Involvement of α5 integrin in survivin-mediated osteosarcoma metastasis
2016-07-25XiaoZhouLiuChengJunLiSuJiaWuXinShiJianNingZhao1SouthernMedicalUniversityGuangzhou510515ChinaDepartmentofOrthopedicsJinlingHospitalMedicalSchoolofNanjingUniversityNanjing1000China
Xiao-Zhou Liu, Cheng-Jun Li, Su-Jia Wu, Xin Shi, Jian-Ning Zhao*1Southern Medical University, Guangzhou 510515, ChinaDepartment of Orthopedics, Jinling Hospital, Medical School of Nanjing University, Nanjing 1000, China
ABSTRACT
Objective: To investigate the role of survivin in osteosarcoma metastasis. Methods: Small interfering RNA (siRNA) was used to knockdown the expression of survivin and α5 integrin in the human osteosarcoma cell line MG63. Western blotting and immunostaining methods was used to assessed the eff ect of survivin knockdown on the expression of α5 integrin through fl ow cytometry and fl uorescence microscopy detection. Meanwhile, the invasion and migration of transfected cells in Transwell and wound healing assays were probed, and the growth situation of these cells transplanted into nude mice was monitored. Results: Knockdown of survivin expression could inhibit the invasion and migration of osteosarcoma MG64 cells in vitro and the expression of α5 integrin on osteosarcoma MG64 cell surface, suggesting that survivin can inhibit the invasion and migration of osteosarcoma cells through downregulation of α5 integrin. Anti-α5 integrin antibody could also markedly decrease the capability of invasion and migration of osteosarcoma MG64 cells. Additionally, knockdown of survivin expression could slow the growth of osteosarcoma MG63 cells transplanted into nude mice. Conclusions: Survivin-directed anti-tumor strategies might be an eff ective method in the treatment of osteosarcoma.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Involvement of α5 integrin in survivin-mediated osteosarcoma metastasis
Xiao-Zhou Liu1,2, Cheng-Jun Li2, Su-Jia Wu2, Xin Shi2, Jian-Ning Zhao1,2*1Southern Medical University, Guangzhou 510515, China
2Department of Orthopedics, Jinling Hospital, Medical School of Nanjing University, Nanjing 210002, China
ABSTRACT
Objective: To investigate the role of survivin in osteosarcoma metastasis. Methods: Small interfering RNA (siRNA) was used to knockdown the expression of survivin and α5 integrin in the human osteosarcoma cell line MG63. Western blotting and immunostaining methods was used to assessed the eff ect of survivin knockdown on the expression of α5 integrin through fl ow cytometry and fl uorescence microscopy detection. Meanwhile, the invasion and migration of transfected cells in Transwell and wound healing assays were probed, and the growth situation of these cells transplanted into nude mice was monitored. Results: Knockdown of survivin expression could inhibit the invasion and migration of osteosarcoma MG64 cells in vitro and the expression of α5 integrin on osteosarcoma MG64 cell surface, suggesting that survivin can inhibit the invasion and migration of osteosarcoma cells through downregulation of α5 integrin. Anti-α5 integrin antibody could also markedly decrease the capability of invasion and migration of osteosarcoma MG64 cells. Additionally, knockdown of survivin expression could slow the growth of osteosarcoma MG63 cells transplanted into nude mice. Conclusions: Survivin-directed anti-tumor strategies might be an eff ective method in the treatment of osteosarcoma.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Keywords:
Survivin
α5 integrin
Osteosarcoma
Metastasis
1. Introduction
Osteosarcoma, the most frequently-encountered primary malignant tumor of bones, represents over 56% of all bone tumors, and it is the third most frequent cause of cancer in adolescents[1-3]. Conventional treatments for osteosarcoma include surgical resection, chemotherapy and radiotherapy, but the prognosis remains poor due to aggressive infi ltration into local tissue and extremelyhigh metastatic rate [4] . Hence, novel therapeutic approaches, such as biological therapies against specifi c targets, are urgently sought [5] .
