Hydroethanolic extract of Smallanthus sonchifolius leaves improves hyperglycemia of streptozotocin induced neonatal diabetic rats
2016-07-25SilmaraBaroniBrunoAmbrosiodaRochaJulianaOliveiradeMeloJurandirFernandoComarSilvanaMartinsCaparrozAssefCiomarAparecidaBersaniAmadoDepartmentofPharmacologyandTherapeuticStateUniversityofMaringParanBrazilDepartmentofBiochemis
Silmara Baroni, Bruno Ambrosio da Rocha, Juliana Oliveira de Melo, Jurandir Fernando Comar, Silvana Martins Caparroz-Assef, Ciomar Aparecida Bersani-Amado*Department of Pharmacology and Therapeutic, State University of Maringá, Paraná, BrazilDepartment of Biochemistry, State University of Maringá, Paraná, Brazil
ABSTRACT
Objective: To evaluate the eff ect of hydroethanolic extract of yacon on the hyperglycemia induced by streptozotocin (STZ) in neonatal rats. Methods: Wistar rats aged two days old received an intraperitoneal injection of STZ (160 mg/kg); after seven weeks, glycosuria was determined and animals with glucose levels above 250 mg/dL were included in the study. Groups of diabetic and non-diabetic rats were treated orally with yacon extract at a dose of 400 mg/kg/d for 14 d. Tests were made for phytochemical characterization, glucose tolerance and toxicity. Results: The results showed that treatment with the extract reduced the glucose levels of fed diabetic rats and did not change the glucose levels of fasting diabetic and normal rats. Additionally, also it was observed that treatment with the extract reduced blood glucose levels of diabetic rats during the oral and intravenous glucose tolerance tests. There was no change in body weight, liver enzymes or mortality with yacon extract treatment. The phytochemical screening revealed the presence of caffeic acid, chlorogenic acid, ferulic acid and gallic acid. Conclusions: The data suggest that yacon extract reduces hyperglycemia, possibly by improving insulin sensibility through its phytochemicals constituents (phenolic compounds).
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Hydroethanolic extract of Smallanthus sonchifolius leaves improves hyperglycemia of streptozotocin induced neonatal diabetic rats
Silmara Baroni1, Bruno Ambrosio da Rocha1, Juliana Oliveira de Melo1, Jurandir Fernando Comar2, Silvana Martins Caparroz-Assef1, Ciomar Aparecida Bersani-Amado1*1Department of Pharmacology and Therapeutic, State University of Maringá, Paraná, Brazil
2Department of Biochemistry, State University of Maringá, Paraná, Brazil
ABSTRACT
Objective: To evaluate the eff ect of hydroethanolic extract of yacon on the hyperglycemia induced by streptozotocin (STZ) in neonatal rats. Methods: Wistar rats aged two days old received an intraperitoneal injection of STZ (160 mg/kg); after seven weeks, glycosuria was determined and animals with glucose levels above 250 mg/dL were included in the study. Groups of diabetic and non-diabetic rats were treated orally with yacon extract at a dose of 400 mg/kg/d for 14 d. Tests were made for phytochemical characterization, glucose tolerance and toxicity. Results: The results showed that treatment with the extract reduced the glucose levels of fed diabetic rats and did not change the glucose levels of fasting diabetic and normal rats. Additionally, also it was observed that treatment with the extract reduced blood glucose levels of diabetic rats during the oral and intravenous glucose tolerance tests. There was no change in body weight, liver enzymes or mortality with yacon extract treatment. The phytochemical screening revealed the presence of caffeic acid, chlorogenic acid, ferulic acid and gallic acid. Conclusions: The data suggest that yacon extract reduces hyperglycemia, possibly by improving insulin sensibility through its phytochemicals constituents (phenolic compounds).
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Keywords:
Yacon extract
Hyperglycemia
Insulin sensibility
Streptozotocin
1. Introduction
Diabetes mellitus is a serious chronic disease, and is considered one of the fi ve leading causes of death worldwide [1,2] . This disease is characterized by high blood glucose levels, caused by a defi ciency in insulin secretion and/or a resistance to insulin action [3] .
