Effects of feeding a diet containing Gymnema sylvestre extract: attenuating progression of obesity in C57BL/6J mice
2016-07-25HyeonJeongKimSeongHoHongSeungHeeChangSanghwaKimAhYoungLeeYoonjeongJangOrkhonselengeDavaadamdinKyeongNamYuJiEunKimMyungHaingChoLaboratoryofToxicologyCollegeofVeterinaryMedicineSeoulNationalUniversitySeoul0886KoreaGrad
Hyeon-Jeong Kim, Seong-Ho Hong, Seung-Hee Chang, Sanghwa Kim,, Ah Young Lee, Yoonjeong Jang, Orkhonselenge Davaadamdin, Kyeong-Nam Yu, Ji-Eun Kim, Myung-Haing Cho,,,,*Laboratory of Toxicology, College of Veterinary Medicine, Seoul National University, Seoul 0886, KoreaGraduate Group of Tumor Biology, Seoul National University, Seoul 0080, KoreaGraduate School of Convergence Science and Technology, Seoul National University, Suwon 69, KoreaAdvanced Institutes of Convergence Technology, Seoul National University, Suwon -70, KoreaInstitute of GreenBio Science Technology, Seoul National University, Pyeongchang-gun, Gangwon-do , Korea
ABSTRACT
Objective: To investigate the eff ect of Gymnema sylvestre extract (GS) on initial anti-obesity, liver injury, and glucose homeostasis induced by a high-fat diet (HFD). Methods: The dry powder of GS was extracted with methanol, and gymnemic acid was identified by high performance liquid chromatography as deacyl gymnemic acid. Male C57BL/6J mice that fed on either a normal diet, normal diet containing 1 g/kg GS (CON+GS) HFD, or HFD containing 1.0 g/kg GS (HFD+GS) for 4 weeks were used to test the initial anti-obesity effect of GS. Body weight gain and food intake, and serum levels about lipid and liver injury markers were measured. Histopathology of adipose tissue and liver stained with hematoxylin and eosin (H&E) and oil-red O were analyzed. After 4 weeks of GS extract feeding, intraperitoneal glucose tolerance test (IPGTT) was performed. Results: The methanol extracts of GS exerted signifi cant anti-obesity eff ects in HFD+GS group. They decreased body weight gain, a lower food and energy effi ciency ratio, and showed lower serum levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL)-cholesterol, very-low density lipoprotein (VLDL)-cholesterol and leptin compared with the HFD group. The decreases of abdominal as well as epididymal fat weight and adipocyte hypertrophy, lipid droplets in liver, and serum levels of aspartate aminotransferase (AST) and alanine transaminase (ALT) were also observed. The CON+GS group showed an eff ect of glucose homeostasis compared to the CON group. Conclusions: This study shows that GS provide the possibility as a key role in an initial anti-obesity eff ects feeding with a HFD.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Effects of feeding a diet containing Gymnema sylvestre extract: attenuating progression of obesity in C57BL/6J mice
Hyeon-Jeong Kim1, Seong-Ho Hong1, Seung-Hee Chang1, Sanghwa Kim1,2, Ah Young Lee1, Yoonjeong Jang1, Orkhonselenge Davaadamdin1, Kyeong-Nam Yu1, Ji-Eun Kim1, Myung-Haing Cho1,2,3,4,5*1Laboratory of Toxicology, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea
2Graduate Group of Tumor Biology, Seoul National University, Seoul 03080, Korea
3Graduate School of Convergence Science and Technology, Seoul National University, Suwon 16229, Korea
4Advanced Institutes of Convergence Technology, Seoul National University, Suwon 443-270, Korea
5Institute of GreenBio Science Technology, Seoul National University, Pyeongchang-gun, Gangwon-do 25354, Korea
ABSTRACT
Objective: To investigate the eff ect of Gymnema sylvestre extract (GS) on initial anti-obesity, liver injury, and glucose homeostasis induced by a high-fat diet (HFD). Methods: The dry powder of GS was extracted with methanol, and gymnemic acid was identified by high performance liquid chromatography as deacyl gymnemic acid. Male C57BL/6J mice that fed on either a normal diet, normal diet containing 1 g/kg GS (CON+GS) HFD, or HFD containing 1.0 g/kg GS (HFD+GS) for 4 weeks were used to test the initial anti-obesity effect of GS. Body weight gain and food intake, and serum levels about lipid and liver injury markers were measured. Histopathology of adipose tissue and liver stained with hematoxylin and eosin (H&E) and oil-red O were analyzed. After 4 weeks of GS extract feeding, intraperitoneal glucose tolerance test (IPGTT) was performed. Results: The methanol extracts of GS exerted signifi cant anti-obesity eff ects in HFD+GS group. They decreased body weight gain, a lower food and energy effi ciency ratio, and showed lower serum levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL)-cholesterol, very-low density lipoprotein (VLDL)-cholesterol and leptin compared with the HFD group. The decreases of abdominal as well as epididymal fat weight and adipocyte hypertrophy, lipid droplets in liver, and serum levels of aspartate aminotransferase (AST) and alanine transaminase (ALT) were also observed. The CON+GS group showed an eff ect of glucose homeostasis compared to the CON group. Conclusions: This study shows that GS provide the possibility as a key role in an initial anti-obesity eff ects feeding with a HFD.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Keywords:
Gymnema sylvestre extract
Obesity
Adipocyte
Body weight gain
High-fat diet
Liver injury
1. Introduction
