Anti Toxoplasma antibodies prevalence and associated risk factors among HIV patients
2016-07-25DechassaTegegneMukarimAbdurahamanTadesseMosissaMotiYohannesDepartmentofMicrobiologyandVeterinaryPublicHealthJimmaUniversityBox307JimmaEthiopiaDepartmentofNaturalResourceManagementJimmaUniversityBox307JimmaEthiopia
Dechassa Tegegne, Mukarim Abdurahaman, Tadesse Mosissa, Moti YohannesDepartment of Microbiology and Veterinary Public Health, Jimma University, P.O. Box 307, Jimma, EthiopiaDepartment of Natural Resource Management, Jimma University, P.O. Box 307, Jimma, Ethiopia
ABSTRACT
Objective: To assess the seroprevalence and associated risk factors of toxoplasmosis among HIV patients in Agaro Town Health Center of Jimma zone. Methods: Convenient sampling was used to collect blood samples from 135 patients attending anti retroviral therapy from February to March 2015. Serum samples were tested for anti-Toxoplasma gondii (T. gondii) antibody by using latex agglutination test. Structured questionnaire was used to collect data on socio-demographic and risk factors associated with toxoplasmosis. Results: Overall seroprevalence of toxoplasmosis was 80.7% (109/135, CI: 74.04-87.36). In multivariate analysis significant association was observed between anti T. gondii seropositivity and raw meat consumption (OR: 3.514, CI: 1.167 10.581, P=0.025), knowledge about toxoplasmosis (OR: 5.225, CI: 1.382, P=0.015) and sex (OR: 4.023, CI: 1.382-19.762, P=0.015). Conclusions: Immuno compromised patients showed high rate of seropositivity and thus, it is highly advisable to introduce routine anti-T. gondii antibodies serological screening test prior to ART commencement.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Anti Toxoplasma antibodies prevalence and associated risk factors among HIV patients
Dechassa Tegegne1*, Mukarim Abdurahaman1, Tadesse Mosissa2, Moti Yohannes11Department of Microbiology and Veterinary Public Health, Jimma University, P.O. Box 307, Jimma, Ethiopia
2Department of Natural Resource Management, Jimma University, P.O. Box 307, Jimma, Ethiopia
ABSTRACT
Objective: To assess the seroprevalence and associated risk factors of toxoplasmosis among HIV patients in Agaro Town Health Center of Jimma zone. Methods: Convenient sampling was used to collect blood samples from 135 patients attending anti retroviral therapy from February to March 2015. Serum samples were tested for anti-Toxoplasma gondii (T. gondii) antibody by using latex agglutination test. Structured questionnaire was used to collect data on socio-demographic and risk factors associated with toxoplasmosis. Results: Overall seroprevalence of toxoplasmosis was 80.7% (109/135, CI: 74.04-87.36). In multivariate analysis significant association was observed between anti T. gondii seropositivity and raw meat consumption (OR: 3.514, CI: 1.167 10.581, P=0.025), knowledge about toxoplasmosis (OR: 5.225, CI: 1.382, P=0.015) and sex (OR: 4.023, CI: 1.382-19.762, P=0.015). Conclusions: Immuno compromised patients showed high rate of seropositivity and thus, it is highly advisable to introduce routine anti-T. gondii antibodies serological screening test prior to ART commencement.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Keywords:
Toxoplasma gondii
HIV
LAT
Seroprevalence
1. Introduction
Toxoplasma gondii (T. gondii) is an obligate intracellular protozoan parasite that can cause toxoplasmosis in animals and humans [1] . Feline species is the defi nitive host and plays a central role in the epidemiology of T. gondii infections by shedding resistant oocysts to the environment and thus serving as a signifi cant source of infection for food animals and humans [2] . It has been estimated that one-third of the world human population is infected with toxoplasmosis and the incidence rate of 400-4 000 congenital toxoplasmosis cases per year has been reported [3] . The principal route of acquisition of infections are raw or under cooked meat, vegetable or other feed or water, soil contaminated with oocyst and vertical transmission [4] . Among these sources of infection, raw or undercooked meat consumption has been reported to account for more than 30%-60% [5] . Toxoplasmosis has been considered as a major public health problem among immune-compromised and pregnant women. It has been reported that the prevalence is higher in warm and humid environment [6] . Pregnant women with acute infection during pregnancy are at risk of exposing the unborn to congenital infection [7]. Wide geographic variation in the prevalence of latent Toxoplasma infection have been reported across the world in Latin America, parts of Eastern/Central Europe, the Middle East, parts of south-east Asia and Africa 30%-75%[8]. Geographical variation of prevalence of toxoplasmosis has been reported in diff erent parts of the world: in United State 15% of childbearing age of women are infected with T. gondii[9], 44% in pregnant women in France [10] , greater than 60% in Indonesia [8] and less than 8.38% in Eastern China [11] .
