Probing the Binding of Torasemide to Pepsin and Trypsin by Spectroscopic and Molecular Docking Methods
2016-07-12WANGYirunFANGQingGUOChenhuiLIUYing
WANG Yi-run, FANG Qing, GUO Chen-hui, LIU Ying,2*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China
2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
Probing the Binding of Torasemide to Pepsin and Trypsin by Spectroscopic and Molecular Docking Methods
WANG Yi-run1, FANG Qing1, GUO Chen-hui1, LIU Ying1,2*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China
2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
Torasemide (TOR) belongs to the pyridine sulfonylurea class of loop diuretics and is widely and effectively used in the treatment of hypertension, heart failure, chronic renal failure and liver disease. One of the adverse reactions caused by TOR was a slight gastrointestinal discomfort in the course of treatment. However, the molecular interactions of TOR with digestive proteases (trypsin and pepsin) rarely reported. The attempt of this paper was to completely investigate the binding characteristics between TOR and trypsin or pepsin at different temperatures under imitated physiological conditions by fluorescence spectroscopy, UV-vis absorption, circular dichroism (CD) and molecular modeling technique. The inner filter effect of all fluorescence data in the paper was eliminated to get accurate binding parameters. It was found that the fluorescence quenching of trypsin and pepsin by TOR was a static quenching type. The Stern-Volmer quenching constants (KSV) of TOR-pepisn and TOR-trypsin were inversely correlated with temperatures. The binding of TOR changed the conformational structures and internal micro-environment of pepsin and trypsin by UV-vis absorption, synchronous fluorescence, three dimensional (3D) fluorescence and circular dichroism (CD) spectroscopy. The results showed the polarity around Tyr residues of pepsin or trypsin was changed more obviously than that around Trp residues, the TOR alters the secondary structure of trypsin and pepsin and reduces the β-sheet content of protein, which may affect its physiological function. The molecular docking results showed that TOR inserted into the active site of pepsin to interact with the catalytic residues Asp32 and Asp215, and caused a decrease in pepsin activity. TOR bound into the primary substrate-binding pocket (S1 binding pocket) of trypsin by hydrophobic forces and affected the function of trypsin by increasing its catalytic activity. Our results offer insights for the binding and toxicity mechanism of TOR with pepsin and trypsininvivo, which provides important information for using the TOR safely.
Torasemide; Pepsin; Trypsin, Multi-spectroscopic techniques; Molecular docking
Introduction
Pepsin is an aspartic protease that acts in food digestion in mammal’s stomach, which composes of two structurally homologous domains, an N-terminal domain (residues 1~172) and a C-terminal domain (residues 173~326)[1]. Both the two domains consist almost entirely of β-sheets. The active binding site of pepsin is located in the cleft between the domains and is formed by residues Asp 32 and Asp 215. The optimal pH of 2 allows pepsin to operate in its natural acidic environment, while at neutral pH, the protein is denatured[2].
Trypsin is a digestive enzyme produced in the pancreas into the small intestine, which maintains the function of human digestive system by hydrolyzing the proteins into smaller peptide fragments and physiological functions such as immune response and blood coagulation. Trypsin contains two domains of nearly equal size and the active sites of trypsin composed by His57, Asp102and Ser195 locating between the two domains. Each domain comprises a set of 6 anti-parallel strands of polypeptide chain tied together into a β-sheet unit by a network of H-bonds[3]. Due to loss of trypsin activity can lead to serious conditions such as cystic fibrosis, trypsin is a well-known target to study the structural effects of drugs, which can bind with it and affect the conformation as well as the activity of trypsin.
Torasemide (TOR) belongs to the pyridine sulfonylurea class of loop diuretics, is a new and effective long-acting loop diuretic promoted for use in hypertension. Due to TOR has higher bioavailability, longer half-life, and a prolonged duration of activity than the other two diuretics (furosemide and bumetanide), it is widely and effectively used in the treatment of hypertension, heart failure, chronic renal failure and liver disease[4]. One of the adverse reactions caused by TOR was a slight gastrointestinal discomfort in the course of treatment. Previous studies have shown that TOR can interact with BSA and then affect the structure and properties of BSA[5]. However, the molecular interactions of TOR with digestive proteases have been rarely reported. What’s more, when TOR enters the human stomach, the digestive proteases may be the indirect binding targets. The conformational changes of pepsin and trypsin induced by TOR could change the activity of pepsin and trypsin, which may reduce the absorption of nutrients. Therefore, the effect of TOR on the activity of proteases needs to be further investigated at the molecular level.
In the present work, our intention is to investigate the fluorescence quenching mechanism and conformational change of pepsin and trypsin induced by TOR in solution under physiological conditions. The mechanism of fluorescence quenching of pepsin and trypsin by TOR was analyzed by the Stern-Volmer equation. The conformational changes of pepsin and trypsin were discussed by UV-vis absorption, synchronous fluorescence spectra, three-dimensional fluorescence spectra and circular dichroism spectra. In addition, molecular docking studies illustrated a specific display of binding information and analysed the change mechanism of pepsin or trypsin activity. The binding study of TOR with pepsin and trypisn can provide useful information to pharmacokinetic and pharmacodynamic behavior of TOR in further experiments and provide a firm basis for its rational use in clinical practice.