Obesity, a major risk factor of various disorders, has greatly increased world widely. According to the World Health Organization, obesity has doubled since 1980, and in 2014, more than 1.9 billion people were overweight and over 600 million were obese [1] . Obesity is related to many health problems such as hypertension, type 2 diabetes, stroke, cardiovascular disease, osteoarthritis, asthma, and even certain types of cancer [2,3] . Therefore, the prevention of obesity is an important issue for public health. Major causes of obesity are an energy imbalance between calorie consumption and expenditure and increased intake of a high-fat diet (HFD) [4,5] . A HFD is known to induce a variety of metabolic disorders such as adipocyte hypertrophy, adipose chronic infl ammation, hepatic steatosis of mature adipocytes, and insulin resistance[6]. As these metabolicdisorders are related to the progression of obesity, many studies have been conducted to fi nd anti-obesity agents. For a long time, several medicinal herbs have been used continuously for prevention or treatment obesity. Gymnema sylvestre extract (GS), a dicotyledonous medicinal herb belonging to the family Asclepiadaceae, is a woody climber found in tropical Africa, Australia, central and southern India and China [7] . GS is known to have antimicrobial and antihypercholesterolemic effects, hepatoprotective properties, and especially, effects on obesity and diabetic mellitus [8,9] . In many phytochemical analyses studies, GS is known to include gymnemic acids, sapoins, stigmasterol, quercitol and the amino acid derivative of choline, trim ethylamine and betaine. Above all, its main active compound is gymnemic acid, saponins and gymnemagenin[10]. In particular, several experimental studies of GS have been performed by using gymnemic acid properties and reported in the various fi elds of chemistry, pharmacology, and biotechnology since the 1930s [11] . In previous studies of obesity, oral administration of GS reduced the serum lipid concentration and the eff ect of atherosclerosis in albino rats fed a HFD [12] , and after GS administration for 8 weeks had antiobesity eff ects such as a decrease in body weight, food consumption, and total cholesterol (TC) and triglyceride (TG) levels in HFD-induced obese rats [13] . Oral administration of GS for 3 weeks also decreased serum TC and TG levels but did not signifi cantly aff ect body weight gain in rats[14]. Moreover, antidiabetic eff ects have also been reported; administration of GS for 7 weeks decreased blood glucose levels but increased serum insulin levels in streptozotocin (STZ)-treated diabetic rats [2] . Many scientifi c studies reported antiobesity and antidiabetic effects of GS, but most studies used a mutant mouse model such as ob/ob or db/db or STZ-treated diabetic mice or the HFD-induced obese mouse model. However, the initial preventive regulation of anti-obesity and antidiabetic eff ects when normal C57BL/6J mice are fed a normal diet or HFD containing GS from the beginning have yet to be confirmed. Therefore, the objective of this study was to evaluate whether gymnemic acid in GS extract had initial anti-obesity and antidiabetic eff ects in mice fed a normal diet or HFD containing GS and identify its role as a functional food additive.
2. Materials and methods
2.1. Chemicals and reagents
Paraformaldehyde 20% solution was purchased from Electron Microscopy Sciences (Hatfield, PA, USA). A total of 10% neutral buff ered formalin solution, sucrose, D-(+)-Glucose, xylenes, Mayer’s hematoxylin solution, eosin Y solution and 0.5% oil red O stock solution in propylene glycol were purchased from Sigma-Aldrich (St. Louis, MO, USA). ELISA kit for leptin assay was purchased from SHIBAYAGI (Gunma Prefecture, Japan) and hemoglobin A1c (HbA1c) assay from Crystal Chem (Downers Grove, IL, USA).
2.2. Plant material and extraction of GS
The methanol extract of GS was off ered in All Season Herbs Pvt, Ltd. The obtained GS powder was stored at 4 ℃ until used. The specimen voucher of GS extract (SOP No: ASH/QC/MS/1012) was retained in the All-Season Herbs Pvt. Ltd., Bangalore-66, India. In this specification sheet, GS extract (Batch No: ASH/GYM/4858) was analyzed for physical, product, microbiological profiles and performed gravimetric assay. Additional specimen voucher of GS extract (Report No: NRPL/QCO/09037) was retained in the Natural Remedies Pvt. Ltd., Bangalore-560 100, India. In this report, GS extract (Batch No: ASH/GYM/5856) was analyzed for gymnemic acid assay by using HPLC and total gymnemic acid assay by using gravimetry protocol.
2.3. Phytochemical analysis of GS
For identify of gymnemic acid in GS, gravimetry and HPLC method were performed. In brief, weigh accurately 2 g of GS into a 100 mL beaker and dissolve completely with 60 mL of water. Add 2 mL of 0.1N NaOH and few drops of 10% sulphuric acid with constant stirring till the pH of the solution reaches 2 to 2.5. Standing for 1 h and filtered, and after dry, the percentage of gymnemic acid was calculated. HPLC as Deacyl gymnemic acid (>95% Pure, Natural Remedies Pvt. Ltd., Bangalore-560 100, India) was performed for quantif ication of gymnemic acid. The method of GS extraction and HPLC were performed based on previous methods [15] .