Literatures indicate that T. gondii are asymptomatic in immunecompetent persons [6] but in immunecompromised individuals as CD4+T cell lymphocyte count decrease, reactivation of the latentT. gondii infection follows causing life threatening disease known as toxoplasmic encephalitis [12-14] . Prevalence rate of Toxoplasma infection among HIV positive individuals across the world has been found to vary from 3%-97% [15,16] . According to the report of UNAIDS, 2011 Ethiopia ranks higher in HIV/AIDS prevalence and about 1.5 million people are infected and live with the virus. The seroprevalence of toxoplasmosis among HIV individuals documented in diff erent parts of Ethiopia include 88.2% in Arba Minch Hospital [16] , 87.4% in Bahir Dar [17] and 60% in South West Ethiopia Mettu Karl hospital [18] .
Routine monitoring of T. gondii antibodies has been gaining great importance in immune-compromised individuals though it is not a routine practice in diff erent health care centers of Ethiopia. Even the existing majority of seroprevalence studies are limited to HIV/AIDS patients alone with little emphasis to associated risk factors. Furthermore, there has been no document of toxoplasmosis seroprevalence study in Agaro town. Therefore, the objective of this study was to detect the level of prevalence of latent toxoplasmosis in HIV positive patients registered and under follow-up by health professionals. In addition, it was to assess socio-demographic risk factors associated with this infection and forward recommendations that will improve health service delivery.
2. Materials and methods
2.1. Study design and area
The study design was cross-sectional based in public health center and convenient sampling was used based on inclusion criteria of patient’s willingness to participate in the study from list of record book of the health center attending anti-retroviral therapy (ART) at Agaro town health center from February to March 2015. Agaro town is located in south western Ethiopia, in Oromia National Regional State, Jimma zone, Agaro Woreda, at a distance of 396 km from Addis Ababa. Its astronomical location is 7º 49’ North Latitude and 36º East Longitude at an elevation ranging from 880 m to 3 360 m above seas level (source Agaro city administration). The area receives mean annual rainfall of about 1 530 mm that comes from the long and short rainy season and the mean annual temperature ranges from 25 ℃-30 ℃. Agaro town has a total population of 28 273.
2.2. Study population and sample size
The study populations were human immunodef iciency virus (HIV) positive patients who were on follow up at Agaro town health center for ART. Presently 706 HIV (457 females and 249 males) positive patients attending anti-retroviral therapy. The total sample size was 135; calculated using Thrusfi eld[19] formula based on prevalence of 88.2% taken from a previous study conducted in Arba Minch Hospital [16] , 0.05 margin of error (d) and at conf idence level of 95%. Accordingly, one month data of patients visiting the health center for therapy and counseling was used to recruit participants of this study.
2.3. Data collection
About f ive milliliters of venous blood without anticoagulant was aseptically collected by trained medical laboratory technician following standard procedure from 135 HIV positive patients. Serum was separated and stored at -20 ℃ until the analyses was conducted at Microbiology laboratory of Jimma University. Trained ART attendant nurse interviewed the participants using structured and pretested questionnaire to collect socio-demographic characteristics and risk factors associated with T. gondii infection.
