Polarization of M1 tumor associated macrophage promoted by the activation of TLR3 signal pathway
2016-07-25BoLiuXiaWangTaiZhongChenGuangLiangLiChangChunTanYongChenShaoQiangDuanDepartmentofPediatricSurgeryYongchuanhospitalofchongqingmedicaluniversityChongqing402160China
Bo Liu, Xia Wang, Tai-Zhong Chen, Guang-Liang Li, Chang-Chun Tan, Yong Chen, Shao-Qiang DuanDepartment of Pediatric Surgery, Yongchuan hospital of chongqing medical university, Chongqing 402160, China
ABSTRACT
Objective: To investigate the correlation between activation of toll-like receptors 3 (TLR3) signaling pathway and tumor-associated macrophage and its eff ect on the tumor growth. Methods: The mice Lewis lung cancer cell lines 3LL and melanoma B16H10 were used to construct the subcutaneous transplantation tumor models and then they were treated with Poly-ICLC. The curative eff ect was observed and then the T cell and macrophage phenotypes infi ltrated in local tumor were detected by fl ow cytometry. After the in vitro culture of mouse bone marrow-derived macrophage, the real-time PCR and western blot were applied to detect the expression of macrophage activation markers and the activation of intracellular signaling pathways. Results: The survival time of mice with brown tumor treated with Poly-ICLC signifi cantly increased and the tumor growth was inhibited. The ratio of local tumor-infi ltrated Treg decreased, while the ratio of CD8+T cell increased significantly. The macrophages surface CD206 expression was down-regulated while the expression of iNOS increased. The Poly-ICLC could promote the expression of M1 markers (IL-1β, TNF-α毩 and iNOS) in bone marrow-derived macrophage and inhibited the expression of M2 molecules (Arg-1, YM-1 and CD206). The phosphorylation level of downstream p65, TBK1 and IRF3 increased significantly. Conclusions: The Poly-ICLC can activate the TLR3 downstream signaling pathway to induce a M1 polarization of tumor associated macrophage, thereby inhibiting the tumor growth.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016 Accepted 15 March 2016
Available online 20 May 2016
Polarization of M1 tumor associated macrophage promoted by the activation of TLR3 signal pathway
Bo Liu*, Xia Wang, Tai-Zhong Chen, Guang-Liang Li, Chang-Chun Tan, Yong Chen, Shao-Qiang Duan
Department of Pediatric Surgery, Yongchuan hospital of chongqing medical university, Chongqing 402160, China
ABSTRACT
Objective: To investigate the correlation between activation of toll-like receptors 3 (TLR3) signaling pathway and tumor-associated macrophage and its eff ect on the tumor growth. Methods: The mice Lewis lung cancer cell lines 3LL and melanoma B16H10 were used to construct the subcutaneous transplantation tumor models and then they were treated with Poly-ICLC. The curative eff ect was observed and then the T cell and macrophage phenotypes infi ltrated in local tumor were detected by fl ow cytometry. After the in vitro culture of mouse bone marrow-derived macrophage, the real-time PCR and western blot were applied to detect the expression of macrophage activation markers and the activation of intracellular signaling pathways. Results: The survival time of mice with brown tumor treated with Poly-ICLC signifi cantly increased and the tumor growth was inhibited. The ratio of local tumor-infi ltrated Treg decreased, while the ratio of CD8+T cell increased significantly. The macrophages surface CD206 expression was down-regulated while the expression of iNOS increased. The Poly-ICLC could promote the expression of M1 markers (IL-1β, TNF-α毩 and iNOS) in bone marrow-derived macrophage and inhibited the expression of M2 molecules (Arg-1, YM-1 and CD206). The phosphorylation level of downstream p65, TBK1 and IRF3 increased significantly. Conclusions: The Poly-ICLC can activate the TLR3 downstream signaling pathway to induce a M1 polarization of tumor associated macrophage, thereby inhibiting the tumor growth.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016 Accepted 15 March 2016
Available online 20 May 2016
Keywords:
1. Introduction
The tumor associated macrophages (TAM) refer to a kind of macrophages which are migrated and infiltrated in local tumor during the occurrence and development of tumor, and can be brought together with other immune cells, tumor cells, fi broblast and interstitial cytokines to form a tumor immune microenvironment. The TAM can secrete a variety of cytokines and inflammatory mediators which play a key role in the formation of tumor microenvironment and tumor invasion and metastasis. According to the activation type of macrophage, it can be divided to two main types M1 and M2. The M1 has the anti-tumor effect which can be exerted by secreting the proinfl ammatory factor, while M2 can promote the growth, invasion and metastasis of tumor through the expression of immune inhibitory signal molecule [1] . The function of M1 and M2 macrophages is entirely different in the tumor microenvironment. The clinical data have showed that M2 TAM can infi ltrate in a wide variety of tumors and the infi ltration number is negatively correlated with the prognosis of patients[2-5]. Therefore, the regulation and control of M2 TAM can be a key factor for improving the prognosis of tumor. This paper aims to investigate the eff ect of Poly-ICLC on the macrophage polarization and tumor growth.