Diabetes mellitus can be classified as diabetes mellitus type 1 (DM1) and diabetes mellitus type 2 (DM2). DM1 is characterized by an absolute deficiency of insulin secretion associated with the autoimmune destruction of pancreatic β cells. DM2, which accounts for over 90% of cases, is caused by a combination of resistance to insulin action and impaired secretion of this hormone [4] . In DM2, muscle and adipose tissue are resistant to insulin and compensatory insulin secretion is insufficient to maintain the physiological levels of glucose[5].
Drug therapy for diabetes is conventionally performed using oral hypoglycemic agents (such as metformin, glyburide, chlorpropamide, and others) and insulin. However, based on recommendations of the World Health Organization [6] , antidiabetic agents derived from plants are an important alternative for the treatment of disease. In recent years, interest in using natural products for pharmacological purposes has increased greatly as a form of complementary therapy. Particularly in the case of diabetes, studies have shown that plant extracts are eff ective in reducing blood glucose, causing fewer adverse eff ects compared to traditional anti-diabetic agents [7,8] .
The yacon plant is native of the Andes, the family Asteraceae (Compositae), the species Smallanthus sonchifolius (S. sonchifolius) Aybar et al. [9] demonstrated the anti-hyperglycemic effect of aqueous extracts obtained from leaves of yacon in normoglycemic rats, transiently hyperglycemic by glucose tolerance or diabetic.Recent studies by our group showed that treatment with extracts of yacon for 14 d reduced blood glucose levels in diabetic animals (DM1) and that this eff ect was not related to reduced food intake or interference of the extract on the intestinal absorption of carbohydrates [10] . In addition, other studies have shown that the butanol, chloroform and methanol fractions of yacon leaves extracts also reduced the blood glucose of diabetic rats [11] . The aim of this study was to evaluate whether the hydroethanolic extract of yacon changes the hyperglycemia induced by streptozotocin (STZ) in neonatal rats.
2. Material and medthods
2.1. Preparation of hydroethanolic extract of yacon
The extracts were prepared from S. sonchifolius leaves acquired from Takashi Kakihara Ltda., Capão Bonito – São Paulo, Brazil. A voucher specimen was deposited at the Herbarium of the State University of Maringá under number HUEM 13021. Hydroethanolic extract from S. sonchifolius was obtained from 10% (w/w) solution with 70% ethanol, stirred mechanically for 5 h. Next, the extract was fi ltered, slowly evaporated to remove the solvent, lyophilized, and stored at -20 ℃. For the assays, the hydroethanolic extract of S. sonchifolius (hydroethanolic extract of yacon) was suspended in water immediately before use.
2.2. Chromatographic procedures
The hydroethanolic extract of yacon was fractionated by means of high performance liquid chromatography (HPLC). The HPLC system (Shimadzu®, Japan) consisted of a system controller (SCL-10AVP), two pumps (model LC10ADVP), a column oven (model CTO-10AVP), and a UV-vis detector (model SPD-10AVP). A reversedphase column C18HRC-ODS (Shimadzu®, Japan), protected with a pre-column GHRC-ODS (Shimadzu®, Japan), was used. The injection volume was 20 μL. The mobile phase was methanol/acetic acid 4% (3/7), with the fl ow 1 mL/min and temperature of 35 ℃. Spectrophotometric monitoring was performed at 254 nm. The identifi cation of each compound was based on a comparison of the retention time of external standards.
2.3. Induction of diabetes
Diabetes was induced in male newborn (2 d old) Wistar rats injected intraperitoneally with STZ (160 mg/kg) dissolved in citrate buf fer (10 mM; pH 4.5). The animals were maintained at a controlled temperature (±22 ℃) and a 12-h-dark-light cycle, with balanced food and free access to water. The protocol for these experiments was approved by the Committee on Experimental Animal Ethics of the State University of Maringá. Seven weeks later, the diabetic state was confi rmed by urine glucose level determination and animals with glycosuria above 250 mg/dL were included in the study.