2.4. Serological method
A rapid slide agglutination Toxo-latex test (SPINREACT, S.A/ S.A.U Ctra Santa Coloma, Spain) was used strictly following manufacturer instructions for qualitative and semi-quantitative detection of anti-Toxoplasma antibodies with analytical sensitivity of 4 IUmL. Briefl y, the test contains latex particles coated with soluble T. gondii antigen that are agglutinated when mixed with serum samples containing antibodies against Toxoplasma infection. In all sample analysis conducted both positive and negative control tests were performed to monitor the performance of the procedures and technical procedure was carried out correctly. The kit has diagnostic sensitivity and specifi city of 96.1% and 89.6%, respectively.
2.5. Data management and analysis
Data was recorded and coded in excel spread sheet before analysis using statistical software SPSS version 22. Descriptive statistics was used to summarize the data and hence, the prevalence of toxoplasmosis was calculated as the number in study population testing positive to serological test divided by the total study unit tested. A logistic regression model was used to check the association of the disease toxoplasmosis with potential risk factors. Noncollinear variables that presented P<0.05 in univariable analysis were off ered to the multivariable regression model. The strength of association was calculated using odds ratio at 95% CI. P<00.05 were considered as statistically signifi cant.
2.6. Ethical approval
Ethical approval was obtained from our University research ethical review committee and it was in accordance with the ethicalstandards of our institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
3. Result
3.1. Socio-demographic characteristics of the study participants
Among 706 HIV positive patients attending ART, 135 (84.4%) was included in this study and the percentage of male and female attendees was 43.7% and 56.3%, respectively. Regardless of the disease condition, the mean age of the participants was (33.83±11.11) years (Table 1).
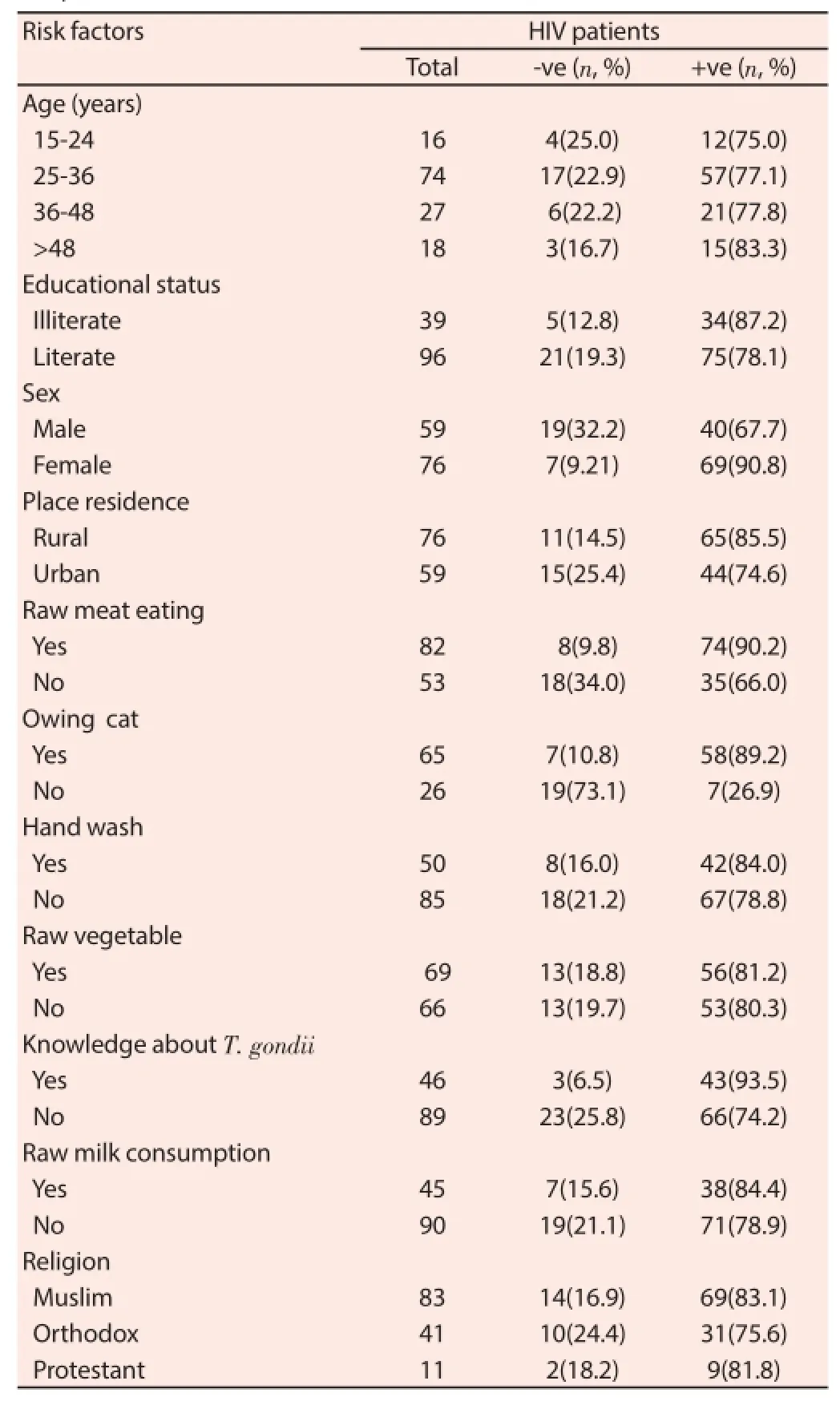
Table 1Distribution of T. gondii infection along with demographic characteristics of HIV patients (n=135).
3.2.1. Seroprevalence of T. gondii infection
The overall seroprevallence of T.gondii infection in this study was 80.7% (109/135, CI: 0.740 0.874). Seroprevalence of T. gondii in relation to diff erent socio-demographic factors is presented in Table 1.
3.2.2. Risk factors associated with T. gondii infection
The bivariate logistic regression analysis showed a significant association between seropositivity to anti-T. gondii antibodies and presence of cat, raw meat consumption, knowledge about T. gondii and sex (Table 1). In multivariate logistic regression analysis, factors such as knowledge about toxoplasmosis, sex and consumption of raw meat were signif icantly associated with seropositivity (Table 2).
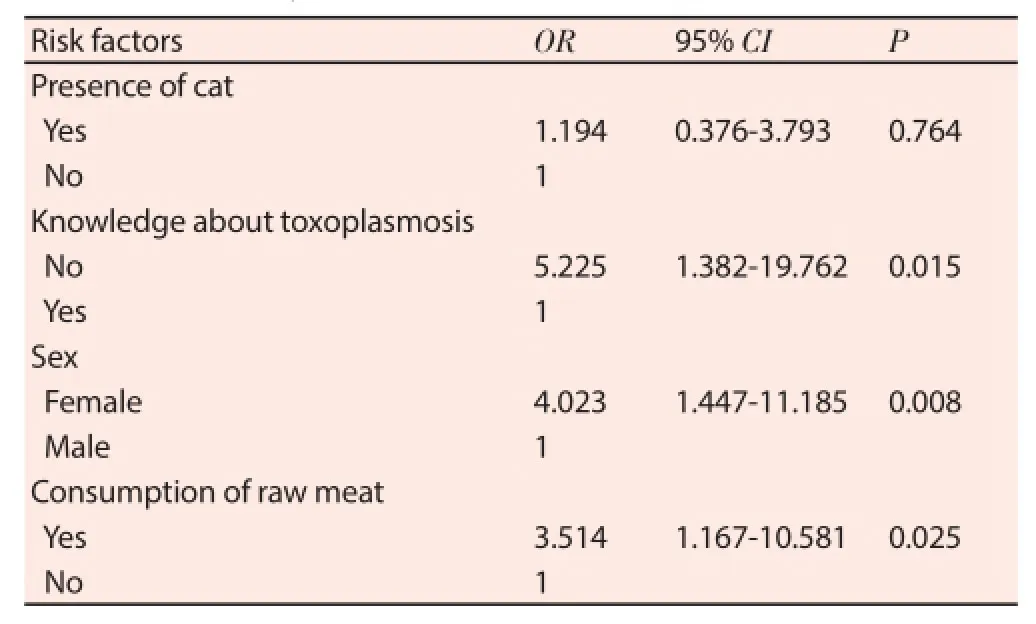
Table 2Multivariate analysis of independent predictor of T. gondii infection among HIV patients (n=135).