2. Materials and methods
2.1. Main reagents
The RT-PCR primer was designed and synthesized by Shanghai Sangon Biotechnology Company. The RT-PCR kit and the ordinary PCR kit were purchased from the Takara Company. The mice Lewis lung cancer cell line 3LL and the melanoma B16H10 cell were bought from ATCC and the trizol was purchased from Invitrogen. The DMEM Medium (high glucose), antibiotics, L-glutamine and the fetal calf serum were purchased from Gibco. The protease inhibitor and phosphatase inhibitors were collected from Roche. For the use of western blot, the p-p65 (S536), p-TBK1 (S172), p-IRF3 (S396) primary antibodies and horseradish peroxidase-labeled secondary antibodies were bought from CST. The CD45, CD3, CD4, CD8, Foxp3, CD206, F4/80 and iNOS for flow cytometry were all purchased from eBioscience.
2.2. Animal model
All the animals involved in this study were purchased from the Chongqing Medical University Laboratory Animal Center (C57 mice, clean, 8 weeks old and weighing 20 g). The collected cells were digested by using the pancreatic enzymes and the final concentration was adjusted at 1X107/mL. After that, the treated cells were placed in the ice until use. The mice were narcotized with 200 L of 0.75% sodium pentobarbital solution per mouse and then were conducted to an inguinal subcutaneous injection of 5X105tumor cells. The mice in treating group were then received an intraperitoneal injection of 50 mg Poly-ICLC at 5th day after the model construction, and a drug administration every other day for four times was conducted. While the mice in control group were also received the same volume injection of PBS and then the tumor size was calculated. The experiment was approved by the Biomedical Ethics Committee of Chongqing Medical University.
2.3. Cell cultivation
The cultivation of mice Lewis lung cancer cell line 3LL and melanoma B16H10 cell was conducted in the DMEM medium contained 10% fetal calf serum, 1% double resistant and 2 mM L-glutamine. Then the cultivated cells were stored in the incubator with 5% CO2, saturated humidity and at 37 ℃ for cultivation. The medium was replaced every 2-3 d and the cells were not transferred to a conventional cultivation until the cell density was moderate. The in vitro macrophage cultivation was carried out by using the mouse bone marrow, and after that the BALB/c mice were conducted to death by broken neck dislocation and 75% alcohol disinfection, the bilateral tibiofi bula was obtained under the aseptic condition. Then the distal insertion of a syringe needle (5 mL injector) contained with 1 mL DMEM was conducted. The DMEM was injected to get the bone marrow cell, and then the collected cells were placed in sterile tube to a repeated stir until that the cells distributed evenly. Then using the ACK to crack red cells and they were fi ltered by a 200-mesh sieve. After washing by DMEM for 2 times, the cells were resuspended by using L929 cell-conditioned medium (contained with 30% L929 cell 3-d cultured supernatant) and then were inoculated in a petri dish. After 7 d, the adherent cells were removed and the remaining cells were the bone marrow-derived macrophage.