2.4. Animals and diets
Eight-week-old C57BL/6J and db/db male mice were purchased from Central Lab Animal Co. (Seoul, Korea), and housed under a 12-h light/dark cycle in a laboratory animal facility with a temperature of (22 ± 1) ℃ and a relative humidity level of 41% ± 2%. They had free access to pelleted food, except when fasted before necropsy. The animal study methods were approved by the Seoul National University Animal Ethics Committee (SNU-141023-1-2). After 1 week of acclimation on a normal diet (D12450K; Research Diets, New Brunswick, NJ, USA), animals were randomly divided. First, C57BL/6J and db/db mice were divided into 3 groups (7 mice/ group): 1) a control (CON) group, a single oral administration of water, 2) a single oral administration of GS 1.0 g/kg body weight, and 3) a single oral administration of GS 1.5 g/kg body weight. Fasting and postprandial glucose test were performed in these groups.
Second, C57BL/6J mice were divided into 4 groups (10 mice/ group): 1) a control (CON) group fed a normal diet, 2) a group fed a normal diet plus GS 1.0 g/kg (CON+GS group), 3) a HFD group, and 4) a group fed a HFD plus GS 1.0 g/kg (HFD+GS group). HFD contains 60% kcal fat (D12492; Research Diets). These groups were fed the diets for 4 weeks, and body weight gain and food intake were assessed twice per week during the experimental period.
2.5. Measurement of serum glucose levels after a single administration of GS
A blood glucose test was performed in C57BL/6J and db/db mice after a single oral administration of GS. The CON groupswere orally administration of water. Blood glucose concentrations were measured with an Accu-Chek glucometer (Roche, Basel, Switzerland) using Accu-Chek test strips.
2.6. Blood and tissue sample collection
After 4 weeks of feeding experimental diets, the mice were killed. Blood samples were gained from the abdominal vein, and organs were collected. Serum was obtained by centrifugation at 13 000 rpm for 35 min and stored -70 ℃ until used for analyses. The liver, epididymal fat, and peritoneal fat tissue were dissected and weighed. Randomly selected liver and epididymal fat tissue samples were fi xed by 4% and 10% neutral buff ered formalin, and the remaining samples were stored at -70 ℃.
2.7. Hematoxylin and eosin (H&E) staining
The liver and adipose tissue were fixed in 10% neutral buffered formalin, paraffin processed, and sectioned at 5 μm. For histopathological analysis, the sections were placed in xylenes and rehydrated through serial alcohol gradients (100%, 95%, 90%, 80%, 70%, and 50%, 2 min each) and then stained with H&E [16] . For histological analyses of the tissue morphology, samples were examined under a light microscope (Nikon Eclipse Ti; Nikon, Tokyo, Japan). ImageJ software was used to quantify the adipocyte number and size (version 1.48; National Institutes of Health, Bethesda, MD, USA).
2.8. Oil red O staining
Liver tissues were fi xed in 4% paraformaldehyde solution at 4 ℃for 1 d; transferred to 10%, 20%, and 30% sucrose solutions for 1 d each; and then embedded with Tissue-Tek OCT (Sakura, Torrance, CA, USA). Frozen liver sections (8 μm) were cut with a microtome (Leica, Nussloch, Germany) and mounted on slides. After drying, the slides were placed in 100% propylene glycol for 3 min and stained with 0.5% oil red O stock solution in propylene glycol for 7 min at 56 ℃. The slides were then placed in 85% propylene glycol solution for 3 min, rinsed in distilled water for 3 changes, counterstained with Mayer’s hematoxylin, and mounted with an aqueous solution [17] .
2.9. Biochemical analysis
Serum TC, TG, high-density lipoprotein (HDL)-cholesterol, lowdensity lipoprotein (LDL)-cholesterol, very-low density lipoprotein (VLDL)-cholesterol, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were analyzed by the Korea Animal Medical Science Institute (Gyeonggi-do, Korea). Atherogenic index was calculated by using the formula of Schulpis and Karikas; TC-HDL-cholesterol/HDL-cholesterol [18] . Serum leptin and HbA1c concentrations were measured by commercial ELISA kit. All procedures were performed following the manufacturer’s instructions.
2.10. Measurement of serum glucose level and intraperitoneal glucose tolerance test (IPGTT)
An intraperitoneal glucose tolerance test was performed in C57BL/6J mice after an intraperitoneal injection of glucose (1 g/ kg body weight) after a 16-h fast. At the time points indicated, blood glucose concentrations were measured with an Accu-Chek glucometer (Roche, Basel, Switzerland) using Accu-Chek test strips before (0 min) and after (15, 30, 60, 90, and 120 min) the glucose injection.
2.11. Statistical analyses
The results are expressed as mean ± SEM. One-way analysis of variance (ANOVA) with a post hoc Student-Newman-Keuls multiple comparison test was performed using GraphPad (San Diego, CA, USA). Body weight gain, postprandial glucose test, IPGTT, and glucose level curves were statistically compared using two-way repeatedmeasures ANOVA with a Bonferroni post hoc test (GraphPad). All results were considered statistically signifi cant at P< 0.05.