1 Experimental
1.1 Materials
Pepesin (from porcine gastric mucosa) and Trypsin (from porcine pancreas) were obtained from Sigma-Aldrich Chemical Co. (USA). Torasemide (TOR), N-α-benzoly-L-arginine ethyl ester (BAEE) and Folin reagent were purchased from Aichun Biological Technology Co. Ltd. (Shanghai, China). Trichloroacetic acid and NaOH were all of analytical purity. The stock solution of TOR (1.0×10-3mol·L-1) was made up in dimethyl sulfoxide (DMSO). Pepesin (1.0×10-4mol·L-1) was dissolved with disodium hydrogen phosphate-citric acid buffer (0.2 mol·L-1, pH 2.0), which is the most common pH for pepsin digests. Trypsin solution (1.0×10-4mol·L-1) was prepared in phosphate buffer solution (0.2 mol·L-1, pH 7.40). NaCl solution was applied to maintain the ionic strength at 0.1 mol·L-1. All of the above stocking solutions were kept in dark at 0~4 ℃. Millipore-Q ultrapure water was used throughout.
1.2 Methods
The methods and parameter settings on UV-Vis spectroscopy, fluorescence spectroscopy, synchronous fluorescence, three-dimensional fluorescence spectra, circular dichroism spectroscopy and molecular docking study were according to Ref.[6].
The activity of pepsin or trypsin was determined by spectrophotometric method. Various concentrations of TOR solution were added in pepsin and the disodium hydrogen phosphate-citric acid buffer was used to control pH at 2.0. After 10 min, 1.0 mL of 5% bovine hemoglobin solution was added for 10 min. Then 5.0 mL of 5% trichloroacetic acid was added to terminate the reaction. After addition of 3.0 mL of NaOH and 300 μL of Fehling’s solution to the supernatant, the mixture was incubated at 37 ℃ for 15 min and the value of OD660 was measured by a spectrophotometer. The activity of pepsin can be calculated according to Ref.[7].
Trypsin was able to catalyze BAEE into N-α-benzoyl-L-arginine (BA), of which the UV absorption is far stronger than of BAEE at 253 nm. Therefore, the activity of trypsin was measured using BAEE as the substrate. By monitoring the absorption increase at 253 nm, the changes in activity of trypsin can be detected. The velocity of the enzymatic reaction (μ) was calculated according to Ref.[8].
2 Results and discussions
2.1 Fluorescence quenching analysis
Fig.1 shows the fluorescence emission spectra obtained for pepsin and trypisn with the addition of TOR at the excitation wavelength of 280 nm at 298 K. It can be seen that with the increase in the concentration of TOR, both fluorescence intensities show remarkable decrease, indicating that TOR can interact with pepsin and trypsin, respectively.

Fig.1 Fluorescence emission spectra of Pepsin and Trypsin by TOR, the inset is the structure of TOR
cpepsin=ctrypsin=1.0×10-5mol·L-1;cTOR(×10-6mol·L-1)
(1→7)=0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0;T=298 K
Fluorescence quenching is classified into static quenching and dynamic quenching. To elucidate the nature of fluorescence quenching, Stern-Volmer equation was used to analyze the fluorescence quenching date according to Ref.[6].
To eliminate the possible inner filter effects, the fluorescence intensity used in this paper was corrected according to Ref.[6]. The corresponding results are displayed in Table 1 and the Stern-Volmer plots are shown in Fig.2. It is observed thatKq(×1013L·mol-1·s-1) obtained for pepsin and trypsin were three orders of magnitude higher than the maximum value of diffusion controlled quenching in water (×1010L·mol-1·s-1). Moreover,KSVvalues of TOR-pepsin system and TOR-trypsin system both declined with rising temperature. These results inferred that the probable quenching mechanism for TOR-pepsin system and TOR-trypsin system were static quenching types.
2.2 UV-visible absorption measurement
It is well-known that UV-visible absorption measurement is a very simple and applicable method to explore the structural change and formation of a complex[1]. The absorption spectral change of pepsin or trypsin in the presence and absence of TOR are shown in Fig.3(a) or Fig.3(b), respectively.