Considering different age groups, the prevalence of T. gondii infection among age groups greater than 48 years was the highest. The prevalence in age category 15-24, 25-36, 37-48 and above 48 were 75% (CI: 0.279-32.209), 77.1% (CI: 0.259-4.129), 77.8% (CI: 0.079-1.472) and 83.3% (CI: 0.279- 32.209), respectively and level of infection was observed to increase with age. However, there was no statistically signifi cance association (P>0.05) observed between seropostivity and the diff erent age groups. Moreover, there was no statistically signifi cant association (P>0.05) between seroplrevalence of Toxoplasma infection and level of education, hand washing, residence, religions, raw vegetable and milk consumption (Table 3). It was observed that 48.1% of the participants had domestic cat at their home. Among HIV patients owning cat, 89.2% (CI: 1.200-7.940) were seropostive for Toxoplasma infection. Assessment of raw meat eating habit revealed that majority (60.7%) had the experience of eating raw meat. Among those with raw meat eating habit, the seroprevalence of T. gondii infection was 89.2% (CI: 1.887-11.995). In addition, knowledge about toxoplasmosis has strong association with T. gondii seroprevalence and the percentage of participants who had no knowhow about toxoplasmosis was 65.9%. Among thosewho has no knowledge about T. gondii, 93.5% (CI: 1.413-17.661) was seropositive. Regarding sex of participants, 56.4% were female attending HIV anti-retroviral therapy. The prevalence of anti T. gondii seropositivity was signifi cantly higher in females than males (P=0.001).

Table 3Risk factors associated with T. gondii infection among HIV patients (n=135).
4. Discussion
Toxoplasma is an opportunistic parasite with grave public health impact in immunocompromised individuals. Acute infection of T. gondii results in IgM anti-T. gondii antibody response followed by enduring solid immunity IgG anti-T. gondii antibody which indicate chronic infection. Chronic infection of T. gondii develops encephalitis in HIV positive patients [20] especially in those individuals with CD4+T lymphocytes count level descend below100 cells/μL [21] . Our study showed that T. gondii antibody in HIV positive individuals attending ART was 80.7%. This seroprevalence rate was high and is in agreement with previous studies conducted in diff erent agro-ecological zones of Ethiopia [16-18,22] .
The higher seroprevalence rates documented in this study might be because of individuals close interaction with cat as a pet animal, low level of awareness about means and route of transmission and poor hygienic conditions. However, the variation in seropositivity across diff erent regions might be attributed to rate of reactivation of latent infection tissue toxoplasmosis when reduced cellular immunity in HIV patients [23] and other sociodemographic factors such as,age of the study participants, type of test method used for detection of antibodies, feeding habit and living standard of individuals are some of the factors responsible for the variability of seroprevalence of toxoplasmosis in diff erent agro ecological zones across the globe. For instance, 19.9% in Malaysia [24] , 28.5% in woman in Democratic republic of Congo, 64.8% in western Romania [25] , 18.2% Southern Iran[26], 46% in Mozambique[27], 38.7% in Nigeria[28] and 54% in Uganda[29].
The seroprevalence of T. gondii antibodies in pre-ART as described also previously by diff erent researchers [16,17] was higher than post-ART of this study. This could be possibly due to convalescing the immune status of patients, thus minimizing the incidence of opportunistic infections [30] .
It has been documented that in immune compromised individuals a previously acquired latent infection can lead to reactivation of toxoplasmosis causing severe encephalitis in about 40% of HIV patients and 10%-30% mortality was reported [31] . In view of this fact, almost all seroprevalence studies conducted so far including the present study, mainly focused on measuring the magnitude of antibody against latent infection and yet encephalitis is the most important sequel. This emphasizes on the need for further study on prevalence of encephalitis in this area and elsewhere for proper management of the cases at health centers.