3. Results
3.1. Effects on blood glucose levels after a single oral administration of GS in C57BL/6J and db/db mice
To evaluate the antidiabetic eff ects after a single oral administration of GS, fasting and post prandial blood glucose levels were measured in C57BL/6J and db/db mice. Figure 1A showed the fasting glucose levels in each group, and there were no significant differences. However, blood glucose levels after a single oral administration of GS at 1.0 g/kg body weight concentrations showed significantly decreased both C57BL/6J and db/db groups (Figure 1B). Furthermore, changes of glucose levels within 2 h in db/db mice also showed signifi cantly decreased at 1.0 g/kg and 1.5 g/kg body weight groups (Figure 1C), and confi rmed to area under the curve (AUC) (Figure 1D). Based on these results, further experiments of GS were performed at 1.0 g/kg body weight concentrations.
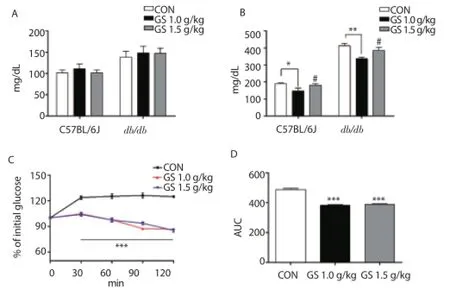
Figure 1. Effects of GS on blood glucose levels after a single oral administration of GS at diff erent concentrations (1.0 g/kg and 1.5 g/kg body weight) in C57BL/6J and db/db mice.
3.2. Effects of GS on body weight gain, food efficiency ratio and energy efficiency ratio in C57BL/6J mice
To investigate whether GS could regulate body weight gain and the food and energy effi ciency ratio, 4 groups of mice: CON, CON+GS, HFD, and HFD+GS were compared. Figure 2A showed the change in body weight during the experimental period. The HFD group had a greater increase in body weight after 6 d than the CON and CON+GS groups. Interestingly, the difference in body weight between the HFD and HFD+GS groups also increased signifi cantly after 21 d. The total body weight gain of the HFD+GS group was significantly reduced compared with the HFD group (P<0.001, Figure 2B). Food intake values were measured, and no signifi cant difference was shown between groups (Figure 2C). However, compared with the HFD group, the energy efficiency ratio was signifi cantly reduced in the HFD+GS group (P<0.001, Figure 2D). Total energy intake values of each experimental diet for 4 weeks were measured, and the value of the HFD and HFD+GS groups were signifi cantly higher than the CON and CON+GS groups (Figure 2E). Interestingly, energy effi ciency ratio values in the HFD+GS group were signifi cantly lower compared with the HFD group (P<0.001, Figure 2F). These results suggested that GS decreased body weight gain induced by HFD in association with decreases in food intake and energy effi ciency ratio.
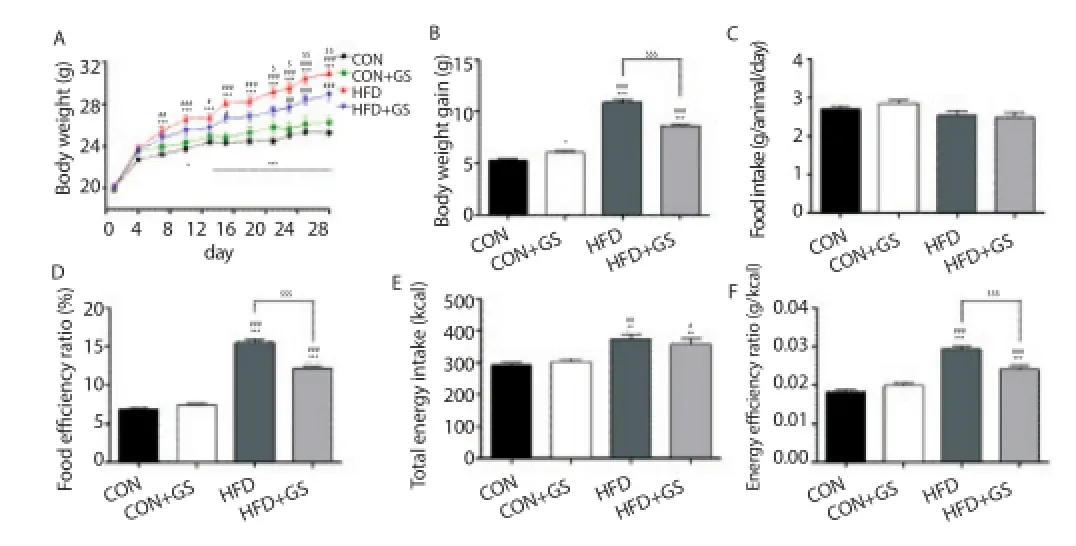
Figure 2. Effects of GS on body weight gain, food efficiency ratio and energy effi ciency ratio in C57BL/6J mice.