Survivin is an inhibitor of apoptosis and has been reported to confer tumor resistance to apoptotic stimuli [6] . It is abundantly expressed during fetal development, but its expression is low in most adult human tissues [7] . In some tumor cells, survivin expression increases aberrantly, which makes it be an attractive anti-tumor target. Elevated survivin expression is associated with enhanced proliferation, reduced apoptosis, resistance to chemotherapy and increased tumor recurrence[8,9]. In an animal model, survivin has been reported to contribute to tumor metastasis [10] . The level of survivin expression was correlated with malignant degree of osteosarcoma [11] , even could be a prognostic indicator for osteosarcoma[12-14]. However, little is known regarding the specifi c role of survivin in osteosarcoma. Inhibition of survivin was previously reported to reduce proliferation and increase apoptosis of the human osteosarcoma cell lines[15,16], and meanwhile, it was also associated with spontaneous pulmonary metastasis of osteosarcoma in a mouse model. Nevertheless, the precise mechanisms thatsurvivin regulates tumor cell metastasis are poorly understood.
McKenzie et al. [17] found that in melanoma, survivin could promote the proliferation of tumor cells via enhancement of integrin expression. The integrin family can not only act as receptors for cell adhesion molecules, but also can mediate cell proliferation, differentiation, apoptosis and motility [18-24] . These cell-cell interactions are crucial for tumor cell motility and growth [20] . Integrin expression is associated with metastatic phenotype of various tumors including osteosarcoma [18-21] . Blocking the expression of integrins with specifi c antibodies can decrease the invasiveness of tumor cells in vitro and in vivo [25] .
In this study, the expression of survivin in the human osteosarcoma cell line MG63 was knocked down, and its eff ect on the properties of these cells important for metastasis was investigated. It was found that knockdown of survivin expression could inhibit MG63 invasion and migration, and downregulate the expression of α5 integrin. Subsequently, it was observed that decreased expression of α5 integrin mimicked the eff ect of survivin knockdown on MG63 invasion and migration. The study further confi rmed that the growth of osteosarcoma MG63 cells transplanted into nude mice was suppressed obviously. All these fi ndings suggest that survivin may promote osteosarcoma metastasis by means of expression of α5 integrin.
2. Materials and methods
2.1. Cell culture
Human osteosarcoma cell line MG63 cells (ATCC, Manassas, VA, USA) were cultured and incubated in DMEM containing 10% FBS at 37 ℃.
2.2. Transwell migration and invasion assays
The migratory response of MG63 cells was measured by Transwell migration assay. Specific operations [26] were as follows: The experiment was performed using a Transwell cell culture plate (Corning Costar, Rochester, NY, USA). The Transwell inserts (8 μm pore size) were pre-coated with Matrigel (Becton Dickinson, Bedford, MA). The upper chamber contained the cells in culture medium (5×105/mL) with 1% FBS, and the lower chamber contained culture medium with 20% FBS. The cells transfected with survivinsiRNA (si-survivin) or scramble RNA were cultured in transwell inserts for 48 h. Non-migrated cells were scraped from the upper surface of the membrane with a cotton swab, and migrated cells on the underside of the fi lters were fi xed in methanol for 15 min and stained with 0.05% crystal violet in PBS for 15 min. The number of cells was counted under a microscope.
2.3. Wound healing assay
MG63 cells were cultured as confl uent monolayers and wounded by scratching with a sterile pipette tip. Wounded monolayers were washed twice with PBS to remove detached cells and incubated in fresh medium for an additional 48 h. Images of the wounds were taken immediately at 0 h and 48 h. Migration of cells into the wound was recorded using a phase contrast microscope. The mean percentage of remaining cell-free area was quantif ied using Image J software, and compared with the area of initial wound to indicate wound healing.
2.4. Flow cytometry
MG63 cells were seeded in 6-well plates at 5×104cells per well and transfected with siRNAs (sense: 5’-C A G A C U U G G C C C A G U G U U U-3’, a n t i s e n s e: 5’-AAACACUGGGCCAAGUCUG-3’) obtained from Shanghai GeneChem Co. Ltd (China). After 24 h, the cells were harvested using 0.05% trypsin solution (Sigma, St. Louis, MO, USA), fi xed in 70% ethanol, washed and stained with anti-human α5 integrin mAb (1: 2 000, ebioscience, San Diego, CA, USA), prior to analysis using a FACSort fl ow cytometer (BD, NJ, USA).