2.4. Treatment of diabetic and normal rats
The hydroethanolic extract of yacon (400 mg/kg body weight) or water was administered by the oral route (gavage), in a single daily dose, in diabetic and normal rats for 14 d. The body weights of rats were evaluated daily, during experimental period.
2.5. Effect of hydroethanolic extract of yacon on glycemia and the activity of plasma phosphatase and transaminases
Blood samples were collected from the inferior vena cava blood in heparinized tubes, and centrifuged at 1 100伊× g for 15 min, and the plasma was separated. The concentration of glucose was determined in aliquots of plasma (10 μL), using the glucose oxidase colorimetric enzymatic method (Gold Analisa®). The activity of alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) was determined in the plasma of diabetic and normal rats, by the colorimetric-kinetic method, using a commercial kit (Gold Analisa®).
2.6. Oral glucose tolerance test (OGTT)
After 14 d of treatment with the hydroethanolic extract of yacon, the animals under fasting for 15 h received an oral glucose solution (1 g of 50% glucose solution/kg body weight). At zero, 15 and 30 min after the administration of glucose solution, blood samples were collected from the tails of animals to determine the concentration of plasma glucose using the glucose oxidase colorimetric enzymatic method (Gold Analisa®).
2.7. Intravenous glucose tolerance test (EGTT)
After 14 d of treatment with the hydroethanolic extract of yacon, the animals under fasting for 15 h received intravenous glucose solution (0.5 g of 50% glucose solution/kg body weight). At zero, 5, 10, 20, 30 and 60 min after glucose infusion, blood samples were collected from the inferior vena cava of the animals and conditioned in heparinized tubes. The plasma glucose concentration was determined by glucose oxidase colorimetric enzymatic method (Gold Analisa®).
2.8. Statistical analysis
The statistical analysis of the data was done using GraphPad Prism® (Graphpad Software Inc., Microsoft Corp.). Results were expressed as mean ± standard error of the mean and analyzed using analysis of variance (ANOVA followed by Tukey’s test) for multiple comparisons. The area under the curve was calculated using the trapezoidal rule. P<0.05 was considered the criterion for signifi cance.
3. Results
3.1. Chemical constituents of hydroethanolic extract of yacon
The HPLC method using a C18 column was commonly used to assess the presence of polyphenols in plant extracts. Four phenolic acids were identifi ed by comparing the retention time of external standards: caff eic acid, chlorogenic acid, ferulic acid and gallic acid (Figure 1).

Figure 1. Chromatograms of the hydroethanolic extract of S. sonchifolius leaves (yacon extract) and injected standards detected by HPLC at 254 nm.
3.2. Effect of hydroethanolic extract of yacon on glycemia in diabetic and normal rats
The effect of yacon on glycemia in diabetic and normal rats in the fed state and in fasting state is shown in Figures 2A and 2B, respectively. The glycemia of the diabetic rats in the fed state [(162.50±7.29) mg/dL] was higher than in normal rats [(128.90±2.91) mg/dL]. The treatment with yacon did not alter the glycemia of normal rats [(116.60±3.56) mg/dL] but significantly reduced the glycemia of the diabetic rats in the fed state by 22.8% [(125.50±3.99) mg/dL]. However, the treatment of normal and diabetic rats in the fasting state with the extract did not change the glucose levels.

Figure 2. Eff ect of treatment with hydroethanolic extract of S. sonchifolius leaves (yacon extract) on glycemia in fed rats (A) and fasting rats (B).
3.3. Effect of hydroethanolic extract of yacon on transient hyperglycemia induced by oral administration of glucose (OGTT)
The glycemia of diabetic and normal rats under fasting was significantly increased 15 and 30 min after glucose load [N0= (70.7±5.5) mg/dL; N15= (121.1±6.3) mg/dL; N30= (131.0±11.5) mg/dL; D0= (93.9±4.2) mg/dL; D15= (184.7±8.6) mg/dl, D30= (206.2±11.9) mg/dL]. The treatment of normal rats with the extract did not alter the plasma glucose concentration; however, there was a reduction in glucose concentration in diabetic rats at 15 and 30 min after glucose loading [DY15= (147.0±9.2) mg/dL; DY30= (156.8±8.6) mg/dL]. The results are shown in Figure 3A, and these results are demonstrated by area under the curve in fi gure 3B (Figure 3B).