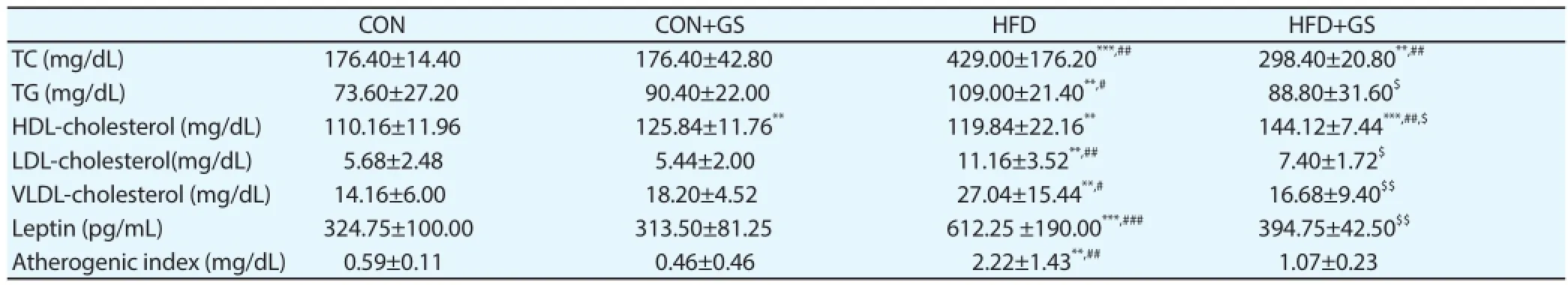
Table 1Serum levels of lipid parameters (n=6-9).
3.3. Effects of GS on serum levels of lipid parameters in C57BL/6J mice
Serum levels of lipid parameters are important for evaluation of obesity. As shown in Table 1, feeding a HFD increased serum TC, TG, LDL-cholesterol, VLDL-cholesterol and leptin levels compared to feeding a normal diet. Interestingly, GS signifi cantly decreased serum TG (P<0.05), LDL-cholesterol (P<0.05), VLDL-cholesterol (P<0.01) and leptin (P<0.01) levels, and increasedserum HDL-cholesterol levels (P<0.05) when feeding with HFD. When feeding a normal diet, however, no signifi cant diff erences between groups except for HDL-cholesterol levels (P<0.01). Atherogenic index was also significantly increased in the HFD group compared to CON and CON+GS groups, but not in the HFD+GS group. These results indicated that the HFD for 4 weeks induced hypercholesterolemia, whereas GS could prevent the increases in serum lipid levels.
3.4. Effects of GS on adipocyte hypertrophy and hyperplasia in C57BL/6J mice
Next, whether the decreased body weight gain in the HFD+GS group was related to adipocyte changes was questioned. Adipocyte hypertrophy and hyperplasia in abdominal fat was analyzed. Histologic examination of epididymal adipose tissue stained with H&E demonstrated that adipocyte size in the HFD group was signifi cantly larger than that in the CON and CON+GS groups, but the HFD+GS group showed a marked decrease in adipocyte size (Figure 3A). We also quantifi ed the number of adipocytes and mean area based on a normalized fi eld. In the HFD group, the number of adipocytes was significantly lower (P<0.001, Figure 3B), and the mean adipocyte area was signifi cantly larger than in the HFD+GS group (P< 0.001, Figure 3C). In the adipocyte area distribution; the graph shape was distributed to forward in CON and CON+GS groups; but the HFD group to widespread. Interestingly, the shape of the distribution of the HFD+GS group was similar to that of the CON and CON+GS groups; therefore, these results indicated that GS acts to prevent adipocyte hypertrophy and hyperplasia. The percentage of total abdominal fat per body weight was signifi cantly increased in the HFD and HFD+GS groups compared with the CON and CON+GS groups (P<0.001, Figure 3D), but signifi cantly decreased in the HFD+GS group compared with the HFD group (P<0.001). The epididymal and peritoneal fat weights of the HFD+GS group were also markedly decreased (P<0.01, P<0.001, Figure 3E, F).

Figure 3. Eff ects of GS extract on adipocyte hypertrophy and hyperplasia.
3.5. Effects of GS on hepatic steatosis and injury in C57BL/6J mice
Hepatic steatosis and injury are usually correlated with obesity, so liver sections from all groups stained with H&E was evaluated (Figure 4A). Representative liver sections of HFD group showed enlarged vacuoles (black arrows), suggesting hepatic lipid deposition, compared with the CON, CON+GS and HFD+GS groups. In addition, the serum AST and ALT levels were also evaluated (Figure 4B, C). AST and ALT levels in the HFD group was signifi cantly increased compared with the CON and CON+GS groups, but signifi cantly decreased in the HFD+GS group compared to HFD group. Consequently, these results indicated that HFD triggered hepatic steatosis and injury, whereas GS significantly suppressed this eff ect.
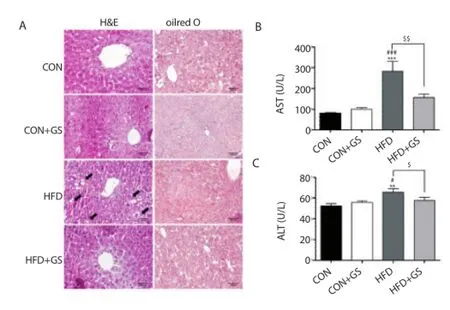
Figure 4. Eff ects of GS on hepatic steatosis and injury.