2.5. Western blotting analysis
The cells transfected with si-survivin were harvested after two days and cell lysates were loaded onto a 4%-12% Bis-Tris gel, electrophoresed and transfered to a polyvinylidenedifluoride membrane. The membrane was subsequently immunoblotted with rabbit polyclonal anti-survivin (Novus) followed by horseradish peroxidase-conjugated goat anti-rabbit IgG (Pierce). The signals were visualized using an enhanced ECL kit from Amersham Pharmacia Biotech.
2.6. Immunofluorescence
Transfected cells were washed, fi xed with methanol, and air-dried, then incubated with rabbit polyclonal anti-survivin antibody (Novus Biologicals) and secondary fl uorescent antibody. The slides were washed and mounted with 4’, 6-diamidino-2-phenylindole. Images were captured by Zeiss Axioplan 2 imaging microscope.
2.7. Xenograft tumor formation
Six-week-old nude mice (Shanghai SLAC Laboratory Animal Co. Ltd, China) were bred and maintained under specif ic pathogen-free conditions in accordance with the Shanghai Medical Experimental Animal Care Commission. MG63 cells (5×106) transfected with either the scramble RNA or si-survivin were inoculated into the left and right fl anks of nude mice, respectively. Three weeks after inoculation, the mice were sacrifi ced and the tumors were weighed.
2.8. Statistical analysis
Statistical analysis was performed using SPSS 19.0 software (SPSS, Chicago, IL, USA). Data were expressed as the mean±SD of at least three independent experiments. Statistical signifi cance wasanalyzed using a Student’s t-test (two-tailed). P<0.05 was considered to be statistically signifi cant.
3. Results
3.1. Survivin was required for human osteosarcoma MG63 cell migration
After transfection with siRNA, the level of survivin in MG63 cells was detected fi rst. As shown in Figure 1A, the content of survivin in the cells transfected with si-survivin was signifi cantly lower than that in the cells transfected with scramble RNA, indicating successful knockdown of survivin expression.