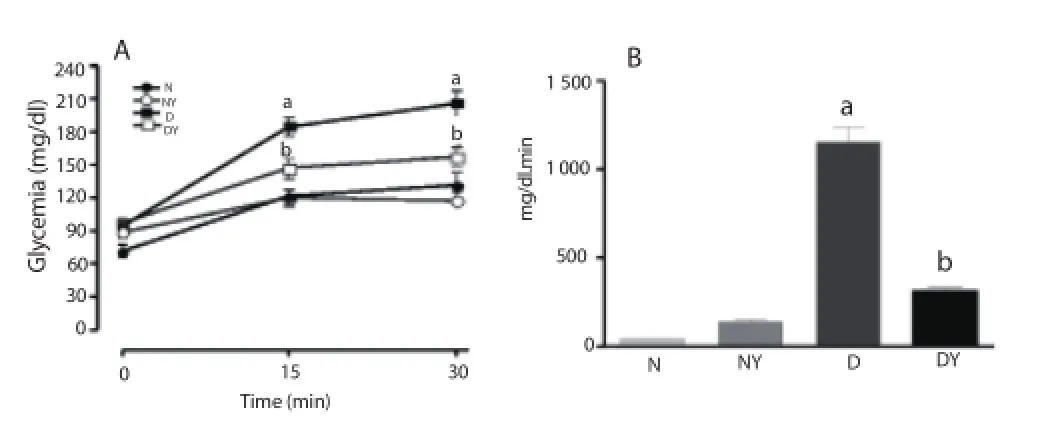
Figure 3. Eff ect of treatment with hydroethanolic extract of S. sonchifolius leaves (yacon extract).
3.4. Effect of hydroethanolic extract of yacon on transient hyperglycemia induced by intravenous administration of glucose (ETTG)
Figures 4A and 4B show changes in plasma glucose levels during ETTG in diabetic and normal rats under fasting. In normal rats, glucose levels were signifi cantly increased fi ve minutes after the glucose load and gradually reduced over the 60 min experiment [N0= (105.7±6.4) mg/dL; N5= (298.3±10.7) mg/dL; N10= (247.5±12.0) mg/dL; N20= (195.5±19.5) mg/dL; N30= (158.8±15.8) mg/dL and N60= (130.6±10.5) mg/dL]. On the other hand, the glycemia of diabetic rats remained increased throughout the period [D0= (145.3±5.7) mg/dL; D5= (357.6±12.2) mg/dL; D10= (322.9±9.5) mg/ dL; D20= (292.6±9.9) mg/dL; D30= (275.3±8.5) mg/dL and D60= (254.4±12.8) mg/dL]. The glycemia in diabetic rats treated with the yacon extract was signifi cantly reduced when compared to diabetic control of glucose at 10, 20, 30 and 60 min after glucose loading [DY10= (303.8±7.1) mg/dL; DY20= (268.8±8.8) mg/dL; DY30= (248.3±9.8) mg/dL and DY60= (214.6±8.7) mg/dL]. The treatment with yacon extract did not change the blood glucose of normal animals.
