Anti-inflammatory activity of the essential oils of Cymbopogon validus (Stapf) Stapf ex Burtt Davy from Eastern Cape, South Africa
2016-07-25PamelaRungquOpeoluwaOyedejiBenedictaNkehChungagSandileSongcaOluwatobiOluwafemiAdebolaOyedejiDepartmentofChemistryFacultyofScienceandAgricultureUniversityofFortHareAlice5700SouthAfricaDepartmentofBiologicalandEnvironmentalSc
Pamela Rungqu, Opeoluwa Oyedeji*, Benedicta Nkeh-Chungag, Sandile Songca, Oluwatobi Oluwafemi, Adebola OyedejiDepartment of Chemistry, Faculty of Science and Agriculture, University of Fort Hare, Alice 5700, South AfricaDepartment of Biological and Environmental Sciences, Faculty of Natural Sciences, Walter Sisulu University, Mthatha 5099, South AfricaDepartment of Chemical and Physical Sciences, Faculty of Natural Sciences, Walter Sisulu University, Mthatha 5099, South AfricaDepartment of Applied Chemistry, Faculty of Sciences, University of Johannesburg, Doornfontein 08, South Africa
ABSTRACT
Objective: To evaluate the essential oil composition and the anti-infl ammatory activity of Cymbopogon validus (C. validus) leaves and fl owers. Methods: A total of 300 g of fresh or dry (leaves and fl owers) of C. validus were cut into small pieces and subjected to hydro-distillation method for approximately 5 h using the Clevenger apparatus. The extracted essential oils were then used for testing the anti-infl ammatory activity. The anti-infl ammatory activity was evaluated by using egg-albumin induced paw edema. Results: The extracted oils had the following yields 2.2% for fresh leaves, 2.0% for dry leaves and 2.4% v/w for dry fl owers. GCMS results revealed that the oils contained artemisia ketone (37.5%), linalool (3.2%-29.6%), northujane (4.4%-16.8%), verbenone (13.5%), naphthalene (1.7%-9.6%), δ毮-cadinene (0.5%-8.1%), hedycaryol (5.4%-7.6%) and α毩-eudesmol (6.5%-6.7%) as the major constituents. C. validus essential oils showed signifi cant (P<0.05) anti-infl ammatory eff ects from the fi rst 30 min after albumin injection compared to aspirin which had a later onset of eff ect. Conclusions: The fi ndings of this study show that the essential oil extracted from C. validus fresh or dry leaves and fl owers have anti-infl ammatory properties; that might be associated with the major components and the minor components found in the essential oils.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Anti-inflammatory activity of the essential oils of Cymbopogon validus (Stapf) Stapf ex Burtt Davy from Eastern Cape, South Africa
Pamela Rungqu1, Opeoluwa Oyedeji1*, Benedicta Nkeh-Chungag2, Sandile Songca3, Oluwatobi Oluwafemi4, Adebola Oyedeji31Department of Chemistry, Faculty of Science and Agriculture, University of Fort Hare, Alice 5700, South Africa
2Department of Biological and Environmental Sciences, Faculty of Natural Sciences, Walter Sisulu University, Mthatha 5099, South Africa
3Department of Chemical and Physical Sciences, Faculty of Natural Sciences, Walter Sisulu University, Mthatha 5099, South Africa
4Department of Applied Chemistry, Faculty of Sciences, University of Johannesburg, Doornfontein 2028, South Africa
ABSTRACT
Objective: To evaluate the essential oil composition and the anti-infl ammatory activity of Cymbopogon validus (C. validus) leaves and fl owers. Methods: A total of 300 g of fresh or dry (leaves and fl owers) of C. validus were cut into small pieces and subjected to hydro-distillation method for approximately 5 h using the Clevenger apparatus. The extracted essential oils were then used for testing the anti-infl ammatory activity. The anti-infl ammatory activity was evaluated by using egg-albumin induced paw edema. Results: The extracted oils had the following yields 2.2% for fresh leaves, 2.0% for dry leaves and 2.4% v/w for dry fl owers. GCMS results revealed that the oils contained artemisia ketone (37.5%), linalool (3.2%-29.6%), northujane (4.4%-16.8%), verbenone (13.5%), naphthalene (1.7%-9.6%), δ毮-cadinene (0.5%-8.1%), hedycaryol (5.4%-7.6%) and α毩-eudesmol (6.5%-6.7%) as the major constituents. C. validus essential oils showed signifi cant (P<0.05) anti-infl ammatory eff ects from the fi rst 30 min after albumin injection compared to aspirin which had a later onset of eff ect. Conclusions: The fi ndings of this study show that the essential oil extracted from C. validus fresh or dry leaves and fl owers have anti-infl ammatory properties; that might be associated with the major components and the minor components found in the essential oils.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Keywords:
Cymbopogon validus
Essential oils
Anti-infl ammation
Artemisia ketone
Linalool
Egg albumin-induced edema
1. Introduction
Cymbopogon genus is one of the most important essential oil yielding genera of the Poaceae family [1] . It comprises of about 180 species, sub species, varieties as well as sub varieties[2]. Cymbopogon species are scattered all around the world and more than 52 species are said to be found in Africa, 45 in India, 6 in Australia and South America, 4 in Europe, 2 in North America and the remaining species are dispersed in South Asia[3]. The plants’ essential oils are known for their pleasant aromas which are used to fl avour foods and beverages; and as fragrances in cosmetic and pharmaceutical industries [4] . The oils are also used in household products and tobacco[5]. Numerous extracts and essential oils extracted from these plants have been tested for repellent and insecticidal properties using diff erent types of arthropods; they have also been used to cure several infectious diseases that are engendered by bacteria, fungi, protozoa and virus. People from the jungle regions of Bolivia Amazon use Cymbopogon plants as repellents against mosquitos [6] . Essential oils from Cymbopogon species like Cymbopogon flexuous (C. flexuous), Cymbopogon citratus (C. citrates), Cymbopogon martini (C. martini), Cymbopogon winterianus (C. winterianus), Cymbopogon nardus (C. nardus), Cymbopogon giganteus (C. giganteus), Cymbopogon schoenanthus (C. schoenanthus) and Cymbopogon parkeri (C. parkeri) contain monoterpenoid components such as geraniol and citronellol which are considered dominant in the oils; while the C. flexuous oils and that of C. parkeri shows the prevalence of sesquiterpenoids such as isontermedeol. The essential oil ofCymbopogon species also reveals that the citralchemotype is common in Cymbopogon Pendulus (C. pendulus), C. flexuous and C. citratus [7] . Cymbopogon validus (C. validus) (Stapf) Stapf ex Burtt Davy is commonly known as a turpentine grass or the African Blue Grass in South Africa, and the Afrikaner people call it ‘Reuse Terpentynegras’. This species belongs to the Poaceae family and has been described as a tufted perennial with culms up to 2.4 m tall. Normally C. validus is found in mountainous grasslands and also in the high-rainfall areas of South Africa where it is known to grow in wet sites, along roads and on the margins of tree communities [8] . It is widespread in the Eastern Cape and is often used as durable thatch [4,8] . C. validus oil is pure therapeutic quality aromatherapy essential oil that is produced by using wild-crafted plants and traditional methods from South Africa [9] . Essential oils from C. validus are used as astringents, skin toners and are also used in anti-ageing preparation for men; these essential oils also have antifungal, antiseptic, as well as anti-viral properties. The oils are also popularly used as a soothing foot bath. C. validus essential oils and decoctions are used traditionally as anti-rodent, fermifuge, emetic, anti-infective, and anti-plasmodic; they also help in treating morning sickness[2,7]. Chagonda et al[7] reported that the major components from wild C. validus essential oils from Zimbabwe were myrcene (23.1%-35.6%), (E)-β-ocimene (10.3%-11.5%), geraniol (3.4%-8.3%), linalool (3.2%-3.7%) and camphene (5.2%-6.0%); in cultivated C. validus essential oils myrcene (11.6%-20.2%), (E)-β-ocimene (6.0%-12.2%), borneol (3.9%-9.5%), geraniol (1.7%-5.0%) and camphene (3.3%-8.3%) were the major components. Naidoo also revealed that C. validus essential oils from Durban contained 毩-cubenene, camphene, citronellal, geraniol, limonene, palmitic acid and sabinene as the major components [10] . The study was aimed at extracting essential oils from both (fresh and dry) parts of C. validus fl owers and leaves, to determine their chemical profi le, characterise the oils for medicinal and then evaluate the biological potential of the oils as anti-infl ammatory agents.
2. Materials and methods
2.1. Plant material
C. validus was collected in the month of April 2013 at the Komga road, near King William’s Town. The plants were taxonomically identifi ed by Dr T. Dold and the voucher specimen was deposited in SelmarSchonland Herbarium Grahamstown (GRA) at Rhodes University and the collection number was PR/PL 02.