Table 1 Stern-Volmer quenching constants for the interaction of TOR with pepsin and trypsin at two different temperatures

SystemT/KKSV/(×105L·mol-1)Kq/(×1013L·mol-1·s-1)RTOR⁃Pepsin2983101 901 611 901 610 99350 9940TOR⁃Trypsin2983101 941 641 941 640 99750 9988
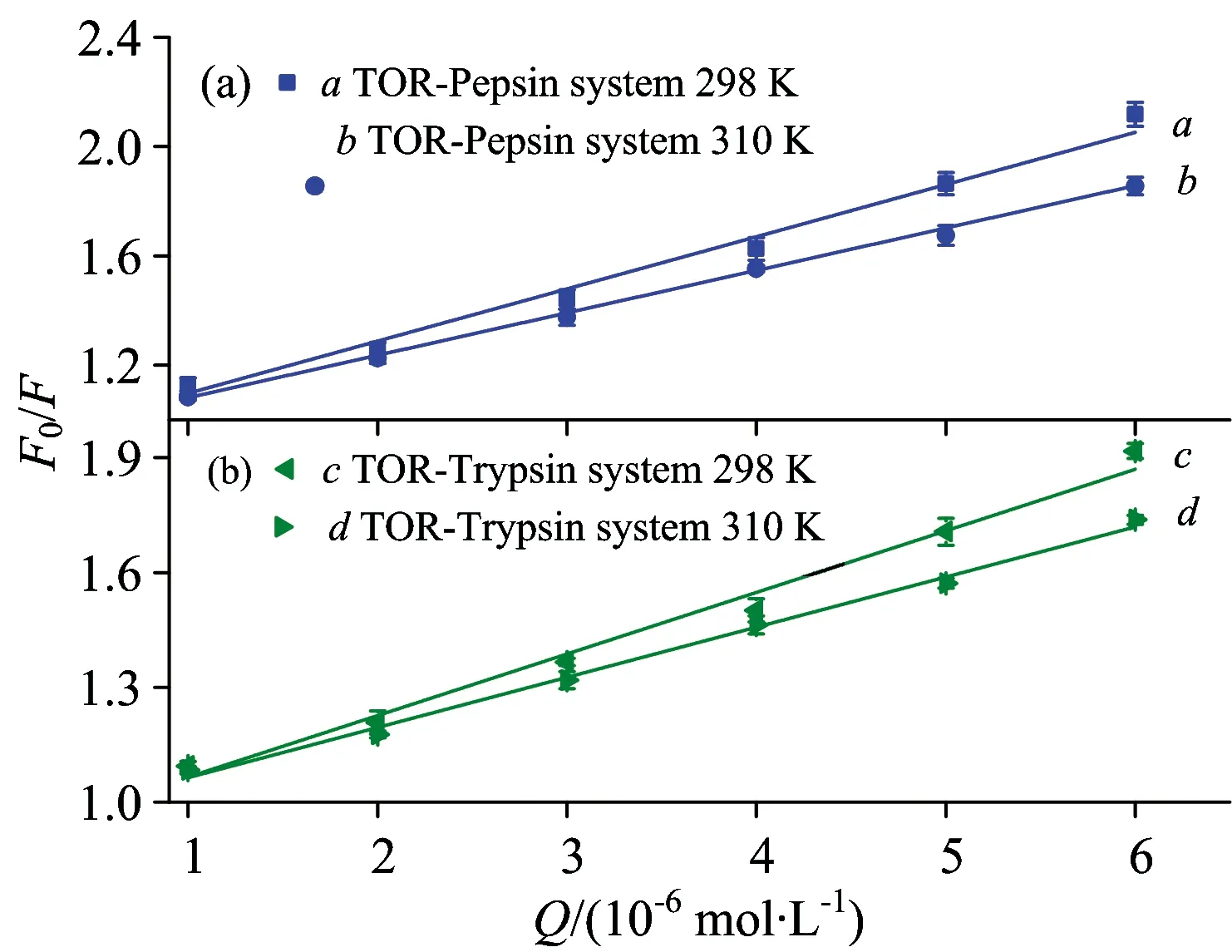
Fig.2 The Stern-Volmer plots for TOR-Pepsin (a) and TOR-Trypsin (b) at different temperatures
cpepsin=ctrypsin=1.0×10-5mol·L-1
The strong absorption baod at around 208 nm reflects the framework conformation of the protein and the weak one at about 280 nm appears owning to the absorption of aromatic amino acids[9]. As shown in Fig.3(a) and Fig.3(b), the absorption peak of pepsin or trypsin at 208 nm decreased sharply with a conspicuously red shift as the TOR is mixed with pepsin or tryspin, indicating that the interaction between TOR and pepsin or trypisn leads to the loosening and unfolding of the protein skeleton structure[10]. In addition, with the gradual addition of TOR to pepsin, the intensity of the peak at 280 nm increased with a red shift, which suggested that TOR can bind to pepsin and form a complex. Fig.3(b) shows different spectra between trypsin, TOR, and TOR-trypsin. If no interaction between trypsin and TOR, the spectra of trypsin and [TOR+trypsin]-TOR should be the same[11]. However, with the addition of TOR, the peak of residues shows a slightly blue shift, implying that there was a change in the microenvironment around the aromatic amino acid residues.
2.3 Conformational investigation
2.3.1 Three-dimensional fluorescence spectra
Three-dimensional (3D) fluorescence spectroscopy is a powerful approach to investigate the characteristic conformational change of enzyme.
Fig.4(a, b) and Fig.4(c, d) show the 3D fluorescence contour map of pepsin and trypsin in the absence and presence of TOR, respectively. Peak 1 displays the intrinsic fluorescence characteristics of Trp and Tyr residues, and peak 2 reflects changes in the polypeptide backbone structure of pepsin or trypsin in the presence of TOR[7]. When TOR solution added into pepsin or trypsin, the fluorescence intensityFof peak 1 (from 986.1 nm to 687.3 nm for pepsin; from 883.8 nm to 699.1 nm for trypsin) and peak 2 (from 242.9 nm to 197.4 nm for pepsin; from 502.4 nm to 348.9 nm fortrypsin) were both decreased, which revealed that the binding of TOR to pepsin or trypsin induced the conformation of the polypeptide backbone altered.