The eff ect of survivin on invasiveness of MG63 cells was evaluated by Transwell assay. The percentage of survivin knockdown cells that migrated was signifi cantly lower than that of cells transfected with scramble RNA (Figure 1B). The effect of survivin on MG63 cell migration was further assessed by wound healing assay. It was found that survivin knockdown profoundly inhibited the MG63 cell migration (Figure 1C). Collectively, these results demonstrate that survivin is required for human osteosarcoma MG63 cell motility and its knockdown greatly attenuate MG63 cell migration, suggesting that survivin might be involved in the process of osteosarcoma metastasis.

Figure 1. Knockdown of survivin expression in MG63 cells by siRNA and its role in cell invasion.
3.2. Knockdown of survivin expression led to down-regulation of α5 integrin in MG63 cells
Considering the possibility that knockdown of survivin expression in MG63 cells might refl ect decreased cell adhesion to extracellular matrix, it was concluded that α5 integrin might be involved in survivin-induced tumor cell metastasis. It was found that the expression of α5 integrin was markedly reduced in si-survivin transfected cells using fl ow cytometry (Figure 2A) and fl uorescent microscopy (Figure 2B), suggesting that survivin is likely an upstream regulator of α5 integrin.
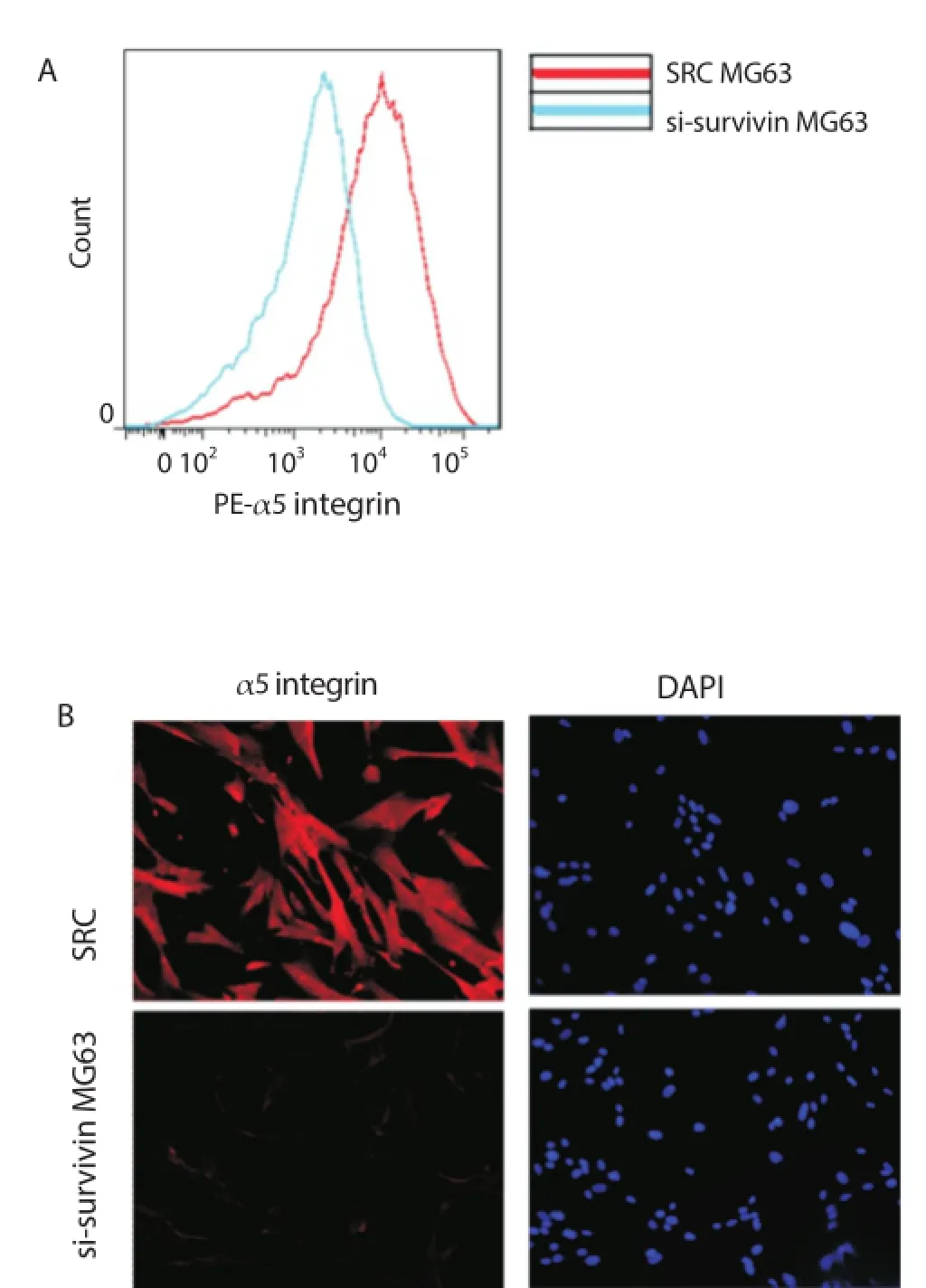
Figure 2. Knockdown of survivin expression led to deceased expression of α5 integrin.
3.3. Knockdown of α5 integrin resulted in decreased metastatic ability of MG63 cells
MG63 cells with α5 integrin targeting siRNA were transduced. The knockdown of α5 integrin expression on the cell surface was confi rmed by fl ow cytometry (Figure 3A), fl uorescent microscopy (Figure 3B) and immunostaining assay.
Transwell and wound healing assays were conducted repeatedly in MG63 cells with knockdown of α5 integrin. As shown in Figure 3C, the cell invasiveness was signifi cantly weakened after knockdown of α5 integrin. Additionally, wound healing assay revealed that the migration of cells was over two fold in α5 integrin knockdown cells than scramble RNA transfected cells (Figure 3D). These results indicate that α5 integrin is related to the migration of human osteosarcoma cells.
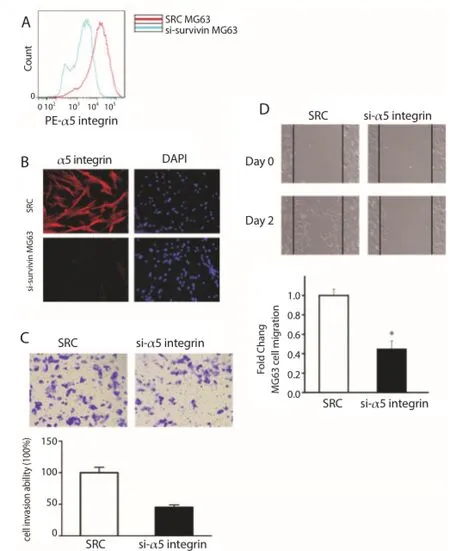
Figure 3. Downregulation of α5 integrin contributed to MG63 cell metastasis.