3.6. Effects of GS on blood glucose levels in C57BL/6J mice
Figure 5 shows the effect of GS on glucose homeostasis in C57BL/6J mice. After 4 weeks, an IPGTT was performed after 16 h of fasting (Figure 5A). The blood glucose levels were all higher in the HFD and HFD+GS groups compared with the CON and CON+GS groups during the 120-min test, indicating glucose intolerance. Interestingly, there was signifi cant diff erence between the HFD and HFD+GS groups at 60 min (P<0.05). The AUC of the IPGTT was also measured, which showed significant increase in the HFD and HFD+GS groups compared with CON and CON+GS groups. Interestingly, this results showed 11.3% decrease in the CON+GS group compared with CON group and 9.2% decrease in the HFD+GS group compared with the HFD group (Figure 5B). Additionally, HbA1c levels were also evaluated after 4 weeks, and the HFD+GS group was signifi cantly decreased compared with the HFD group (P<0.05, Figure 5C). These results indicated that there was an effect on glucose homeostasis in C57BL/6J mice fed the HFD containing GS for 4 weeks.
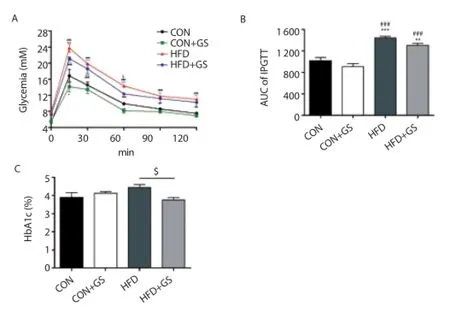
Figure 5. Eff ects of GS on glucose homeostasis.
4. Discussion
Obesity is a major risk factor of various diseases such as type 2 diabetes, hypertension, cardiovascular disease, arthritis, and certain types of cancer [2,19] . Recently, the imbalance between energy consumption and expenditure has been known as a major cause of obesity and this imbalance could be facilitated by eating a HFD [20,21] . It is well known that HFD results in increased body weight, adipocyte hypertrophy, hepatic steatosis, and visceral adiposity [22] . Furthermore, HFD influences the serum levels of several biochemicals such as TC, TG, LDL-cholesterol, and VLDL-cholesterol[19]. For these reasons, the HFD-induced obesity model is widely used to investigate anti-obesity effects. GS is one ofthe traditional medicinal plants used to treat diverse diseases[23]. A number of reports demonstrated that GS has antidiabetic, antiinfl ammatory, antioxidant, anti-atherosclerotic, anticancer, and antiobesity activities [2,10] . Recently, the anti-obesity effect of GS has been evaluated in many studies using HFD-induced obesity or ob/ ob mouse models. However, a limitation of these models is that the animals are already obese; making it diffi cult to evaluate an initial anti-obesity eff ect. Therefore, in the present study, we evaluated the initial anti-obesity eff ect by feeding a normal diet containing GS and HFD containing GS to determine whether GS could regulate the increase in body weight, adipocyte hypertrophy, and hepatic steatosis. To set the concentration of GS, a single oral administration of GS at 1.0 g/kg and 1.5 g/kg body weight concentration was performed in C57BL/6J and db/db mice. In these results, blood glucose levels were signifi cantly decreased in both C57BL/6J and db/db mice, especially at 1.0 g/kg body weight concentration. At the concentration of 1.5 g/kg body weight showed marginal eff ect compared to 1.0 g/kg body weight concentration. Based on these results, the optimal concentration of further experiments was 1.0 g/kg body weight in this study. Our present study clearly showed that the HFD+GS group had signifi cantly decreased body weight gain and food and energy effi ciency ratio compared with the HFD group; however, there was no diff erence in food intake among all groups. According to the food effi ciency ratio and energy effi ciency ratio, the change in body weight was the most important factor, as there was no significant change in food and energy intake. Thus, food efficiency ratio and energy efficiency ratio could be eff ective parameters to predict an anti-obesity eff ect [24] and these results indicated that GS could attenuate body weight gain related to food efficiency ratio and energy efficiency ratio. Moreover, several studies have shown that GS affects the serum levels of lipid parameters; TG, TC, HDL-cholesterol, LDL-cholesterol and leptin that related to obesity. Oral administration of GS (100 mg/ kg body weight daily) to STZ diabetic rats decreased TG, TC, and LDL-cholesterol but increased HDL-cholesterol levels [25] , and GS (120 mg/kg, orally) fed to HFD rats for 21 d decreased serum lipid, apolipoprotein A, and leptin levels and increased HDL-cholesterol levels [10] . In the present study, serum levels of TC, TG, LDL-cholesterol and VLDL-cholesterol were markedly decreased and HDL-cholesterol was increased in the HFD+GS group compared with the HFD group. In particular, serum leptin levels are correlated with body weight changes, adiposity, and the proportion of body fat mass[26]. In this study, serum leptin levels in the HFD+GS group were also markedly decreased, indicating that GS may regulate the decrease of body weight gain and fat accumulation in adipose tissue. Atherogenic index is used for predictors for metabolic disturbances like dyslipidemia, hypertension, atherosclerosis and cardiovascular diseases [27] . In the HFD group, serum levels of atherogenic index was significantly increased compared to CON and CON+GS groups. This result confirmed that feeding a HFD for 4 weeks induce