2.2. Extraction of essential oils
A total of 300 g of fresh or dry (leaves and fl owers) of the plant material were cut into small pieces and subjected to hydro-distillation method for approximately 5 h using the Clevenger apparatus. The extracted essential oils were stored in sealed sample vials and stored in a refrigerator at 4 ℃ until the time of analysis and bioassays.
2.3. Analysis of essential oils
GC-FID was performed on a HP5890-栻 instrument, equipped with a DB-5MS (30 m伊0.25 mm; 0.25 μm film thickness) fused silica capillary column. Hydrogen was used as carrier gas adjusted to a linear velocity of 32 cm/s (measured at 100 ℃). Split fl ow was adjusted to give a 20:1 ratio and septum sweep was a constant 10 mL/min. The oven was programmed as follows: 60 ℃-240 ℃ at 3 ℃/min. The samples were injected using the splitless technique using 2 μL of oil in hexane (2:1 000). Injector and detector were set at 250 ℃. The GC was equipped with FID and connected with to an electronic integrator HP 5896 Series 栻. The percentage of the samples was computed from the GC peak areas without using correction for response factors.
GC-MS was performed on a Hewlett Packard-6890 system equipped with a HP-5MS fused capillary column (30 m伊0.25 mm; 0.25 μm fi lm thickness), coupled to a selective mass detector Hewllet Packard-5973. Helium (1 mL/min.) was used as carrier gas; oven temperature program: 60 ℃-240 ℃ at 3 ℃/min; splitless during 1.50 min; sample volume 2 μL of the oil solution in hexane (2:1 000). Injector and detector temperature was 250 ℃. EIMS: electron energy, 70 eV; ion source temperature and connection parts: 180 ℃.
2.4. Identification of compounds
Identifi cation of compounds was done by matching their mass spectra and retention indices with those recorded in NIST11 library and by comparison of retention indices and mass spectra with literature values [11-13]
2.5. Bio-assay (anti-inflammation)
Both female and male Wistar rats weighing 195-240 g were used. The rats were obtained from the South African Vaccine Producers and were housed in the animal holding facility at the Zoology Department of Walter Sisulu University in Mthatha. Ethical clearance for the study was obtained from the Walter Sisulu University Research Ethics Committee DVC (AA&R)/DRD/SREC: Reference No: 31. The animals were kept under standard conditions with each cage housing 5 rats; room temperature was maintained at 24 ℃ and lighting was by daylight only. Animals had free access to food and water throughout except 8 h before experimentation when animals were only given only water. A total of 5 rats were randomly assigned to one of 5 groups, control group treated with 1 mL 0.09% NaCl, standard group treated with 100 mg/kg Aspirin, C.V.F.L group treated with 1 mL of 2% essential oil from fresh leaves of C. validus, C.V.D.L group treated with 1 mL of 2% essential oil from dry leaves of C. validus and C.V.D.F group treated with 1 mL of 2% essential oil from dry fl owers of C. validus. All treatments were administered in 1 mL volumes.
Aspirin was procured from Reckitt Benckiser Pharmaceutical (PTY) LTD/(EDMS) BPK Elansfontein -South Africa.
2.6. Anti-inflammatory activity: fresh egg albumin - induced right hind paw edema
Animals were randomly distributed to one of the 5 groups as indicated earlier. Baseline right hind paw diameter (paw size) was determined for each rat using a pair of YATO digital caliper [14-17] . Rats were administered with oral doses of drugs as per assigned group. A total of 30 min later the right hind paw of each rat was injected with 1 ml of 50% (v/v) fresh egg albumin. Paw sizes were again measured 30, 60, 90 and 120 min after albumin injection. Change in paw size was calculated as:
Δ △paw size = paw size after albumin injection at pre-determined times - paw size before albumin injection.
2.7. Statistical analysis
One way of Analysis with Turkey-Kramer Multiple Comparisons Test was performed using GraphPadInstat (version 3.05 for Windows 95, GraphPad Software, San Diego California USA, www.graphPad. com to determine the diff erence between each treatment group and control. The P value for the overall relationship was 0.001 and was considered signifi cant. Results were expressed as mean ± standard error of the mean.