3.4. Anti-α5 integrin antibody could effectively reduce MG63 motility
The invasiveness and migration of MG63 cells in Transwell and wound healing assays were measured under the action of anti-α5 integrin antibody (M200). It was found that the invasiveness and migration of tumor cells could be inhibited in the treatment of MG63 tumor cells using α5 integrin (Figure 4), suggesting that anti-α5 integrin can eff ectively block the metastasis of MG63 cells.
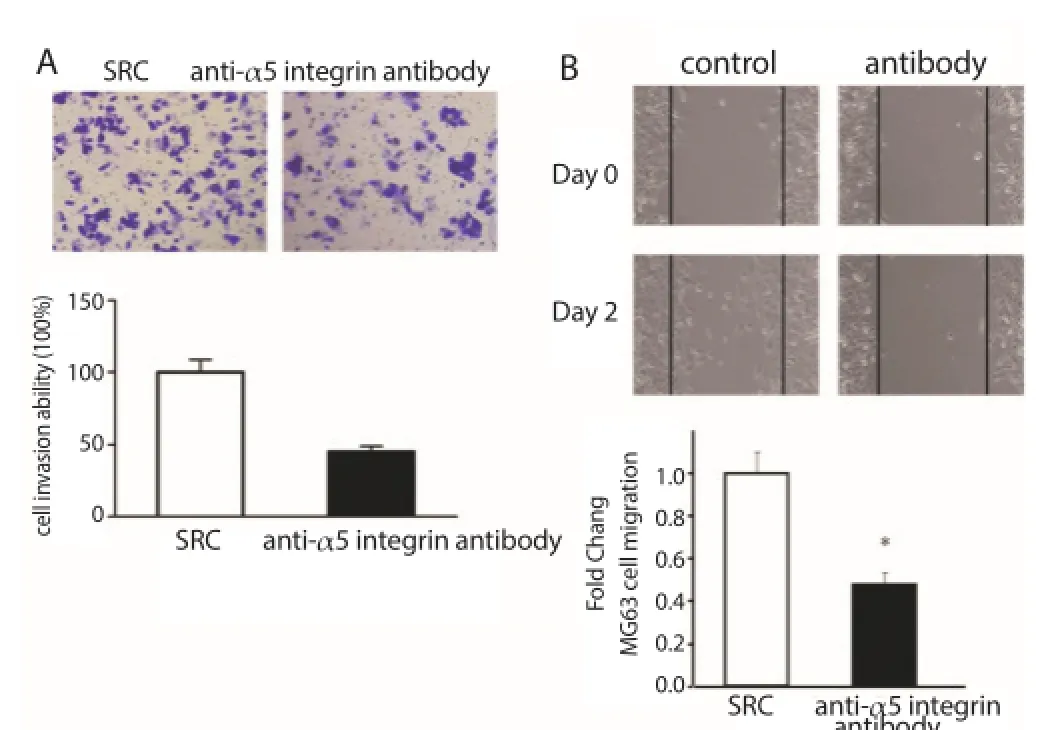
Figure 4. Anti-α5 integrin antibody decreased migration and invasiveness of MG63 cells.
3.5. Knockdown of survivin expression could reduce the tumor growth in vivo
As shown in Figure 5, the tumor volume of survivin knockdown was smaller than that of cells transduced with scramble RNA. These results suggest that inhibition of survivin expression may suppress xenograft tumor formation in osteosarcoma cells in vivo.
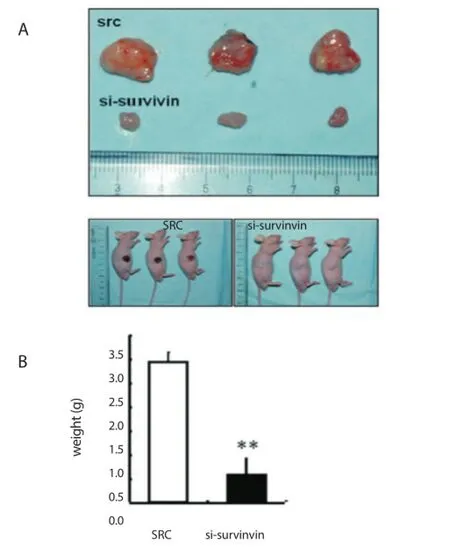
Figure 5. Tumorigenicity of scramble RNA and si-survivin cells (1×106) after subcutaneous injection in the fl anks of nude mice (n=10).