Fig.3 UV-Vis absorption spectra of pepsin (a) and trypsin (b) in the presence of different concentration of TORcpepsin=ctrypsin=1.0×10-5 mol·L-1;cTOR(×10-6 mol·L-1) (1→6): 0.0, 1.0, 2.0, 3.0, 5.0, 6.0
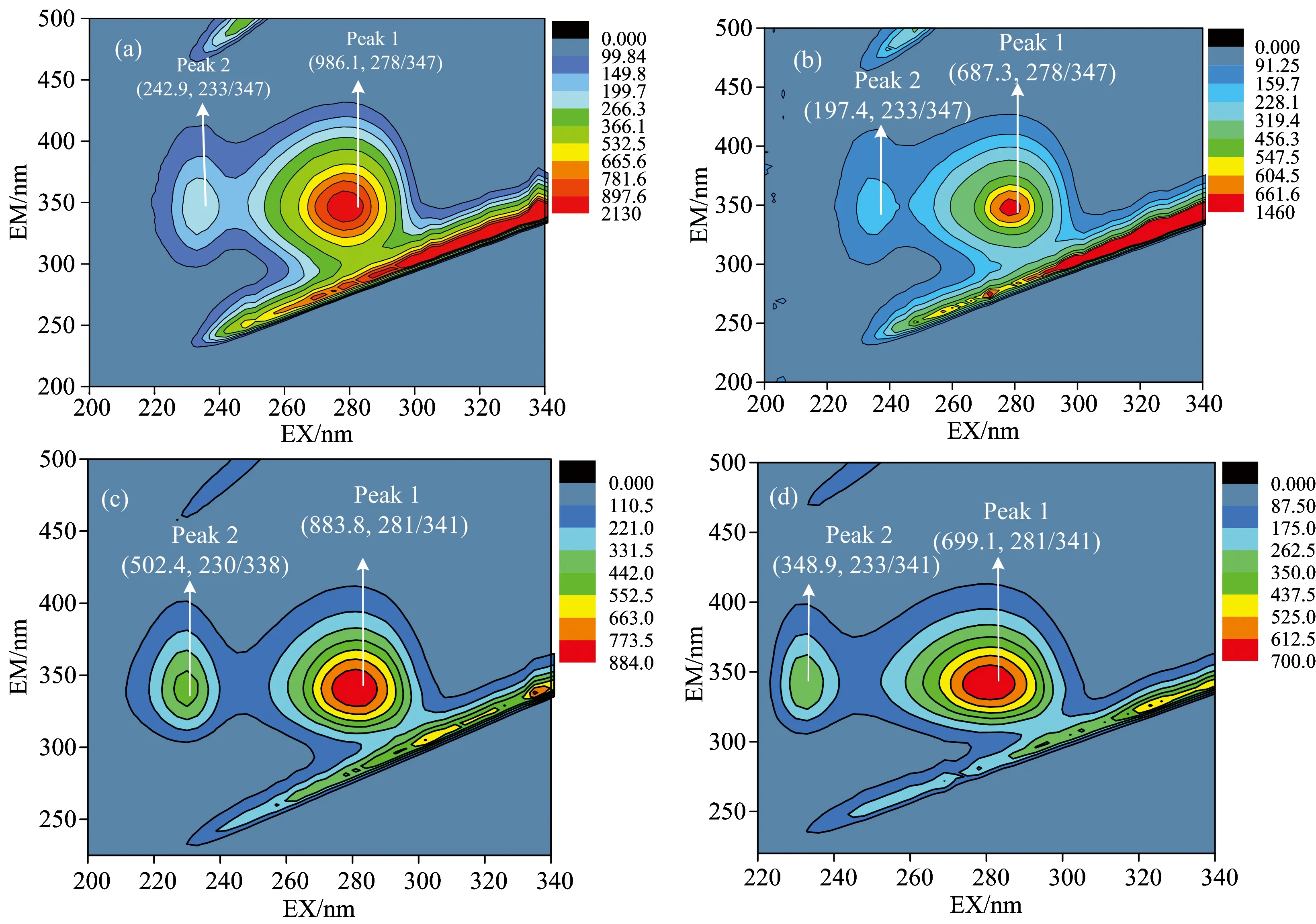
Fig.4 The three-dimensional fluorescence contour map of pepsin (a), TOR-pepsin (b), trypsin (c) and TOR-trypsin (d)cTOR=1.0×10-6 mol·L-1; cpepsin=ctrypsin=1.0×10-5 mol·L-1
2.3.2 Synchronous fluorescence
The effect of TOR on the synchronous fluorescence spectra of pepsin or trypsin are shown in Fig.5(a, b) or Fig.5(c, d), respectively. It can be seen that the peak position of Trp residues for pepsin or trypsin has no significant shift change, which indicated that the interaction of TOR with pepsin or trypsin did not obviously affect the micro-environment around Trp. Whereas, a blue shift (from 292 nm to 287.4 nm) of Tyr residues of pepsin [Fig.5(b)] was observed upon addition of TOR. The blue shift indicated that hydrophobicity of the Tyr residues of pepsin was increased. In addition, a slight red shift (from 298.4 to 30.2 nm ) of Tyr residue of trypsin was also shown in Fig.5(d), indicating that Tyr residues were placed in a less hydrophobic environment and more exposed to the solvent. These results indicated that TOR can bind and induce the conformation changes of pepsin or trypsin. Moreover, the polarity around Tyr residues of pepsin or trypsin changed more obviously than that around Trp residues after binding to TOR.