metabolic disturbances and GS could prevent these metabolic disturbances markedly. Adipocyte hypertrophy and hepatic steatosis are induced by HFD, and they play critical roles in the development of the metabolic syndrome, such as infl ammation and insulin signaling problems [28] . Recent studies have shown that adipose tissue is not a simple energy store but is also an endocrine organ that secretes agents such as adipokines and growth factors, which have an important role in homeostasis regulation[25]. When adipose tissue is expanded by hypertrophy, hyperplasia, or both, it promotes infl ammation and macrophage infi ltration and can lead to the development of obesity [26] . Furthermore, imbalanced lipogenesis and interaction between adipose tissue and liver may cause hepatic steatosis [6] , and the prevention of adipocyte hypertrophy and hepatic steatosis is an important factor for evaluating anti-obesity eff ects. In our histological examination of adipose tissue, adipocyte mean area and density were signifi cantly decreased in the HFD+GS group. Epididymal and peritoneal as well as abdominal fat weight was also decreased compared with the HFD group. Furthermore, the number of fat droplets in liver tissue was signifi cantly decreased in the HFD+GS group compared with the HFD group. To investigate whether GS could prevent liver injury induced by HFD, we measured serum levels of AST and ALT, representative biomarkers of liver injury and also indicators of liver function [25,29] . Both AST and ALT levels were decreased in the HFD+GS group compared with the HFD group; therefore, we suggest that GS plays a key role in suppression of adipocyte hypertrophy and hepatic steatosis induced by HFD. In addition, to evaluate whether GS could regulate the initial glucose homeostasis induced by HFD, glucose and HbA1c levels and performed IPGTT were measured. The hypoglycemic effects of GS were identified in many studies, especially those targeting type 2 diabetes. Oral administration of GS (20 mg/kg body weight) to STZ diabetic rats decreased plasma glucose levels by more than 50% and HbA1c by 40% [30] , and GS (100 mg/kg) fed to STZ diabetic rats for 7 weeks decreased blood glucose levels and increased serum insulin levels [2] . In this study, GS decreased blood glucose level during IPGTT in the CON+GS and HFD+GS groups. Therefore, further mechanism studies that how to regulate the initial progression of obesity and glucose homeostasis are needed. In conclusion, GS had several initial anti-obesity eff ects when fed with a normal diet and HFD for 4 weeks; decreased body weight gain, serum levels of lipid parameters, epididymal and peritoneal fat weight, percentage of body weight that is abdominal fat, adipocyte hypertrophy and hyperplasia, and hepatic steatosis and injury. These results provide the possibility of GS as a food additive that could play a key role in an initial anti-obesity eff ect. Taken together, our results strongly suggest that GS seems to have initial anti-obesity as well as preventive eff ects against obesity.
Conflict of interest statement
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the Bio-Synergy Research Project (NRF-2012M3A9C4048819) of the Ministry of Science, ICT and Future Planning through the National Research Foundation. H.J.K, S.H.H, S.K, A.Y.L, Y.J, O.D are supported by BK21 PLUS Program for Creative Veterinary Science Research and Research Institute for Veterinary Science, Seoul National University.
References
[1] World Health Organization. Global Status Report On Noncommunicable Diseases 2014. [Online]. Available from: http://www.who.int/nmh/ publications/ncd-status-report-2014/en/[Accessed on 10 Oct 2015].
[2] Pothuraju R, Sharma RK, Chagalamarri J, Jangra S, Kumar Kavadi P. A systematic review of Gymnema sylvestre in obesity and diabetes management. J Sci Food Agric 2013; 94(5): 834–840.
[3] Kumar V, Bhandari U, Tripathi CD, Khanna G. Evaluation of antiobesity and cardioprotective eff ect of Gymnema sylvestre extract in murine model. Indian J Pharmacol 2012; 44(5): 607-613.
[4] Xu Y, Zhang M, Wu T, Dai S, Xu J, Zhou Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caff eine in rats fed with a high-fat diet. Food Funct 2015; 6(1): 296–303.
[5] KimJ, Jang JY, Cai J, Kim Y, Shin K, Choi EK,et al. Ethanol extracts of unroasted Coffea canephora robusta beans suppress adipogenesis in preadipocytes and fat accumulation in rats fed a high-fat diet. Food Sci. Biotechnol 2014; 23(6): 2029-2035.
[6] Gao M, Ma Y, Liu D. High-fat diet-induced adiposity, adipose infl ammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One 2015; 10(3): e0119784.
[7] Leach MJ. Gymnema sylvestre for diabetes mellitus: a systematic review. J Altern Complement Med 2007; 13(9): 977–983.
[8] Tiwari P, Mishra BN, Sangwan NS. Phytochemical and pharmacological properties of Gymnema sylvestre: an important medicinal plant. Biomed Res Int 2014; 2014: 830285.
[9] Vermaak I, Viljoen AM, Hamman JH. Natural products in anti-obesity therapy. Nat Prod Rep 2011; 28(9): 1493-1533.
[10] Kishore L, Kaur N, Singh R. Role of Gymnema sylvestre as alternative medicine. J Homeop Ayurv Med 2014; 3(4): 172-180.