3. Results
3.1. Chemical composition of essential oils
The pale yellow oils extracted from C. validus had a pleasant odor. The percentage yields were as follows 2.2% v/w for fresh leaves, 2.0% v/w for dry leaves and 2.43% v/w for dry flowers. Table 1 presents the examined constituents of the essential oils as well as their percentage compositions. A total of 32 components were identified in the essential oil of C. validus; 17 components accounted for 85.2% in fresh leaves, 20 components for 91.3% in dry leaves and 16 components for 89.6% in dry fl owers. The main components identifi ed in the essential oil of C. validus fresh leaves were artemisia ketone (37.5%), verbenone (13.5%), naphthalene (9.6%) and northujane (4.4%); linalool (29.6%), northujane (12.3%),δ毮-cadinene (8.1%) and α毩-eudesmol (6.7%) were the major components in dry leaves; while in dry flowers linalool (28.0%), northujane (16.8%), hedycaryol (7.6%) and α毩-eudesmol (6.5%) were the major components. The GC-MS results also showed that the fresh leaves oil of C. validus contained 8.6 % monoterpenes, 55.4% oxygenated monoterpenes, 5.4% sesquiterpenes, 1.8% oxygenated sesquiterpenes, 14% others in fresh leaves; while in dry leaves the oils were mostly composed of 1.9% monoterpenes, 31.0% oxygenated monoterpenes, 3.1% sesquiterpenes, 41.1% oxygenated sesquiterpenes, 14.2% other. C. validus dry fl ower’s oil was mostly composed of 1.9% monoterpenes, 28.0% oxygenated monoterpenes, 12.0% sesquiterpenes and 29.6% oxygenated sesquiterpenes and 18.5% other (Table 2).
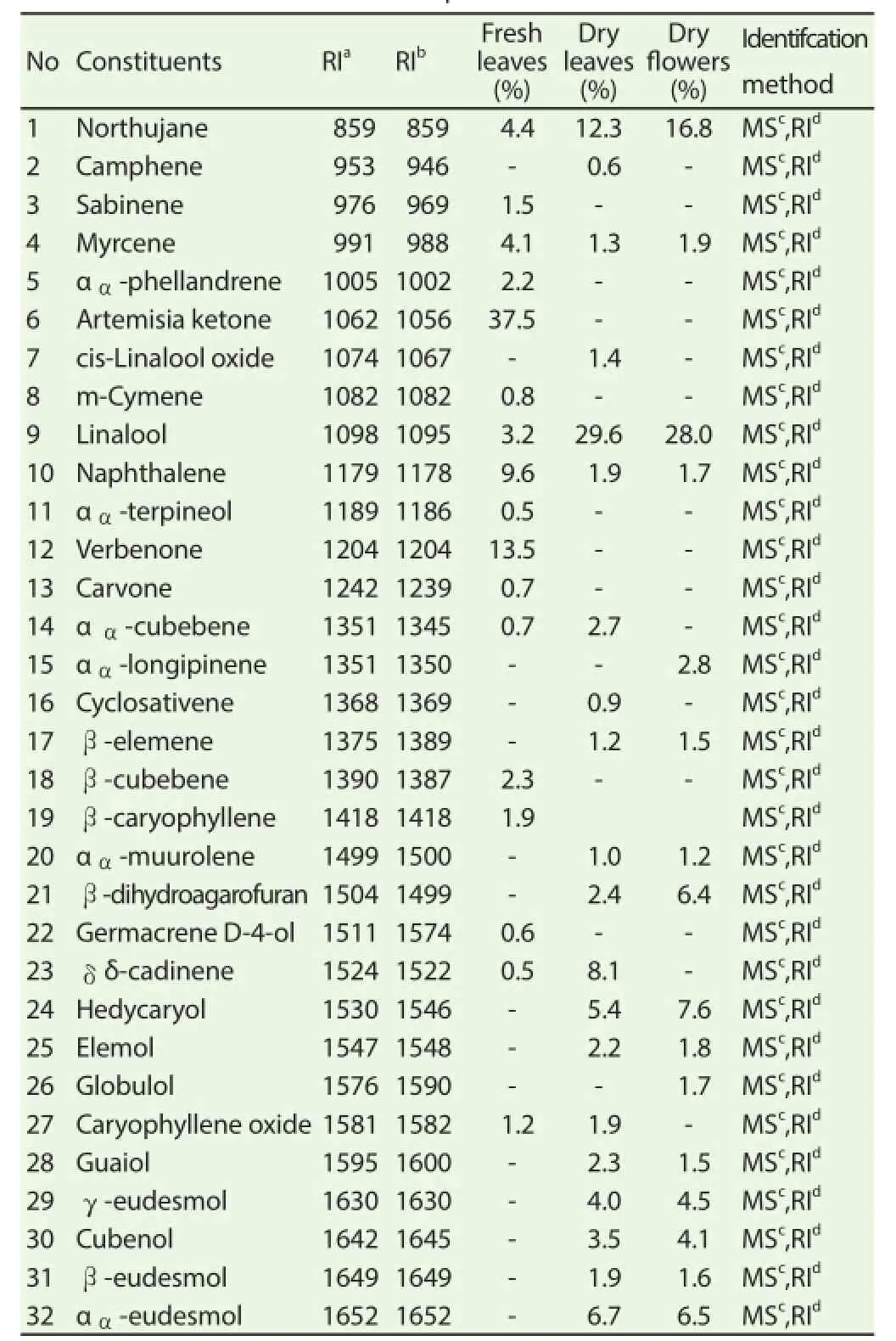
Table 1Chemical constituents from diff erent parts of C. validus essential oil extracts.