2.3.3 Circular dichroism (CD) spectra
Further evidence for the content fluctuations of different parts of the secondary structure of pepsin or trypsin upon addition of TOR was confirmed by CD spectroscopy (Fig.6, Fig.7). The calculated contents of different secondary structure were expressed in terms of mean residue ellipticity according to Ref.[6] and listed in Table 2. As shown in Fig.6, with addition of TOR, the shape of pepsin does not change significantly and the negative molar ellipticity decrease. From Table 2, the proportion of β-sheet content in pepsin exhibits an decrease from 54.5% to 45.3% with increasing the molar ratio of TOR to pepsin from 0∶1 to 1∶1.
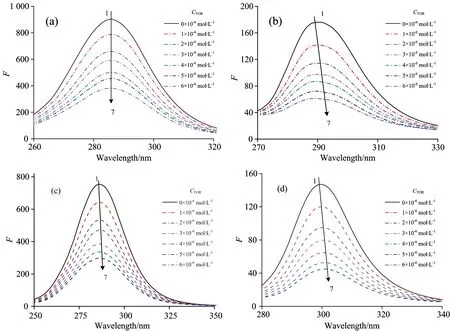
Fig.5 Synchronous fluorescence spectra of pepsin [(a) Δλ=60 nm; (b) Δλ=15 nm] and trypsin [(c) Δλ=60 nm; (d) Δλ=15 nm] in the presence of different concentration of TOR
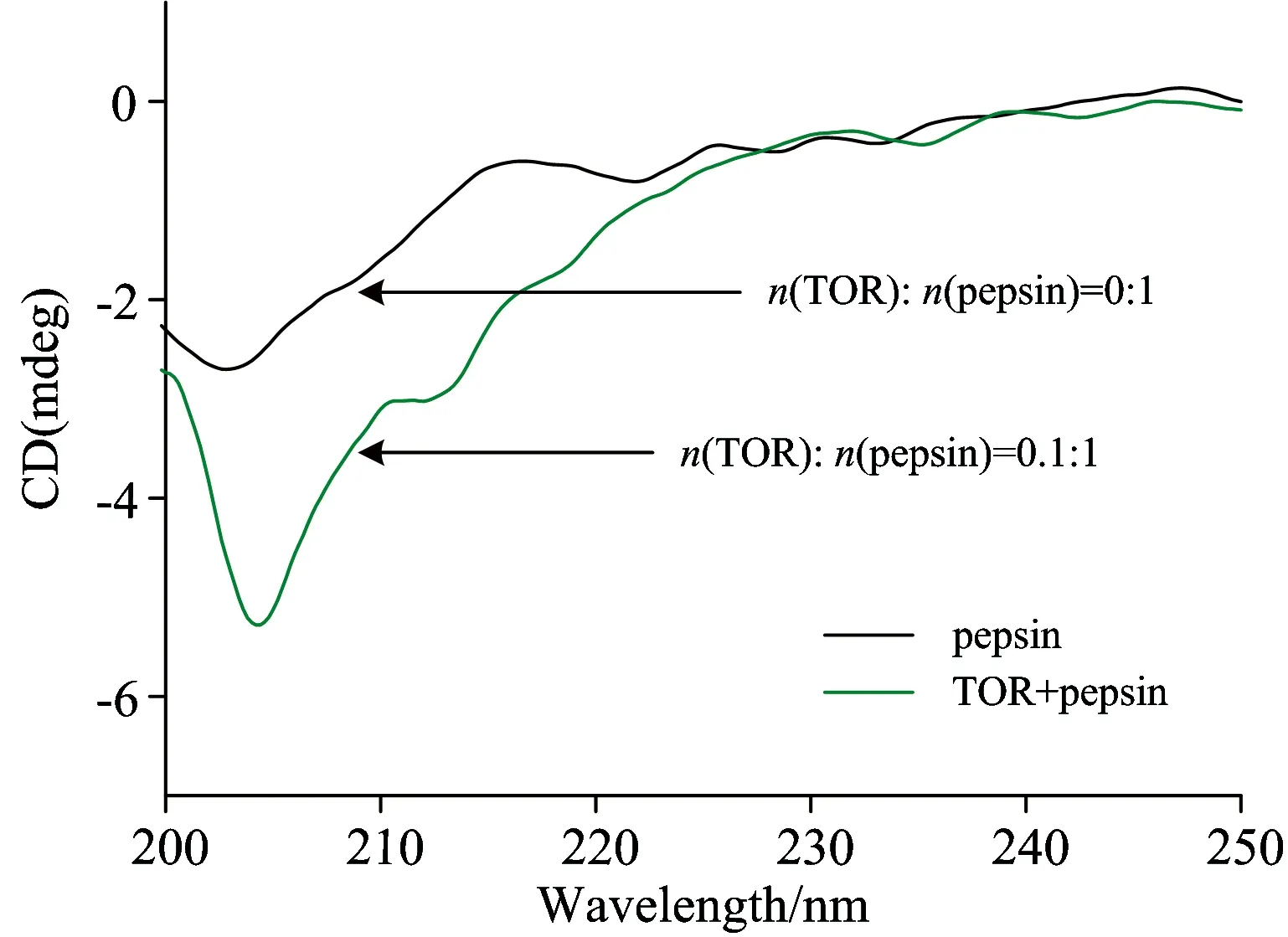
Fig.6 The CD spectra of pepsin in the absence and presence of TOR
At the same time, Fig.7 shows the CD spectra of trypsin in the absence and presence of TOR. It could be observed from Fig.7 that the CD signal between 200 and 250 nm changed and the intensity increased with addition of TOR. As the data showed in Table 2, when TOR was added into the trypsin solution, the contents of secondary structures fluctuated in different trends. The content of α-helix increased (from 18.5% to 24.5%) and β-sheet decreased (from 43.1% to 35.2%). These results suggested that TOR alter the secondary structure of trypsin and pepsin and causes partial unfolding and loosening the polypeptide chain, which may affect its physiological function.