In this study, latent toxoplasmosis seroprevalence was found to increase with age and the diff erence was not statistically signifi cant. This fi nding is in agreement with reports [1,16,32] . This might be due to increased risk of exposure to infection with increasing age. In contrast, the report of [33] indicates signifi cant association between seropositivity and age of patients.
The common traditional taboo of Ethiopia is raw/under cooked meat consumption accounts four times more likely seropostivity for toxoplasmosis. This finding is consistent with the reports of [16,17,22,34] . However, Muluye et al. [34] reported the absence of statistically significant association between seroprevalenceof T. gondii infection and the habit of raw or undercooked meat consumption.
This inconsistency of raw meat consumption in predicting the seroprevalence of T. gondii in individuals depends on seroprevalence of T. gondii in meat producing animals which is 70.48% in sheep, 58.3% in goat in central Ethiopia [35] and 3.6% in cattle in Tanzania [36] , 64% in chicken in Ghana [37] . Furthermore, the area is warmer and humid environment which is favorable climate for survival of the oocyst to maintain its lifecycle. As a result of this fact there is a possible contamination of the environment with T. gondii oocyst excreted by house or street cats available in the area. Our study revealed that contaminated environment with cat excreted oocyst is three times more likely for T. gondii antibodies seropostivity and this finding is in agreement with previous report of [17] and in contrast, with the fi nding of Yesuf and Melese [18] showed no association of presence of cat with T. gondii seropositivity.
Preponderance of the study participants had no knowledge about toxoplasmosis which is one of the independent predictor for the seroprevalence of T. gondii. Even though some of the participants had knowledge about the parasite, they did not know the potential predisposing factors and role of cat in the epidemiology of the toxoplasmosis. Our study also revealed that the odds of T.gondii seropositivity was four times higher in female in contrast with previous fi nding[1]. The study area is well known for its cash crop type of Agriculture whereby gardening and cropping is conducted by women which is the main contributing factor for the high seroprevalence of the disease in women. The ratio of female to male currently attending ART at Agaro health center is 1.8:1 this might be another attributing factor for the activation of latent toxoplasmosis higher in women.
The overall seroprevalence of T. gondii infection antibodies among HIV patients was high and some potential independent risk factors are signifi cantly associated with seropositivity of T. gondii infection. Furthermore, raw meat consumption is a potential source of the parasites and the recommendation should be to avoid raw meat consumption to prevent possible ingestion of viable cysts in meat which modulate immune system conversely decrease CD4+cells leading to activation of the latent toxoplasmosis.
Considering this fact, we recommend toxoplasmosis chemoprophylaxis in all HIV positive individuals to prevent encephalitis. Other hygienic procedures such as hand washing after gardening/farming to minimize the contamination of oocyst need to be included in counseling package of patients. Knowledge, attitude and practice about toxoplasmosis were low and thus, education of the public is needed to create good awareness about T. gondii transmission and risk factors. Moreover, it is highly advisable for health offi cers in charge to include routine serological screening test for determination of anti-T. gondii antibodies among HIV infected individuals pre-ART.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Shimelis T, Tebeje M, Tadesse E, Tegbaru B, Terefe A. Sero-prevalence of latent Toxoplasma gondii infection among HIV-infected and HIV-uninfected people in Addis Ababa, Ethiopia : A comparative crosssectional study. BMC Res Notes 2009; 5: 3-7.
[2] Gajadhar AA, Scandrett WB, Forbes LB. Overview of food- and waterborne zoonotic parasites at the farm level. Rev Sci Tech Off Int Epiz 2006; 25(2): 595-606.
[3] Fong MY, Wong KT, Rohela M, Tan LH, Adeeba K, Lee YY, et al. Unusual manifestation of cutaneous toxoplasmosis in a HIV-positive patient. Trop Biomed 2010; 27(3): 447-450.
[4] Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol 2010; 26(4): 190-196.