2.4. Flow cytometry
The single-cell suspension of local tumor tissue was obtained from the collagen digestion and after the isolation by Percol, the mononuclear cell was obtained. The steps for surface antigen staining were conducted strictly in accordance with the Best Protocol (eBioscience). At first, they were blocked by using the CD16/32 antibody for 10 min at 4 ℃ and after that, were washed by PBS to obtain 300 mL PBS heavy suspension. Finally, they were conducted to a detection by using BD fl ow cytometry. The data were analyzed and drawned by applying the FlowJo software.
2.5. Real-time PCR
After cell collection, the total RNA was extracted by trizol method and was dissolved in 20 μL DEPC treating water. Then the concentration of total RNA was detected by using NanoDrop (Thermo, American). The reverse transcription system had 20 μL materials and after adding in 1.5 μg total RNA, they were removed the genomic DNA by using RT-PCR Kit (Takara) and then were conducted to a reverse transcriptional reaction according to the manual operation steps. The obtained reverse transcripts were diluted 10 times and subsequently conducted to a real-time PCR amplification by using real-time qPCR (ABI 7500, American) and SYBR Green kit (Takara). With the β-actin as a reference, the relative gene expression level was calculated by using 2-△△Ctmethod. The primers were synthesized by Shanghai Sangon Biotechnology Company (Table 1).

Table 1The sequence of real-time PCR primer.
2.6. Western blot
The macrophage was processed by using 100 ng/mL of Poly-ICLC for 30 min, and after 1 h, the cells were collected. The precooling PBS, after washing, were conducted to a cell lysis by using lysis buffer contained with 1 M tris–HCl pH 7.5, 1% TritonX-100, 1% NP-40, 10% SDS, 0.5% sodium deoxycholate, 0.5 M EDTA, 10 μg/mL leupeptin,10 μg/mL aprotinin, 1 mM PMSF, 40 mmol/L DTT, phosphatase inhibitors and protease inhibitor. After centrifugation at 4 ℃ 12 000 r/min for 20 min, the supernatant was collected and then tested by Bradford method. The total protein was boiled for 10 min for the denaturation, and the electrophoretic separation and trarsmembran of it were then carried out in the SDS-PAGE gel. The PVDF membrane was blocked in tris buffered saline Tween (TBST) of 5% skimmed milk powder for 2 h and then added in primary antibodies for overnight incubation at 4 ℃. After the TBST was washed for 5 min for 3 times, the second antibody (horseradish peroxidase labeled goat anti rabbit IgG, 1:1 000) was added in and then was incubated at room temperature for 2 h. When the sufficient rinse of TBST for 10 min for total 3 times was finished, the coloration was carried out by using electrochemiluminescence method.
2.7. Statistical analysis
All the experimental data were processed by using GraphPad Prism software and were showed as Mean ± SE. The analysis of the survival curves was tested by applying the Long-Rank. The Means of two groups were tested by using t-test and P<0. 05 was considered statistically signifi cant.
3. Results
3.1. Significant inhibition of tumor growth by Poly-ICLC
In the subcutaneous tumor animal models of 3LL and B16F10, the tumor growth ratio of mice in Poly-ICLC treating group signifi cantly decreased and the survival time of mice with brown tumor was significantly longer than that in PBS control group with that the tumor size also had signifi cant dif ference between two groups after 15-d growth of brown tumor (Table 2), which showed that the Poly-ICLC could inhibit the tumor growth.
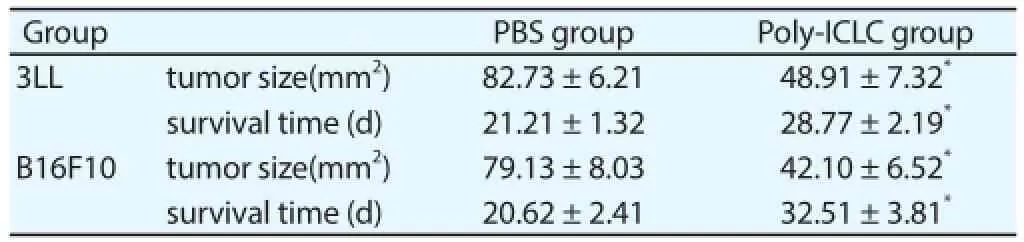
Table 2The eff ect of Poly-ICLC on tumor growth.
