas N, Sergentanis T, Sakellariou S, Agrogiannis G, Chatzipantelis P, et al. Estimation of maspin's subcellular localization in invasive ductal breast cancer via light microscopy and computerized image analysis: a comparative study. J Buon 2013;18(2):342-351.
[19] Gomes LR, Fujita A, Mott JD, Soares FA, Labriola L, Sogayar MC. RECK is not an independent prognostic marker for breast cancer. BMC Cancer 2015; 8(15):660.
[20] Jackson HW, Hojilla CV, Weiss A, Sanchez OH, Wood GA, Khokha R. Timp3 defi cient mice show resistance to developing breast cancer. PLoS One 2015; 10(3):e0120107.
Tel: 13885033479
E-mail: nienyj@hotmail.com
Foundation project: This study was supported by National Natural Science Foundation of China (Grant No.: 81560269)
doi:Document heading 10.1016/j.apjtm.2016.03.025
*Corresponding author:Ying-Jie Nie, Associate Chief Technician, Central Lab, Guizhou Provincial People’s Hospital.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
- Polarization of M1 tumor associated macrophage promoted by the activation of TLR3 signal pathway
- Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Mechanism of low molecular weight GTP binding protein RAC1 in injury of neural function of rats with cerebral ischemia reperfusion
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