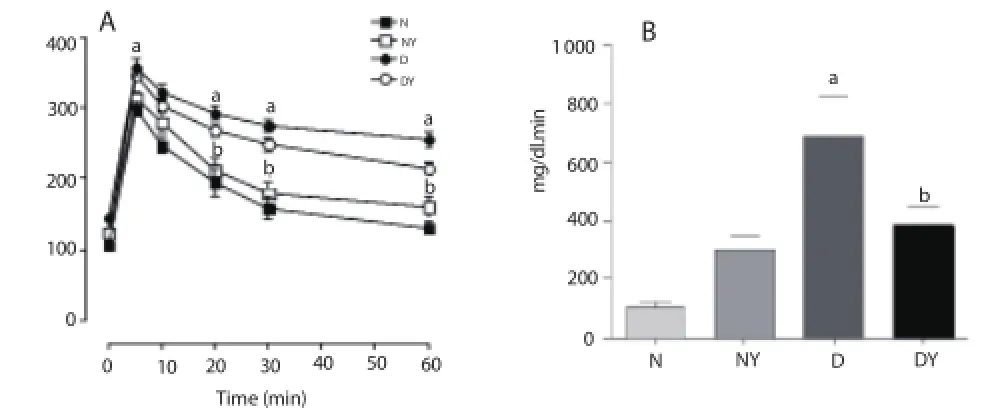
Figure 4. Eff ect of hydroethanolic extract of S. sonchifolius leaves (yacon extract) on serum glucose in rats after intravenous glucose overload (0.5 g/kg body weight).
3.5. Effect of hydroethanolic extract of yacon on body weight and liver injury of diabetic and normal rats
The treatment of diabetic and normal animals with yacon extract did not change the body weight, without any signifi cant diff erences between groups (data not shown). Additionally, the activity of liver enzymes (AST, ALT and ALP) was not significantly increased in the plasma of diabetic and normal rats treated with yacon extract (Table 1). Mortality was not observed in the treated groups (data not shown).

Table 1Eff ect of oral treatment with the hydroethanolic extract of yacon (Y-400 mg/ kg/d) for 14 d, on AST, ALT, and ALP.
4. Discussion
Ethnobotanical studies show that around 800 plants are used as traditional remedies for the treatment of diabetes due to their effectiveness, fewer side effects and relatively lower cost[12]; in this context, the use of leaves and/or yacon roots has shown antihyperglycemic activity [13,14] .
Baroni et al. [10] found that the crude extract of yacon leaves significantly reduced blood glucose levels of diabetic rats in the experimental model induced by STZ. It was also shown that the hydroethanolic fraction of the extract was able to reduce the glycogen content of the liver and skeletal muscle, and restored the activity of glucose 6-phosphate dehydrogenase in type 1 diabetic rats [13] . Additionally, Genta et al. [11] also showed that the methanolic, butanolic and chloroform yacon extract, tested in a type 1 diabetes model induced by STZ, increased the plasma levels of insulin, reducing the blood glucose. However, the above results are associated with a fasting state in experimental models of DM1, which is diff erent to the results of the present study in which the eff ect of the hydroethanolic yacon extract was observed in the fed state in a newborn STZ-diabetes model. This experimental model has been widely used as a tool for the research of compounds with anti-diabetic actions, as well as to study the mechanism involved in disease, as it exhibits several phases of DM2, such as impaired glucose tolerance, and mild, moderate or severe glycemia. It has been reported that blood glucose changes and chronic hyperglycemia develop in rats that receive STZ injection on the second day after birth[15-18].
The results of this study demonstrated that the hydroethanolic extract of yacon (400 mg/kg) signifi cantly (P<0.05) reduced blood glucose level in fed diabetics rats when compared with the control rats (normal). To confi rm this anti-hyperglycemic eff ect, oral and intravenous glucose tolerance tests were conducted, which can identify insulin sensitivity [8] . Yacon reduced the blood glucose levels of diabetic rats after glucose loading in both tests, but did not change the glucose levels in normal rats. These results are possibly due to the reduction of insulin resistance. An insulin-resistant state is one of the biggest risk factors for developing diabetes [19] ; therefore, reducing insulin resistance can positively contribute to the control of diabetes mellitus complications.
The improvement of glycemia using the hydroethanolic extract of yacon may be due to its phytochemical constitution. Genta et al.[11] showed that the hypoglycemic effect of butanolic yacon extract was attributed to the presence of phenolic compounds such as caff eic acid and chlorogenic acid. Other authors have also shown that plants rich in phenolic compounds, fl avonoids, terpenoids and coumarin have potential hypoglycemic eff ects [20-23] . Among these compounds, caffeic acid in particular is associated with reduced blood glucose [24] and chlorogenic acid to recover glucose intolerance and insulin resistance [25] . Additionally, it was also shown that the gallic acid increases plasma insulin [26] . Our results showed that the phytochemical analysis of the hydroethanolic extract yacon identifi ed the presence of phenolic compounds such as caff eic acid, ferulic acid, gallic acid and chlorogenic acid, which may possibly contribute to the anti-hyperglycemic eff ect observed.