3.2. Increase of CD8+T cell ratio and the decrease of Treg (CD3+)
In order to defi ne the mechanism of Poly-ICLC on inhibiting tumor growth, the infi ltration of local tumor T cell was detected by using fl ow cytometry. The result showed that compared with control group, the ratio of Treg of local tumor in mice with brown tumor in treating group notably decreased among the CD45+CD3+cells, while the ratio of CD8+T cell increased signifi cantly, there were signifi cant diff erence between them (Table 3).

Table 3The infi ltration of Treg and CD8+T cells in the local tumor.
3.3. Changes of macrophage phenotypes
Meanwhile, the significant difference was also found in the expression of M1 and M2 macrophage phenotypes in local tumor. Compared with the PBS group, the expression of CD206 (M2-type markers) in macrophage (CD45+F4/80+cells) in treating group was significantly inhibited, while, the expression of iNOS (M1-type markers) increased markedly (Table 4). The results revealed that Poly-ICLC could improve the tumor immune microenvironment.

Table 4Expression of M1/M2 type markers in the macrophage.
3.4. M1 macrophage polarization induced by Poly-ICLC
The Poly-ICLC was used to in vitro processed the bone marrowderived macrophage and after 24 h, the changes of the expression of M1/M2 markers were detected by real-time PCR. As shown in Table 5, the expression of M1 polarization genes iNOS, TNF-毩α and IL-1β of cells treated with Poly-ICLC increases signifi cantly compared with the control group, while the expression of M2 type markers CD206, YM-1 and Arg-1 decreases notably and there is a concentration dependence.

Table 5M1 macrophage polarization induced by Poly-ICLC.
3.5. Activation of toll-like receptors 3 (TLR3) downstream signaling pathways by Poly-ICLC
In order to further discuss the forming pattern of macrophage M1 polarization induced by Poly-ICLC, the TLR3 downstream signaling pathway was tested and the result showed that the Poly-ICLC could caused the signifi cant increased phosphorylation levels of p65, TBK1 and IRF3 of TL3 downstream signaling pathways in macrophage (Table 6), which further confi rm that Poly-ICLC could activate TLR3 downstream signaling pathways in macrophages.
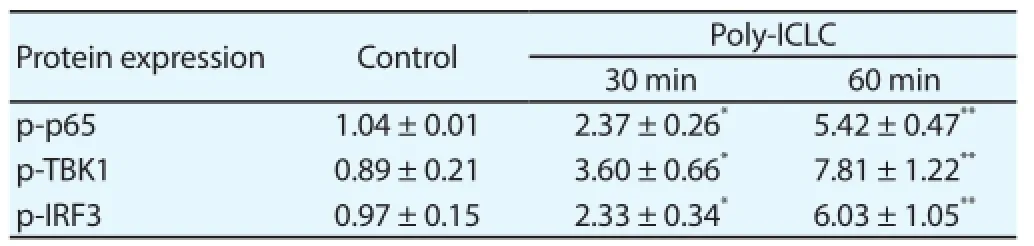
Table 6Phosphorylation of p65, TBK1 and IRF3Poly-ICLC promoted by Poly-ICLC.
4. Discussion
A large number of animal experiments and clinical test have verifi ed that M2 type TAM can promote the growth, infi ltration and migration of tumor, while the M1 type TAM possesses the effect of promoting immune activation and inhibiting tumor growth. Therefore, it may be a new breakthrough point for the future cancer treatment to further explore the transformation between these two cell types, confi rm their signal transduction pathways and molecular mechanisms, to alter local neoplasm M2 macrophages to M1 macrophages polarization through artifi cial means.