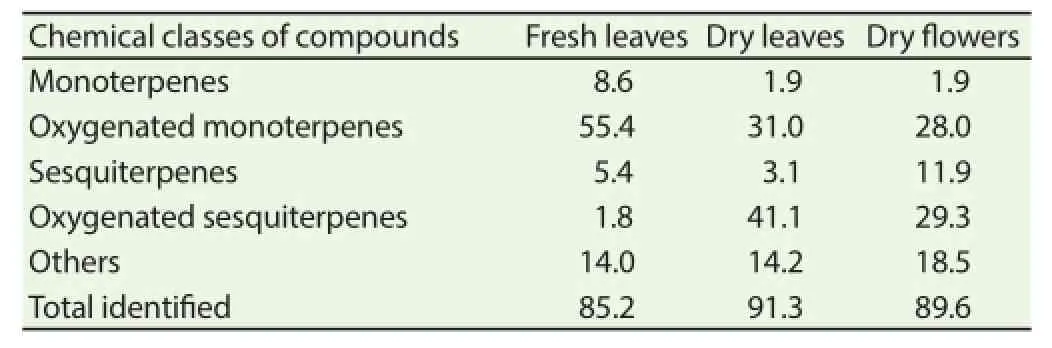
Table 2Chemical classes of compounds found on different parts of C. validus essential oil (%).
3.2. Anti-inflammatory of paw edema inhibition
Figure 1 illustrated the anti-infl ammation eff ects of essential oils of C. validus on fresh egg albumin-induced infl ammation: measured 30, 60, 90 and 120 min after injection of the phlogistic agent. All the essential oils showed significant (P<0.01) anti-inflammatory effects from 30 to 120 min. Results obtained with essential oils were better than those obtained with aspirin during the experimental period. On the other hand Figure 2 showed a comparison of the anti-infl ammatory eff ect of aspirin with oils from fresh; dry leaves and dry fl owers of C. validus. The fresh leaves oil showed signifi cantly (P<0.05 and P<0.01) greater anti-inflammatory effect compared to aspirin throughout the experimental period. Essential oil from the dry fl owers also showed signifi cantly (P<0.05) greater eff ects than aspirin during the 60 and 120 min while the anti-infl ammatory eff ects of oil from dry leaves was not diff erent from results obtained with aspirin.

Figure 1. Anti-infl ammatory eff ects of C. validus oil (fresh and dry leaves and dry flowers) on albumin-induced paw edema, at different time of inhibition.*P<0.05,**P<0.01 compared to control animals.