4. Discussion
Survivin can protect osteosarcoma cells from apoptosis and enhance proliferation in vitro[15,16], but its role in osteosarcoma is not well understood. In this study, the role of survivin in the human osteosarcoma metastasis was explored, and siRNA knockdown of survivin expression was established in the human osteosarcoma cell line. It was found that knockdown of survivin expression could reduce the invasion and migration of this human osteosarcoma cell line in vitro, suggesting that survivin may be required for the metastasis of human osteosarcoma.
α5 integrin is a sort of protein that can make cells adhere to the extracellular matrix. The results in this study displayed that knockdown of survivin expression could decrease the expression of α5 integrin on the cell surface. On the contrary, knockdown of α5 integrin could also reduce the invasion and migration of this human osteosarcoma cell line, suggesting that knockdown of survivin expression may decrease the metastasis via inhibition of α5 integrin expression, thus reducing the capability of tumor cells adhering to the extracellular matrix.
In this study, a mouse model of osteosarcoma was established by transplantation of the human osteosarcoma cell line into nude mice so as to probe the role of survivin in osteosarcoma metastasis in vivo. It was found that knockdown of survivin expression could slow the tumor growth of the transplanted human osteosarcoma cell line, indicating that survivin may involve in the process of osteosarcoma proliferation.
The results in this study also revealed that inhibition of survivin was detrimental to human osteosarcoma cell lines, which were consistent with the previous studies [15,16] . Upregulation of survivinmediated α5 integrin could promote the metastasis of melanoma [17] . It is reported that survivin is associated with spontaneous pulmonary metastasis of osteosarcoma in another mouse model [10] . All of these fi ndings point to a potential target for osteosarcoma. As survivin and α5 integrin are required for osteosarcoma metastasis, suppressing the expression of survivin or α5 integrin in tumor cells or blocking the activity of these proteins may reduce the tumor metastasis. For instance, survivin siRNA, antagonizing survivin function by dominant-negative surviving and anti-α5 integrin antibody all are conductive to the development of osteosarcoma treatment. Additionally, it was also found in this study that blocking α5 integrin antibody in vitro could inhibit the invasion and migration of the human osteosarcoma cell line.
It still needs further studies about the role of survivin in tumor cells and downstream signaling processes involved in survivininduced metastasis. Survivin is expressed in some adult cells, but its inhibition is not associated with restrictive side eff ects in animal models[7]. In recent years, a small molecule inhibitor has been reported to inhibit the proliferation of human osteosarcoma cells and induce its apoptosis in a subcutaneous xenograft tumor model via downregulation of survivin pathway [27] . Hence, studying the drugs targeting to survivin may provide a new idea for osteosarcoma.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer 2014; 14(11): 722-735.
[2] He JP, Hao Y, Wang XL, Yang XJ, Shao JF, Guo FJ, et al. Review of the molecular pathogenesis of osteosarcoma. Asian Pac J Cancer Prev 2014; 15(15): 5967-5976.
[3] Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res 2014; 162: 65-92.
[4] Zhou W, Hao M, Du X, Chen K, Wang G, Yang J. Advances in targeted therapy for osteosarcoma. Discov Med 2014; 17(96): 301-307.
[5] Zhang FY, Tang W, Zhang ZZ, Huang JC, Zhang SX, Zhao XC. Systematic review of high-dose and standard-dose chemotherapies in the treatment of primary well-diff erentiated osteosarcoma. Tumour Biol 2014; 35(10): 10419-10427.
[6] Soleimanpour E, Babaei E. Survivin as a potential target for cancer therapy. Asian Pac J Cancer Prev 2015; 16(15): 6187-6191.
[7] Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther 2006; 5(5): 1087-1098.
[8] Pavlidou A, Kroupis C, Dimas K. Association of survivin splice variants with prognosis and treatment of breast cancer. World J Clin Oncol 2014; 5(5): 883-894.
[9] Cai YP, Ma W, Huang XJ, Cao LY, Li H, Jiang Y, et al. Effect of survivin on tumor growth of colorectal cancer in vivo. Int J Clin Exp Pathol 2015; 8(10): 13267-13272.