[5] Jones J, Lopez A, Wilson M. Congenital Toxoplasmosis. Am Fam Physician 2003; 67(10): 2131-2138.
[6] Studenicova C, Bencaiova G, Holková R. Seroprevalence of Toxoplasma gondii antibodies in a healthy population from Slovakia. Eur J Int Med 2006; 17: 470-473.
[7] Dubey JP. Toxoplasmosis of animals and humans. Boca Ration, Florida, U.S.A,. 2nd edition CRC 2010.
[8] Pappasa G, Roussosa N, Falagasa ME. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol 2009; 39(12): 138513-138594.
[9] Jones JL, Kruszon-moran D, Wilson M, Mcquillan G, Navin T, Mcauley JB. Toxoplasma gondii infection in the United States: Seroprevalence and risk factors. Am J Epidemiol 2001; 154(4): 357-365.
[10] Berger F, Goulet V, Le Strat Y, Desenclos JC. Toxoplasmosis among preganant women in France: Risk factors and change of prevalence between 1995 and 2003. Rev Epidemiol Sante Publique 2009; 57(4): 241-248.
[11] Wang L, He L, Meng D, Chen Z, Wen H, Fang G, et al. Seroprevalence and genetic characterization of Toxoplasma gondii in cancer patients in Anhui Province, Eastern China. Parasit Vectors 2015; 8: 162.
[12] Kodym P, Malý M, Beran O, Jilich D, Rozsypal H, Machala L, et al. Incidence, immunological and clinical characteristics of reactivation of latent Toxoplasma gondii infection in HIV-infected patients. EpidemiolInfect 2015; 143(3): 600-607.
[13] Luma HN, Clet B, Tchaleu N, Mapoure YN, Temfack E, Doualla MS, et al. Toxoplasma encephalitis in HIV/AIDS patients admitted to the Douala general hospital between 2004 and 2009: a cross sectional study. BMC Res Notes 2013; 6: 146.
[14] Dubey JP, Lindsay DS. Biology of Toxoplasma gondii in cats and other animals. Kluwer Academic Publishers 2004.
[15] Nissapatorn V. Review toxoplasmosis in HIV/AIDS: A living legacy. Southeast Asian J Trop Med Public Heal 2009; 40(6): 1158-1178.
[16] Yohanes T, Debalke S, Zemene E. Latent Toxoplasma gondii infection and associated risk factors among HIV-Infected individuals at Arba Minch hospital, South Ethiopia. AIDS Res Treat 2014; 2014: 1-6.
[17] Walle F, Kebede N, Tsegaye A, Kassa T. Seroprevalence and risk factors for Toxoplasmosis in HIV infected and non-infected individuals. Parasites&Vectors 2013; 6(1): 1-8.
[18] Yesuf KM, Melese ZT. Prevalence of Toxoplasmosis in HIV/AIDS patients in Mettu Karl hospital. Am J Heal Res 2015; 3(3): 183-188.
[19] Thrusfield M V. Veterinary epidemiology. Blackwell Publishing Professional, Ames, Iowa, USA 2005.
[20] Zangerle R, Allerberger F, Pohl P, Fritsch P, Dierich MP. High risk of developing toxoplasmic encephalitis in AIDS patients seropositive to Toxoplasma gondii. Med Microbiol Immunol 1991; 180(2): 59-66.
[21] George SM, Malik AK, Hilli F Al. Cerebral Toxoplasmosis in an HIV positive patient: A case report and review of pathogenesis and laboratory diagnosis. Bahrain Med Bull 2009; 31(2): 1-5.
[22] Aleme H, Getachew T, Fekade D, Berhe N, Medhin G. Infectious diseases&therapy sereoprevalence of immunoglobulin-G and of immunoglobulin-M anti-Toxoplasma gondii antibodies in human immunodefi ciency virus infection/acquired immunodefi ciency syndrome patients at Tikur Anbessa. Infect Dis Ther 2013; 1(4): 1-5.
[23] Zhou P, Chen Z, Li H, Zheng H, He S, Lin R, et al. Toxoplasma gondii infection in humans in China. Parasites Vectors 2011; 4(165): 1-9.