[11] Porchezhian E, Dobriyal RM. An overview on the advances of Gymnema sylvestre: chemistry, pharmacology and patents. Pharmazie 2003; 58(1): 5–12.
[12] Bishayee A, Chatterjee M. Hypolipidaemic and antiatherosclerotic eff ects of oral Gymnema sylvestre R. Br. Leaf extract in albino rats fed on a high fat diet. Phytother Res 1994; 8(2): 118–120.
[13] Reddy RM, Lathan PB, Vijaya T, Rao DS. The saponin-rich fraction of a Gymnema sylvestre R. Br. aqueous leaf extract reduces cafeteria and highfat diet-induced obesity. Z Naturforsch C 2012; 67(1-2): 39–46.
[14] Shigematsu N, Asano R, Shimosakan M, Okazaki M. Effect of administration with the extract of Gymnema sylvestre R. Br leaves on lipid metabolism in rats. Biol Pharm Bull 2001; 24(6): 713-717.
[15] Kusum D, Nimisha J. A validated HPLC method for estimation of Gymnemic acids as Deacyl gymnemic acid in various extracts and formulations of Gymnema sylvestre. Int J Phytomed 2014; 6(2): 165-169.
[16] Kim JE, Lim HT, Minai-Tehrani A, Kwon JT, Shin J, Woo CG, et al. Toxicity and clearance of intratracheally administered multiwalled carbon nanotubes from murine lung. J Toxicol Env Health Part A 2010; 73(21-22): 1530–1543.
[17] Jung TS, Kim SK, Shin HJ, Jeon BT, Hahm JR, Roh GS. α-lipoic acid prevents non-alcoholic fatty liver disease in OLETF rats. Liver Int 2012; 32(10): 1565–1573.
[18] Giuliano Ide C, Coutinho MS, Freitas SF, Pires MM, Zunino JN, Ribeiro RQ. Serum lipids in school kids and adolescents from Florianópolis, SC, Brazil--Healthy Floripa 2040 study. Arq Bras Cardiol 2005; 85(2): 85-91.
[19] Mopuri R, Meriga B. Anti-lipase and anti-obesity activities of Terminalia paniculata bark in high calorie diet-induced obese rats. Global J Pharmacol 2014; 8(1): 114-119.
[20] Lee MR, Kim BC, Kim R, Oh HI, Kim HK, Choi, KJ, et al. Anti-obesity eff ects of black ginseng extract in high fat diet-fed mice. J Ginseng Res 2013; 37(3): 308–314.
[21] Li Q, Liu Z, Huang J, Luo G, Liang Q, Wang D, et al. Anti-obesity and hypolipidemic eff ects of Fuzhuan brick tea water extract in high-fat dietinduced obese rats. J Sci Food Agric 2013; 93(6): 1310–1316.
[22] JinD, Xu Y, Mei X, Meng Q, Gao Y, Li B, et al. Antiobesity and lipid lowering eff ects of theafl avins on high-fat diet induced obese rats. J Funct Food 2013; 5(3): 1142–1150.
[23] Gulab ST, Rohit S, Bhagwan SS, Mukeshwar P, GBKS P, Prakash SB. Gymnema sylvestre: An alternative therapeutic agent for management of diabetes. J App Pharm Sci 2012; 2(12): 1-6.
[24] Choi HK, Won EK, Jang YP, Choung SY. Antiobesity effect of Codonopsis lanceolata in high-calorie/high-fat-diet-induced obese rats. Evid Based Complement Alternat Med 2013; 2013: 210297.
[25] Kang Y, Lee HY, Kim JH, Moon DI, Seo MY, Park SH, et al. Antiobesity and anti-diabetic eff ects of Yerba Mate (Ilex paraguariensis) in C57BL/6J mice fed a high-fat diet. Lab Anim Res 2012; 28(1): 23–29.
[26] Yoon YI, Chung M, Hwang JS, Han M, Goo TW, Yun EY. Allomyrina dichotoma (Arthropoda: Insecta) Larvae confer resistance to obesity in mice fed a high-fat diet. Nutrients 2015; 7(3): 1978-1991.
[27] Ikewuch CJ, Ikewuchi CC. Alteration of plasma lipid profiles and atherogenic indices by Stachytarpheta jamaicensis L. (Vahl). Biokemistri 2009; 21(2): 71-77.
[28] Yang ZH, Miyahara H, Takeo J, Katayama M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and infl ammation in mice. Diabetol Metab Syndr 2012; 4(1): 32-46.
[29] Mohajeri D, Sefidan AM. Inhibitory effects of Solanum lycopersicum L. on high fat diet-induced fatty liver disease in rats. AdvBiores 2013; 4(4): 33–39.
[30] Daisy P, Eliza J, Mohamed Farook KA. A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J Ethnopharmacol 2009; 126(2): 339–344.
Tel: +82-2-880-1276
Fax: +82-2-873-1268
Email: mchotox@snu.ac.kr
Foundation project: This work was supported by the Bio-Synergy Research Project (NRF-2012M3A9C4048819) of the Ministry of Science, ICT and Future Planning through the National Research Foundation.
doi:Document heading 10.1016/j.apjtm.2016.03.0037
*Corresponding author:Myung-Haing Cho, Laboratory of Toxicology, College of Veterinary Medicine, Seoul National University, Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
- Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
- Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