[10] Zhang L, Ye YY, Yang DJ, Lin JH. Survivin and vascular endothelial growth factor are associated with spontaneous pulmonary metastasis of osteosarcoma: Development of an orthotopic mouse model. Oncol Lett 2014; 8(6): 2577-2580.
[11] Wang W, Luo H, Wang A. Expression of survivin and correlation with PCNA in osteosarcoma. J Surg Oncol 2006; 93(7): 578-584.
[12] Osaka E, Suzuki T, Osaka S, Yoshida Y, Sugita H, Asami S, et al. Survivin as a prognostic factor for osteosarcoma patients. Acta Histochem Cytochem 2006; 39(3): 95-100.
[13] Osaka E, Suzuki T, Osaka S, Yoshida Y, Sugita H, Asami S, et al. Survivin expression levels as independent predictors of survival for osteosarcoma patients. Acta Histochem Cytochem 2006; 39(3): 95-100.
[14] Liu Y, Teng Z, Wang Y, Gao P, Chen J. Prognostic significance of Survivin expression in osteosarcoma patients: A Meta-Analysis. Med Sci Monit 2015; 21: 2877-2885.
[15] Wu YF, Liang XJ, Liu YY, Gong W, Liu JX, Wang XP, et al. Antisense oligonucleotide targeting survivin inhibits growth by inducing apoptosis in human osteosarcoma cells MG-63. Neoplasma 2010; 57(6): 501-506.
[16] Liang XJ, Da MX, Zhuang ZQ, Wu WG, Wu Z, Wu YF, et al. Effects of Survivin on cell proliferation and apoptosis in MG-63 cells in vitro. Cell Biol Int 2009; 33(1): 119-124.
[17] McKenzie JA, Liu T, Jung JY, Jones BB, Ekiz HA, Welm AL, et al. Survivin promotion of melanoma metastasis requires upregulation of α5 integrin. Carcinogenesis 2013; 34(9): 2137-2144.
[18] Bianchi-Smiraglia A, Paesante S, Bakin AV. Integrin β5 contributes to the tumorigenic potential of breast cancer cells through the Src-FAK and MEK-ERK signaling pathways. Oncogene 2013; 32(25): 3049-3058.
[19] Caccavari F, Valdembri D, Sandri C, Bussolino F, Serini G. Integrin signaling and lung cancer. Cell Adh Migr 2010; 4(1): 124-129.
[20] Multhaupt HA, Leitinger B, Gullberg D, Couchman JR. Extracellular matrix component signaling in cancer. Adv Drug Deliv Rev 2015; doi: 10.1016/j.addr.2015.10.013.
[21] Stucci S, Tucci M, Passarelli A, Silvestris F. Avβ3 integrin: Pathogenetic role in osteotropic tumors. Crit Rev Oncol Hematol 2015; 96(1): 183-193. [22] Slack-Davis JK, Parsons JT. Emerging views of integrin signaling: implications for prostate cancer. J Cell Biochem 2004; 91(1): 41-46.
[23] Paulhe F, Manenti S, Ysebaert L, Betous R, Sultan P, Racaud-Sultan C. Integrin function and signaling as pharmacological targets in cardiovascular diseases and in cancer. Curr Pharm Des 2005; 11(16): 2119-2134.
[24] Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 2006; 18(5): 516-523.
[25] Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 2015; 25(4): 234-240.
[26] Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J 2006; 20(11): 1892-1894.
[27] Duan DP, Dang XQ, Wang KZ, Wang YP, Zhang H, You WL. The cyclooxygenase-2 inhibitor NS-398 inhibits proliferation and induces apoptosis in human osteosarcoma cells via downregulation of the survivin pathway. Oncol Rep 2012; 28(5): 1693-1700.
Tel: 86-13951896799
Fax: 86-25-80860015
E-mail: zhaojianning.0207@163.com
Foundation project: This study is financially supported by Clinical Science and Technology Foundation of Jiangsu Province (BL2012002), Natural Science Foundation of Jiangsu Province (BK2012776) and National Natural Science Foundation of China (No. 81000814).
doi:Document heading 10.1016/j.apjtm.2016.03.022
*Corresponding author:Jian-Ning Zhao, Southern Medical University, Guangzhou 510515, Guangdong Province; Department of Orthopedics, Jinling Hospital, Medical School of Nanjing University, Nanjing 210002, Jiangsu Province.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
- Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
- Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