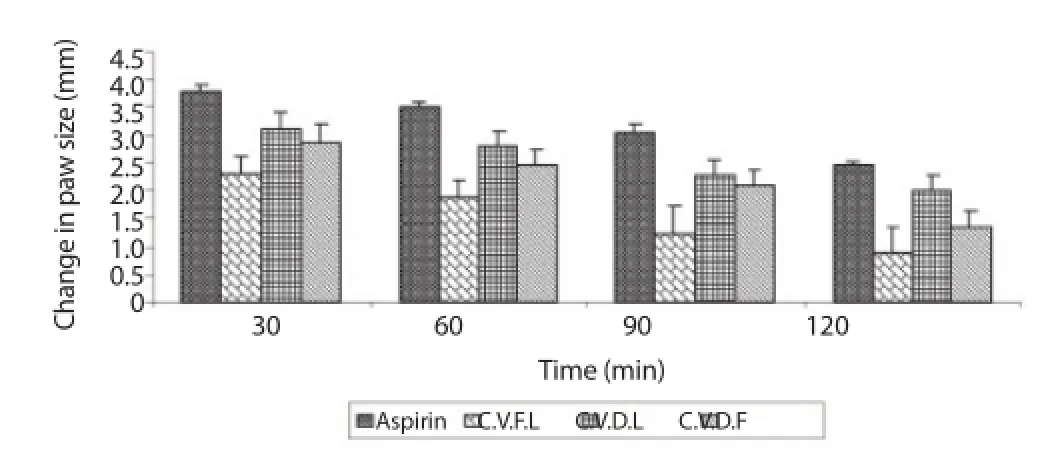
Figure 2. Anti-infl ammatory eff ects of C. validus oil (fresh and dried leaves; and fresh buds and dried buds) on fresh egg albumin-induced paw edema, at diff erent time of inhibition.*P<0.05,**P<0.01 compared to aspirin treated animals.
4. Discussion
4.1. Chemical composition of essential oils
GC/MS results revealed that the oils contained artemisia ketone (37.5%), linalool (3.2%-29.6%), northujane (4.4%-16.8%), verbenone (13.5%), naphthalene (1.7-9.6%), δ毮-cadinene (0.5%-8.1%), hedycaryol (5.4%-7.6%) and α毩-eudesmol (6.5%-6.7%) as the major components. The GC/MS results also revealed that oxygenated monoterpenes represented 5 of the 32 compounds corresponding to 55.4% of the fresh leaves oil, oxygenated sesquiterpenes represented 11 of the 32 compounds corresponding to 41.1% of the dry leaves, while the dry fl ower’s oil represented 8 of the 32 compounds corresponding to 29.3%. The main constituents in our C. validus essential oil were quite diff erent from the C.validus essential oil derived from Zimbabwe and Durban. For example, Chagonda, et al reported that the essential oil of wild C. validus from Zimbabwe had the following major constituents myrcene (23.1%-35-6%), (E)-β-ocimene (10.3%-11.5%), geraniol (3.4%-8.3%), linalool (3.2%-3.7%) and camphene (5.2%-6.0%) while in cultivated C. validus essential oil myrcene (11.6%-20.2%), (E)-β-ocimene (6.0%-12.2%), borneol (3.9%-9.5%), geraniol (1.7%-5.0%) and camphene (3.3-8.3%) as major constituents [7] . Naidoo revealed that the essential oil of C. validus from Durban had α毩 -cubenene, camphene, citronellal, geraniol, limonene, palmitic acid and sabinene as major constituents [10] . When comparing our results to the essential oil of C. validus reported by Chagonda, et al and Naidoo [7,10] , it was observed that our C. validus essential oil showed a very distinct composition which was mainly characterized artemisia ketone (37.5%), linalool (3.2%-29.6%), northujane (4.4%-16.8%), verbenone (13.5%), naphthalene (1.7%-9.6%), δ毮-cadinene (0.5%-8.1%), hedycaryol (5.4%-7.6%) and α毩-eudesmol (6.5%-6.7%). It was also observed that our C. validus essential oil comprised of oxygenated monoterpenes as their major oil constituents, while the essential oil of C. validus from Durban consisted of more than 50% of sesquiterpene constituents [10] ; Chagonda et al found that the oils from the wild and cultivated C. validus predominately comprised of monoterpenes [7] . Then it was concluded that the 3 plants from 2 diff erent provinces (Eastern Cape and Kwazulu Natal) and one from Zimbabwe are 3 diff erent chemotypes of the same plant species. The observed diff erence in chemical composition and content of the essential oil of C. validus could be due to climatic and soil variation, stage of vegetative cycle and seasonal variation[19], literature does support the identifi cation of diff erent chemotypes of plant species based on variation of their chemical constituents. Therefore, our results support the fact that plant species from the same genus can diff er because of their geographical location.