Pattern recognition receptors (PPR) plays a key role in the mutual recognition and function of pathogen-associated molecular pattern (PAMP) of the host cell surface and pathogene surface, and in the innate immune response activation [6] . Then, the TCR is considered as the most important PPR which can identify a variety of pathogens, activate the natural immune response, remove viruses or exert the antitumor eff ect [7-9] . TLR3 ligand is a double-stranded RNA which usually does not exist in the mammalian body. It is the product of viral replication, therefore, it is regarded as a crucial ‘danger signal’of inducing the body's natural immune response.
The Poly(I:C), a dsRNA analogue, consists of a ploy (I) and a ploy (C). The Poly-ICLC is produced by using Poly l lysine and carboxymethylcellulose to stabilize Poly(I:C) and both them are the TLR3 agonists. The Poly-ICLC can induce the Th1 cytokine secretion, such as IL-12, TNF-毩α, IFN-毭, IL-6 and type I interferon [10-12] , and induce the expression of chemokines, such as the expression of CXCL9, CXCL10, MCP1, MIP1-a and MIP1–b. It is closely associated with the immune cells recruitment[13]. The patients with glioma tumor treated by a combination therapy of intramuscular poly-ICLC, radiation and temozolomide, the result showed that Poly-ICLC could improve the eff ect of radiation and chemotherapy and had no signifi cant toxic and side eff ect [14] . The Poly-ICLC can also block immunosuppressive molecules signals or other danger signals and then further improve the results of cancer vaccines. It has been used as the immunologic adjuvant in the treatment of lymphoma, liver cancer, colon cancer and leukemia etc., which has achieved a certain curative eff ect [15-17] . The result obtained in this study also showed that the Poly-ICLC could extend the survival time of mice with brown tumor, inhibit the tumor growth and had the antitumor eff ect.
The TLR3 can express in a variety of tissue cells including epithelial cells, tumor cells and immune cells etc. The previous study has reported that Poly-ICLC can induce the tumor cell apoptosis by activating the downstream signaling pathway [18] , while the activation of TLR3 in lymphocyte can induce an innate immune response [19] . The result showed that the ratio of Treg in local tumor of mice treated with Poly-ICLC decreased signifi cantly, while, the ratio of CD8+T cell increased, which could showed that the immune suppressive microenvironment of it had been improved. The Poly-ICLC could caused a increased expression of iNOS (macrophage phenotype M1 markers) in local tumor and inhibit the expression of CD206 (M2 markers), which revealed that Poly-ICLC might have an eff ect on inducing the M1 polarization of TAM.
Through the in vitro cultivation of mouse bone marrow-derived macrophage and after 24-h proceeding by Poly-ICLC, the expression of proinflammatory factor iNOS, TNF-毩α and IL-1 β in macrophage signifi cantly increased, while the expression of inhibitory molecules CD206, YM-1 and Arg-1 decreased notably with that the expression diff erence was positively correlated with the concentration of Poly-ICLC, which showed that Poly-ICLC could directly aff ect the macrophage and induce the changes in the state of immune function of macrophage. Besides, the western blot result revealed that 0.5 and 1 h after received the stimulation from Poly-ICLC, the phosphorylation levels of p65, TBK1 and IRF3 of TL3 downstream signaling pathways in macrophage increased significantly, which further proved that Poly-ICLC could activate TLR3 downstream signaling pathway in macrophages to cause the M1 polarization.
In conclusion, in the subcutaneous transplantation tumor models of mice Lewis lung cancer 3LL and C16B10 melanoma cells, Poly-ICLC can be used as a TLR3 agonist to activate the downstream signaling pathway, induce the polarization of M1 TAM, improve the local inhibitory tumor microenvironment and then exert theantitumor effect. Poly-ICLC can effectively activate the innate immune cells, which has a wide application prospect in anti- tumor treatment.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunolo 2012; 33(3): 119-126.
[2] Cui R, Shi XY. Expression of pyruvate kinase M2 in human colorectal cancer and its prognostic value. Int J Clin Exp Pathol 2015; 8(9): 11393-11399.