Interestingly, treatment with hydroalcoholic extract of yacon for 14 d (400 mg/kg/d) did not cause liver damage and mortality, and also did not aff ect the weight gain of animals. The non-toxicity eff ect was also observed in the study by Oliveira et al. [27] , who showed that polar yacon extract (rich in phenolic compounds such as chlorogenic acid, caffeic acid and coumarin compounds) produced toxicity only at high doses (1 000 mg/kg) following prolonged treatment (90 d). The authors suggest that the polar extract is safe for oral administration since it presents low or absent sesquiterpene lactones. Among the strategies for diabetes control are normalization of glucose-induced insulin secretion and restoring glucose transport into insulin sensitive tissues. Our results showed that the hydroethanolic extract of yacon has the potential ability to control hyperglycemia probably through one or a combination of the above mechanisms. However, further work should be carried out to determine the toxicity profi le, isolate the bioactive compound(s) in the plant and determine the exact mechanism(s) of action.
Conflict of interest statement
We declare that we have no confl ict of interest.
Acknowldgement
The authors are grateful for the fi nancial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
References
[1] Gipsen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 2000; 23: 542–549.
[2] Shengxia X, Xiaoming C, Jianxin L, Liqin J. Protective eff ect of sulfated Achyranthes bidentata polysaccharides on streptozotocin-induced oxidative stress in rats. Carbohyd Polyms 2009; 75: 415–419.
[3] Warren RE, Frier BM. Hypoglycemia and cognitive function. Diabetes Obes Metab 2005; 7(5): 493-503.
[4] American Diabetes Association. Diagnosis and classifi cation of diabetes mellitus. Diabetes Care 2014; 37(Suppl. 1): S81–S90.
[5] Kim JO, Lee GD, Kwon JH, Kim KS. Anti-diabetic eff ects of new herbal formula in neonatally streptozotocin-induced diabetic rats. Biol Pharm Bull 2009; 32(3): 421–426.
[6] WHO. Traditional medicine strategy 2002-2005. Geneva: World Health Organization; 2002. p. 1-6.
[7] Gupta RK, Kesari AN, Murthy PS, Chandra R, Tandon V, Watal G. Hypoglycemic and anti-diabetic eff ect of ethanolic extract of leaves of Annona squamosa L. in experimental animals. J Ethnopharmacol 2005; 99(1): 75–81.
[8] Zhang L, Xu J, Song H, Yao Z, Ji G. Extracts from Salvia-Nelumbinis naturalis alleviate hepatosteatosis via improving hepatic insulin sensitivity. J Transl Med 2014; 12: 236–246.
[9] Aybar MJ, Riera AS, Grau A, Sanches SS. Hypoglycemic effect of the water extract of Smallanthus sonchifolius (Yacon) leaves in normal and diabetic rats. J Ethnopharmacol 2001; 74(2): 125–132.
[10] Baroni S, Suzuki-Kemmelmeier F, Caparroz-Assef SM, Cuman RKN, Bersani-Amado CA. Effect of crude extracts of leaves of Smallanthus sonchifolius (yacon) on glycemia in diabetic rats. Brazilian J Pharm Sci 2008; 44(3): 521-530.
[11] Genta SB, Cabrera WM, Mercado MI, Grau A, Catalán CA, Sáncheza SS. Hypoglycemic activity of leaf organic extracts from Smallanthus sonchifolius: constituents of the most active fractions. Chem-Biol Interact 2010; 185(2): 143–152.
[12] Naru R, Raghnver I, Chitme HR, Chandra R. Anti-diabetic activity of Nyctanthes arbotritis. Pharmacog Magazine 2008; 4(16): 335-340.