4.2. Anti-inflammatory activity
The anti-infl ammatory eff ect of C. validus essential oils in Wistar rats using the fresh egg albumin-induced rat paw edema model was analyzed. The essential oil from C. validus (i.e. fresh; dry leaves and dry fl owers) showed anti-infl ammatory action by reducing the paw volume signifi cantly (P<0.05 and P<0.01). Fresh egg albumininduced infl ammation occurs in 3 phases. An initial phase during the fi rst 1.5 h is caused by the release of histamine and serotonin [20] . The second phase involves the release of bradykinin from 1.5 h to 2.5 h, whilst the third phase involves the release of prostaglandins and that occurs from 2.5 h to 6.0 h after albumin injection [21] . The current study lasted for only 120 min due to rapid resolution of infl ammation. This corresponds to the fi rst phase of infl ammation, indicating that the oil from the fresh; dry leaves and dry fl owers of C. validus exert their anti-infl ammatory properties by inhibiting the release of histamine and serotonin. C. validus essential oils exhibited signifi cant (P<0.01) anti-infl ammatory activity as compared to the control group throughout the 2 h period of experimentation. Aspirin showed no eff ectiveness during the fi rst 30 min, but it was eff ective (P<0.05) at 60, 90 and 120 min. Linalool, a major constituent in the oil could have contributed to the observed anti-infl ammatory eff ects. Indeed a few studies have revealed an eff ect of (-) - Linalool on chronic inflammation, which significantly reduced Complete Freund’s Adjuvant induced paw edema at a dose of 200 mg/kg [22-25] . Artemisia ketone could also be attributed with the antiinfl ammatory eff ect of the oil as other studies reveal that ketonescan reduce pain and infl ammation[26]. This inhibition may also be associated to the presence of α毩-eudesmol, naphthalene and δ毮-cadinene for example previous reports reveal that α毩-eudesmol was responsible for the inhibition of neurogenic infl ammation in models of the electrical stimulation [27] , while naphthalene derivatives are currently used for the treatment of infl ammatory disorders [28] ; Moreover, δ毮-cadinene which was one of the main components in the Teucrium essential oil exhibited anti-infl ammatory activity [29] . Contents of essential oil from C. validus may exert their eff ects by inhibiting the release of mediators such as histamine and serotonin during the fi rst phase. Thus, both fresh and dry parts of the plant proved to have signifi cant anti-infl ammatory properties
Conflict of interest statement
The authors declare no confl ict of interest.
Acknowledgements
The authors are grateful to Govan Mbeki Research office, UFH, Directorates of Research, Walter Sisulu University (WSU) and NRFIKS for fi nancial support.
References
[1] Padalia RC, Verma RS, Chanotiya CS, Yadav A. Chemical fi ngerprint of the fragrant volatiles of nineteen Indian cultivars of Cymbopogon Spreng (Poaceae). Rec Nat Prod 2011; 5(4): 290-299.
[2] Akhila A, editor. The essential oil-bearing grasses: the genus Cymbopogon. London: CRC Press; 2009.
[3] Khanuja SSP, Shasany AK, Pawar A, Lal RK, Darokar MP, Naqvi AA, et al. Essential oil constituents and RAPD markers to establish species relation in Cymbopogon Spreng (Poaceae). Biochem Sys Eco 2005; 33(2): 171-186.
[4] Van Oudshoorn F. Guide to the grasses of Southern Africa. Pretoria: Briza Publications; 1999.
[5] Bose NSC, Nirmala T, Amani K. Screening of antimicrobial activity of Cymbopogon caesius and Cymbopogon coloratus essential oils. Int J Biopharma Res 2013; 02(01): 55-57.
[6] Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils a review. Bioresour Tech 2010; 101(1): 372-372.
[7] Chagonda LS, Makanda C, Chalchat, JC. The essential oils of wild and cultivated Cymbopogon validus (Stapf) Stapf ex Burtt Davy and Elionusrus muticus (Spreng.) Kunth from Zimbabwe. Flavour Fragr J 2000; 15(2):100-104.
[8] Nόbrega de Almeida R, Agra MDF, Negromonte Souto Maior F, De Sousa DP. Essential oils and their constituents: anticonvulsant activity. Molecules 2011; 16(3): 2726-2742.
[9] Kumar S, Dwivedi S, Kukreja AK, Sharma JR. Bagchi GD. Cymbopogon: the aromaticgrass monograph. Lucknow : Central Institute of Medicinal and Aromatic Plants Publications; 2000.
[10] Naidoo N, Thangaraj K, Odhav B, Baijnath H. Chemical composition and bilogical activity of the essential oil from Cymbopogon narddus (L). Rendle. Afric J Tradit Complement Altern Med 2009; 6: 395.
[11] Adams RP. Identification of Essential Oil Components by ion trap mass Spectroscopy. New York: Academic Press; 2012.