2.4 Molecular docking study
To obtain the specific binding sites of TOR on pepsin or >trypsin and to analyse the inhibition mechanism of pepsin or trypsin activity, we had run docking programs to simulate the binding mode of pepsin or trypsin and ligand. TOR was docked with pepsin or trypsin using DockingServer (http://www.docking server.com). The crystal structure of pepsin(PDB code 3PEP) and trypsin (PDB code 2ZQ1) were taken from RCSB Protein Data Bank (http://www.rcsb.org/pdb). The structure of TOR (Pubchem CID 41781) was download from PubMed NCBI website (https:// pubchem. Ncbi. Nlm. nih. gov/).
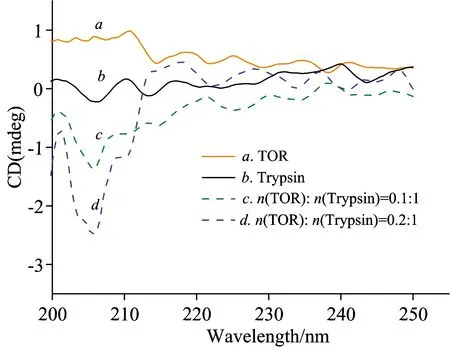
Fig.7 The CD spectra of trypsin in the absence and presence of TOR
cTrypsin=1×10-5mol·L-1;cTOR(×10-6mol·L-1):c, 1;d, 2;T=298 K

Table 2 The influence of TOR on the secondary structure of pepsin and trypsin
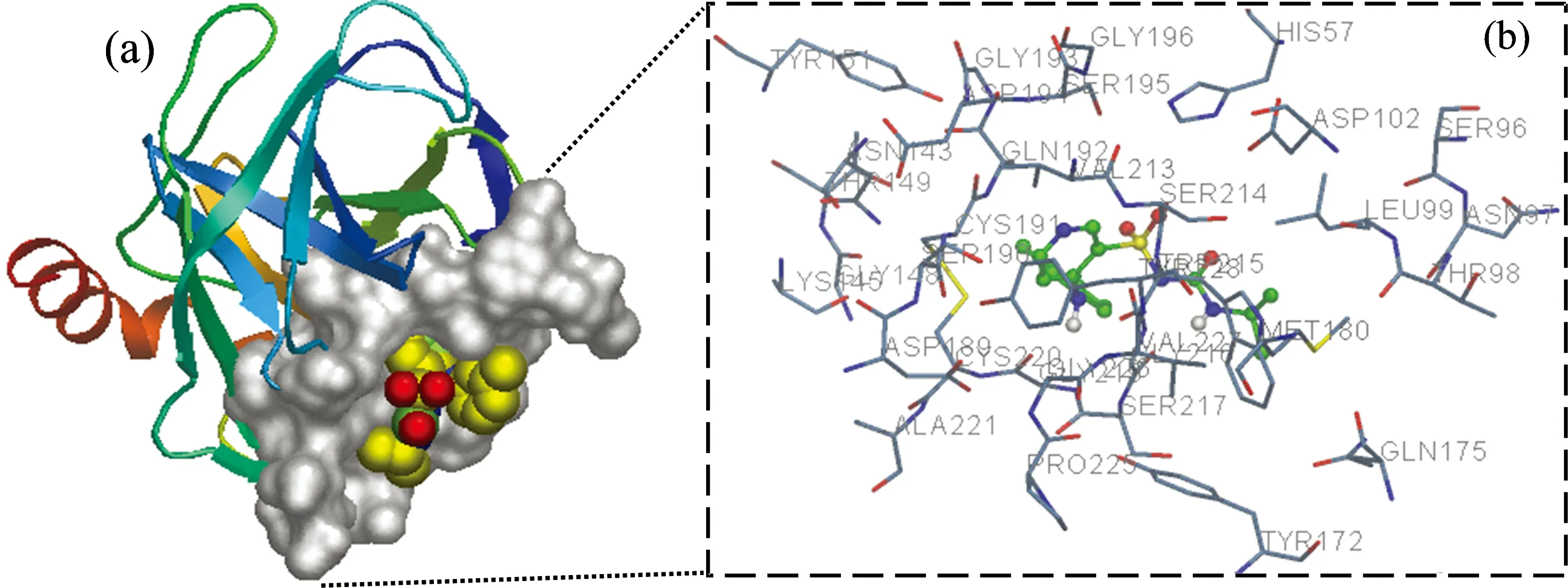
Fig.8 (a) The binding site of TOR in pepsin; (b) Molecular modeling of the interaction between TOR and pepsin. The atoms of TOR are marked with sticks model for clarity and the atoms of amino acid residues of pepsin are represented by solid lines
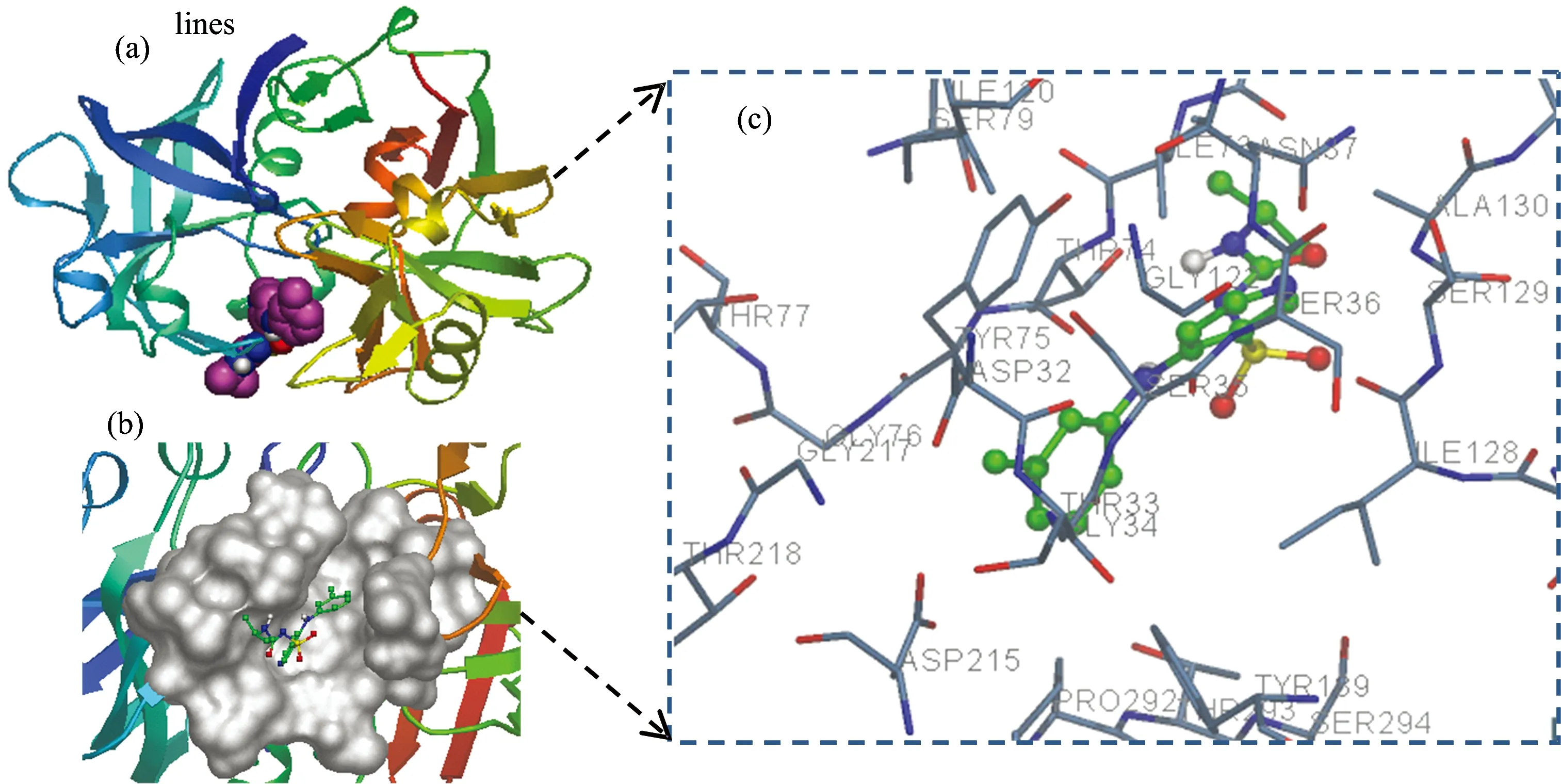
Fig.9 (a) The binding site of TOR in trypsin; (b) Detailed illustration of the binding between TOR and trypsin in hydrophobic surface model; (c) Molecular modeling of the interaction between TOR and trypsin. The atoms of TOR are marked with sticks model for clarity and the atoms of amino acid residues of trypsin are represented by solid lines