[24] Brandon-Mong G-J, Sri NAACM, Sharma RS, Andiappan H, Tan T, Lim YA, et al. Seroepidemiology of toxoplasmosis among people having close contact with animals. Front Immunol 2015; 6(143): 1-6.
[25] Olariu TR, Petrescu C, Darabus G, Lighezan R, Mazilu O. Seroprevalence of Toxoplasma gondii in Western Romania. Infect Dis (Auckl) 2015; 47(8): 580-583.
[26] Davarpanah MA, Mehrabani D, Neirami R, Ghahremanpoori M, Darvishi M. Toxoplasmosis in HIV/AIDS patients in Shiraz, southern Iran. Iran Red Crescent Med J 2007; 9(1): 22-27.
[27] Domingos A, Ito LS, Coelho E, Lucio JM, Matida LH, Ramos Jr AN. Seroprevalence of Toxoplasma gondii IgG antibody in HIV/AIDS-infected individuals in Maputo, Mozambique. Rev Saude Publica 2013; 47(5): 890-896.
[28] Ogoina D, Onyemelukwe GC, Musa BO, Obiako RO. Seroprevalence of IgM and IgG Antibodies to Toxoplasma infection in healthy and HIV-positive adults from Northern Nigeria. J Infect Dev Ctries 2013; 7(5): 398-403.
[29] Lindström I, Kaddu-mulindwa DH, Kironde F, Lindh J. Prevalence of latent and reactivated Toxoplasma gondii parasites in HIV-patients from Uganda. Acta Trop 2006; 100(3): 218-222.
[30] Sukthana Y, Chinatana T, Lekkla A. Toxoplasma gondii antibody in HIV-infected persons. J Med Assoc Thail 2000; 83: 681-684.
[31] Ammassari A, Murri R, Cingolani A, De Luca A, Antinori A. AIDS-associated cerebral toxoplasmosis: an update on diagnosis and treatment. Curr Top Microbiol Immunol 1996; 219: 209-222.
[32] Osunkalu VO, Akanmu SA, Ofomah NJ, Onyiaorah IV, Adediran AA, Akinde RO, et al. Seroprevalence of Toxoplasma gondii IgG antibody in HIV-infected patients at the Lagos University Teaching Hospital. HIV/ AIDS-Reasearch Palliat Care 2011; 3: 101-105.
[33] Falusi O, French AL, Seaberg EC, Tien PC, Watts DH, Minkoff H, et al. Prevalence and predictors of Toxoplasma seropositivity in women with and at risk for human immunodefi ciency virus infection. Clin Infect Dis 2002; 35: 1414-1417.
[34] Muluye D, Wondimeneh Y, Belyhun Y, Moges F, Endris M, Ferede G, et al. Prevalence of Toxoplasma gondii and associated risk factors among people living with HIV at Gondar University Hospital, Northwest Ethiopia. ISRN Trop Med 2013; 2013: 1-5. Doi: http://dx.doi. org/10.1155/2013/123858.
[35] Gebremedhin EZ, Agonafi r A, Tessema TS, Tilahun G, Medhin G, Vitale M, et al. Seroepidemiological study of ovine toxoplasmosis in East and West Shewa Zones of Oromia Regional State, Central Ethiopia. BMC Vet Res 2013; 9(117): 1-8.
[36] Schoonman LB, Wilsmore T, Swai ES. Sero-epidemiological investigation of bovine toxoplasmosis in traditional and smallholder cattle production systems of Tanga Region, Tanzania. Trop Anim Heal Prpduction 2010; 42(4): 579-589.
[37] Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol 2008; 38: 1257-1278.
E-mail: dachassat@yahoo.com
Foundation project: The study was funded by Jimma University, College of Agriculture and Veterinary Medicine (NO. 6223).
doi:Document heading 10.1016/j.apjtm.2016.03.034
*Corresponding author:Dechassa Tegegne, Department of Microbiology and Veterinary Public Health, Jimma University, P.O.Box 307, Jimma, Ethiopia.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
- Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
- Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