[12] Joulain D, Koenig AW. The Atlas of spectral data of sesquiterpene hydrocarbons. Hamburg : E.B.-Verlag; 1998.
[13] ESO 2000. The complete database of essential oil. B.A.C.I.S: The Netherlands; 1999.
[14] Winyard PG, Willoughby DA. Inflammation protocols. Vol 225. Totowa, NJ: Humana Press; 2003.
[15] Dubner R, Hylden JKL, Nahin, RL, Traub RJ. Neuronal plasticity in superficial dorsal horn following peripheral tissue inflammation and nerve injury. In: Cervero F, Bennett GJ, Headley PM, editors. Processing of Sensory Information in the Superficial Dorsal Horn of the Spinal Cord. New York: Springer US; 1989. p. 429-442.
[16] Greenwald RA, Diamond HS. CRC handbook of animal models for rheumatic diseases vol 1. Boca Raton: CRC Press; 1988.
[17] Cai C, Chen Y, Zhong S, Ji B, Wang J, Bai X, et al. Anti-inflammatory activity of N-Butanol extract from Ipomoea stolonifera in vivo and in vitro. PloS one 2014; 9(4): e95931
[18] Adams RP. Identification of essential oil components by gas chromatography/ mass spectrometry. Carol Stream: Allured Publishing Corporation; 2007.
[19] Viuda-Martos M, Ruíz-Navajas Y, Fernández-Lόpez J, Pérez-Álvarez JA. Chemical composition of the essential oils obtained from some spices widely used in Mediterranean region. Acta Chim Slov 2007; 54: 921-926.
[20] Lin CC, Lin W, Chang CH, Namba T. Anti-inflammatory and hepatoprotective eff ects of Ventilago leiocarpa. Phytother Res 1995; 9(1): 11-15.
[21] Suba V, Murugesan T, Kumaravelrajan R, Mandal SC, Saha BP. Antiinflammatory, analgesic and antinoperoxidative efficacy of Barleria lupulina Lindl extract. Phytother Res 2005; 19: 695-699.
[22] Erdemoglu N, Küpeli E, Yesilada E. Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. J Ethnopharmacol 2003; 89(1):123-129.
[23] Bounihi A, Hajjaj G, Alnamer R, Cherrah Y, Zellou A. In vivo potential anti-inflammatory activity of Melissa Offinalis L. essential oils. Adv Pharmacol Sci 2013; 2013:1-7.
[24] Batista PA, Werner MF, Oliveira EC, Burgos L, Pereira P, Brum LF, et al. The antinociceptive effect of (-) - linalool in models of chronic Infl ammatory and neurophatic hypersensitivity in mice. J Pain 2010; 11(11):1222-1229.
[25] de Cássia da Silveira e Sá R, Andrade LN, de Soussa DP. A review on antiinfl ammatory activity of monoterpenes. Molecules 2013; 18(1):1227-1254.
[26] Ruskin DN, Kawamura M, Masino SA. Reduced pain and infl ammation in juvenile and adult rats fed ketogenic diet. PloS One 2009; 4(12): e8349.
[27] Asakura K, Kanemasa T, Minagawa K, Kagawa K, Yagami T, Nakajima M, et al. αα-Eudesmol, a P/Q-type Ca2+channel blocker, inhibits neurogenic vasodilation and extravasation following electrical stimulation of trigeminal ganglion. Brain Res 2000; 873(1): 94-101.
[28] Sharma S, Singh T, Mittal R, Saxena KK, Srivastava VK, Kumar A. A study of Anti-infl ammatory activity of some novel αα-amino naphthalene β-amino naphthalene derivatives. Arch Pharm (Weinheim) 2006; 339(3):145-152.
[29] Menichini F, Conforti F, Rigano D, Farmisano C, Piozzi F, Senatore F. Phytochemical composition anti-infl ammatory and antitumour activities of four Teuricrium essential oils from Greece. Food Chemistry 2009; 115(2): 679-686.
Tel: +27-764260280
E-mail: ooyedeji@ufh.ac.za
Foundation project: It was fi nancially supported by Govan Mbeki Research and Development Centre (GMRDC) (UFH), Directorates of Research, Walter Sisulu University (WSU) and NRF-IKS (UID-82640).
doi:Document heading 10.1016/j.apjtm.2016.03.031
*Corresponding author:Opeoluwa Oyedeji, Ph.D, Department of Chemistry, Faculty of Science and Agriculture, University of Fort Hare, Alice 5700, South Africa.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
- Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
- Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