From the docking results of TOR-pepsin system (Fig.8), TOR was surrounded by resides Asp32, Tyr 75, Asp 215, Gly 34, Ser 35, Ser 36, Asn 37, Ile 128 and Ser 129. It was reported that the catalytic site of pepsin is formed by residues Asp32 and Asp215[1]. The binding of TOR into the pepsin cavity influenced the micro-environment of the pepsin activity site, which resulted in the reduction of pepsin activity. On the other hand, there were hydrogen bonds between TOR and Thr 74, Ile 128, Asp 32 of pepsin with a distance in the range of 2.80~2.92 Å, which played an important role in increasing the binding affinity of TOR to pepsin. Besides, the hydrophobic interactions between TOR and Ile 73, Tyr 75, Ala 130 were also involved in the association.
The binding mode and binding site of TOR-trypsin complex are given in Fig.9. TOR inserted into the primary substrate-binding pocket (S1 binding pocket) of trypsin to interact with residues Cys191, Asp215, Gly217, Asp 189, and Thr218. The active site of trypsin formed by His 57, Asp 102 and Ser 195 was also around TOR. There are hydrophobic interactions between TOR and Leu 99, Cys 220, Val 282, Glu 287, Trp 215 of trypsin with a distance in the range of 3.41~3.66 Å. The hydrogen bonds (N3, N1, H1 of TOR with Gly 216, Gly216, Trp 215, respectively) were also presented. However, the hydrophobic forces played a major role in the biding of TOR to tyrpsin.