[3] Komura T, Sakai Y, Harada K, Kawaguchi K, Takabatake H, Kitagawa H, et al. Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4+T cells with clinical impact. Cancer Sci 2015; 106(6): 672-686.
[4] Zhang BC, Zhang YF, Zhao J, Wang ZG, Wu TT, Ou WL, et al. M2-polarized macrophages contribute to the decreased sensitivity of EGFRTKIs treatment in patients with advanced lung adenocarcinoma. Med Oncol 2014; 31(8): 127.
[5] Becker M, Müller CB, De Bastiani MA, Klamt F. The prognostic impact of tumor-associated macrophages and intra-tumoral apoptosis in nonsmall cell lung cancer. Histol Histopathol 2014; 29(1): 21-31.
[6] Sellge G, Kufer TA. PRR-signaling pathways: Learning from microbial tactics. Semin Immunol 2015; 27(2): 75-84.
[7] Jiménez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE. The critical role of toll-like receptors - From microbial recognition to autoimmunity: a comprehensive review. Autoimmuy Rev 2016; 15(1): 1-8.
[8] van Egmond M, Vidarsson G, Bakema JE. Cross-talk between pathogen recognizing Toll-like receptors and immunoglobulin Fc receptors in immunity. Immunol Rev 2015; 268(1): 311-327.
[9] Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy 2009; 1(6): 949-964.
[10] Gesuete R, Packard AE, Vartanian KB, Conrad VK, Stevens SL, Bahjat FR, et al. Poly-ICLC preconditioning protects the blood-brain barrier against ischemic injury in vitro through type I interferon signaling. J neurochem 2012; 123(Suppl 2): 75-85.
[11] Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res 2014; 2(8): 720-724.
[12] Martins KA, Steffens JT, van Tongeren SA, Wells JB, Bergeron AA, Dickson SP, et al, Dickson SP2, Toll-like receptor agonist augments virus-like particle-mediated protection from Ebola virus with transient immune activation. PloS One 2014; 9(2): e89735.
[13] Zhu X, Fallert-Junecko BA, Fujita M, Ueda R, Kohanbash G, Kastenhuber ER, et al. Poly-ICLC promotes the infiltration of effector T cells into intracranial gliomas via induction of CXCL10 in IFN-alpha and IFN-gamma dependent manners. Cancer Immunol Immunother 2010; 59(9): 1401-1409.
[14] Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 2010; 16(8): 2443-2449.
[15] Martins KA, Bavari S, Salazar AM. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev Vaccines 2015; 14(3): 447-459.
[16] Glavan TM, Pavelic J. The exploitation of Toll-like receptor 3 signaling in cancer therapy. Curr Pharm Des 2014; 20(42): 6555-6564.
[17] Rosenfeld MR, Chamberlain MC, Grossman SA, Peereboom DM, Lesser GJ, Batchelor TT, et al. A multi-institution phase栻study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro Oncol 2010; 12(10): 1071-1077.
[18] Gambara G, Desideri M, Stoppacciaro A, Padula F, De Cesaris P, Starace D, et al. TLR3 engagement induces IRF-3-dependent apoptosis in androgen-sensitive prostate cancer cells and inhibits tumour growth in vivo. J Cell Mol Med 2015; 19(2): 327-339.
[19] Ho V, Lim TS, Lee J, Steinberg J, Szmyd R, Tham M, et al. TLR3 agonist and Sorafenib combinatorial therapy promotes immune activation and controls hepatocellular carcinoma progression. Oncotarget 2015; 6(29): 27252-27266.
Tel: 13617614001
E-mail: zsfvaca@126.com
Foundation project: It was supported by Natural Science Foundation Project of Yongchuan District of Chongqing (Gran No. YCSTC2015nc5026).
Tumor-associated macrophage TLR3
Poly-ICLC
M1
doi:Document heading 10.1016/j.apjtm.2016.03.019
*Corresponding author:Bo Liu, MsC, Attending Physician, Department of Pediatric Surgery, Second people's Hospital of Chongqing City, Chongqing 402160, China.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
- Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
- Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