[13] Baroni S, Comar JF, Kemmelmeier FS, Mito MS, Melo JO, Rocha BA, et al. Benefi cial eff ects of a hydroethanolic extract of Smallanthus sonchifolius leaves on the metabolic changes in diabetic rats. Int J Pharm Bio Sci 2014; 5(3): 183–196.
[14] Oliveira GO, Braga CP, Fernandes AAH. Improvement of biochemical parameters in type 1 diabetic rats after the roots aqueous extract of yacon [Smallanthus sonchifoliu (Poepp. & Endl.)] treatment. Food Chem Toxicol 2013; 59: 256–260.
[15] Cuman RKN, Bersani-Amado CA, Fortes ZB. Influence of type 2 diabetes on the infl ammatory response in rats. Inflamm Res 2001; 50(9): 460–465.
[16] Ozturk M, Bolkent S, Kaya Dagistanli F, Tuncdemir M, Yilmazer S, Akkan AG. Effects of 5-aminoimidazole-4-carboxamide riboside on pancreas in neonatal streptozotocin diabetic rats. Acta Diabetol 2006; 43: 61–65.
[17] Turk N, Dagistanli FK, Sacan O, Yanardag R, Bolkent S. Obestatin and insulin in pancreas of newborn diabetic rats treated with exogenous ghrelin. Acta Histochem 2012; 114(4): 349–357.
[18] Kaya-Dagistanli F, Ozturk M. The role of clusterin on pancreatic beta cell regeneration after exendin-4 treatment in neonatal streptozotocin administered rats. Acta Histochem 2013; 115(6): 577–586.
[19] Salman ZK , Refaat R , Selima E , El Sarha A , Ismail MA. The combined eff ect of metformin and L-cysteine on infl ammation, oxidative stress and insulin resistance in streptozotocin-induced type 2 diabetes in rats. Eur J Pharmacol 2013; 714(1-3): 448–455.
[20] He CN, Wang CL, Guo SX. Study on chemical constituents in herbs of Anoectochilus roxburghii 栻. Chin J Chin Materia Medica 2005; 30(10): 761–776.
[21] Jung M, Park M, Lee H-Ch, Kang Y, Kang ES, Kim SK. Anti-diabetic agents from medicinal plants. Curr Med Chem 2006; 13: 1203–1218.
[22] Zhang Y, Cai J, Ruan H, Pi H, Wu J. Anti-hyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J Ethnopharmacol 2007; 114(2): 141–145.
[23] Singh RK, Mehta S, Jaiswal D, Rai PK, Watal G. Anti-diabetic effect of Ficus engalensis aerial roots in experimental animals. J Ethnopharmacol 2009; 123(1):110–114.
[24] Hsu FL, Chen YC, Cheng JT. Caffeic acid as active principle from the fruit of Xanthium strumarium to lower plasma glucose in diabetic rats. Planta Med 2000; 66(3): 228-230.
[25] Rodríguez de Sotillo DV, Hadley M. Chlorogenic acid modifi es plasma and liver concentrations of: cholesterol, triacylglycerol, and minerals in (fa/fa) Zuckeri rats. J Nutr Biochem 2002; 13(12): 717-726.
[26] Punithavathi VR, Prince PSM, Kumar R, Selvakumari J. Antihyperglycemic, anti-lipid peroxidative and antioxidant eff ects of gallici acid on streptozotocin induced diabetic Wistar rats. Eur J Pharmacol 2011; 650: 465-471.
[27] Oliveira RB, Paula DAC, Rocha BA, Franco JJ, Gobbo-Neto L, Uyemura SA, et al. Renal toxicity caused by oral use of medicinal plants: the yacon example. J Ethnopharmacol 2011; 133(2): 434-441.
Tel: þ55-44-3011-5166
E-mail: cabamado@uem.br
Foundation project: It was financially supported by the Conselho Nacional de Desenvolvimento Científi co e Tecnológico, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
doi:Document heading 10.1016/j.apjtm.2016.03.033
*Corresponding author:Ciomar Aparecida Bersani-Amado, Department of Pharmacology and Therapeutic, State University of Maringá, Paraná, Brazil.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
- Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
- Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