2.5 Effect of TOR on enzyme activity
The effect of pepsin or trypsin structure by TOR we have investigated, to further study the effect of enzyme physiological function by TOR, we set the activity of pepsin or trypsin as 100% or 1 in the absence of TOR and other activities changes in the presence of different concentrations of TOR were calculated accordingly[12].
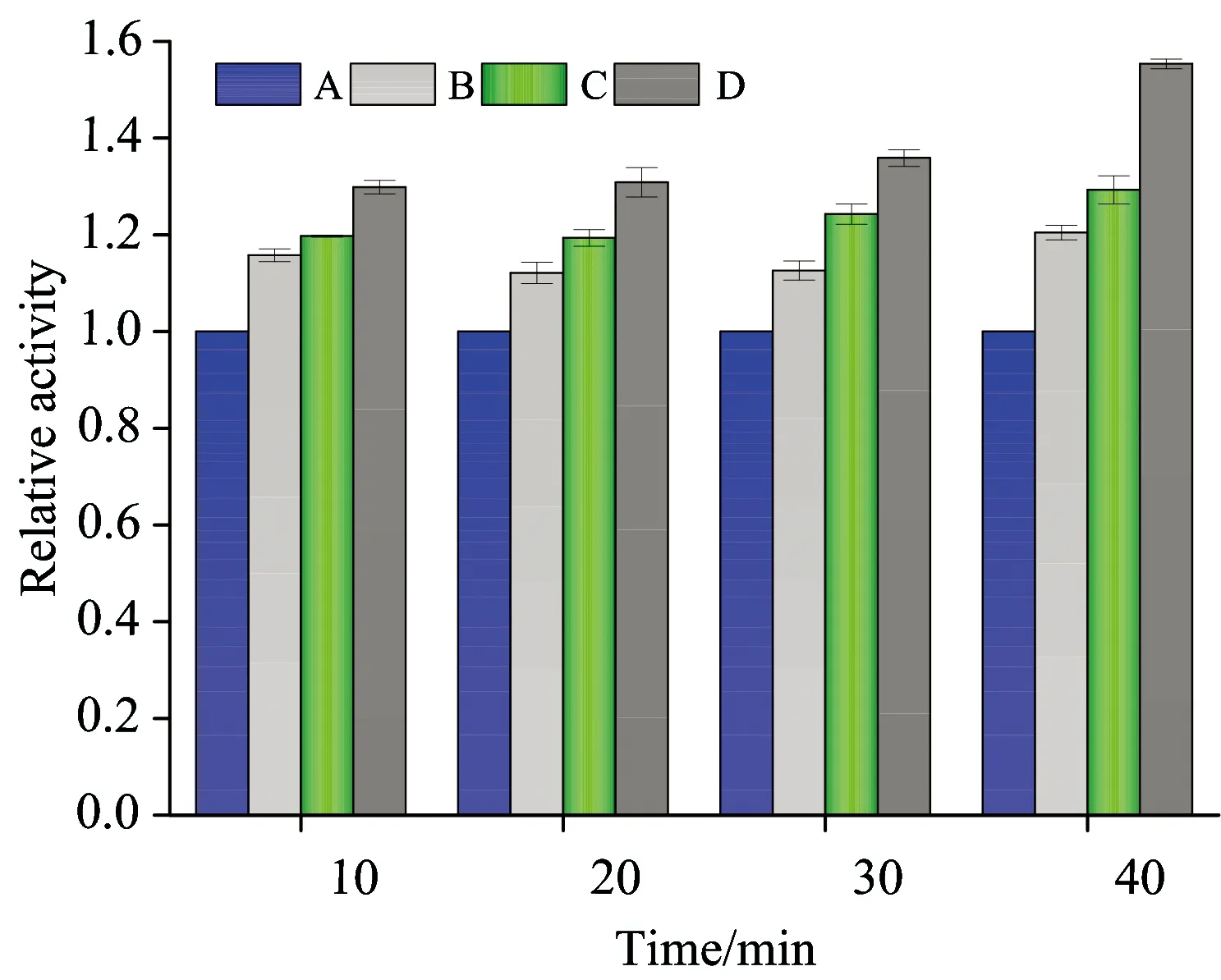
Fig.10 Effect of interaction time of TOR with trypsin on the activity of trypsin
cTrypsin=1×10-5mol·L-1;cTOR(×10-6mol·L-1): A, 0; B, 1; C, 2; D, 3
As showed in Fig.10, pepsin activity decreased significantly with TOR concentration increased. When the concentration of TOR reached 4.0×10-5mol·L-1, the activity of pepsin was decreased to approximately 60%. Based on the docking and synchronous fluorescence results, TOR bound directly to the enzyme cavity site and this influenced the micro-environment of the pepsin activity site, resulting in reduced pepsin activity.
On the other hand, the activity of trypsin increased with the addition of TOR. It is probably because the hydrophobic forces played a major role in the biding of TOR to trypsin, which is conducive the substrate bind into the enzyme activity center, leading to the more exposure of the catalytic activity and expression of the trypsin activity increased finally[9].
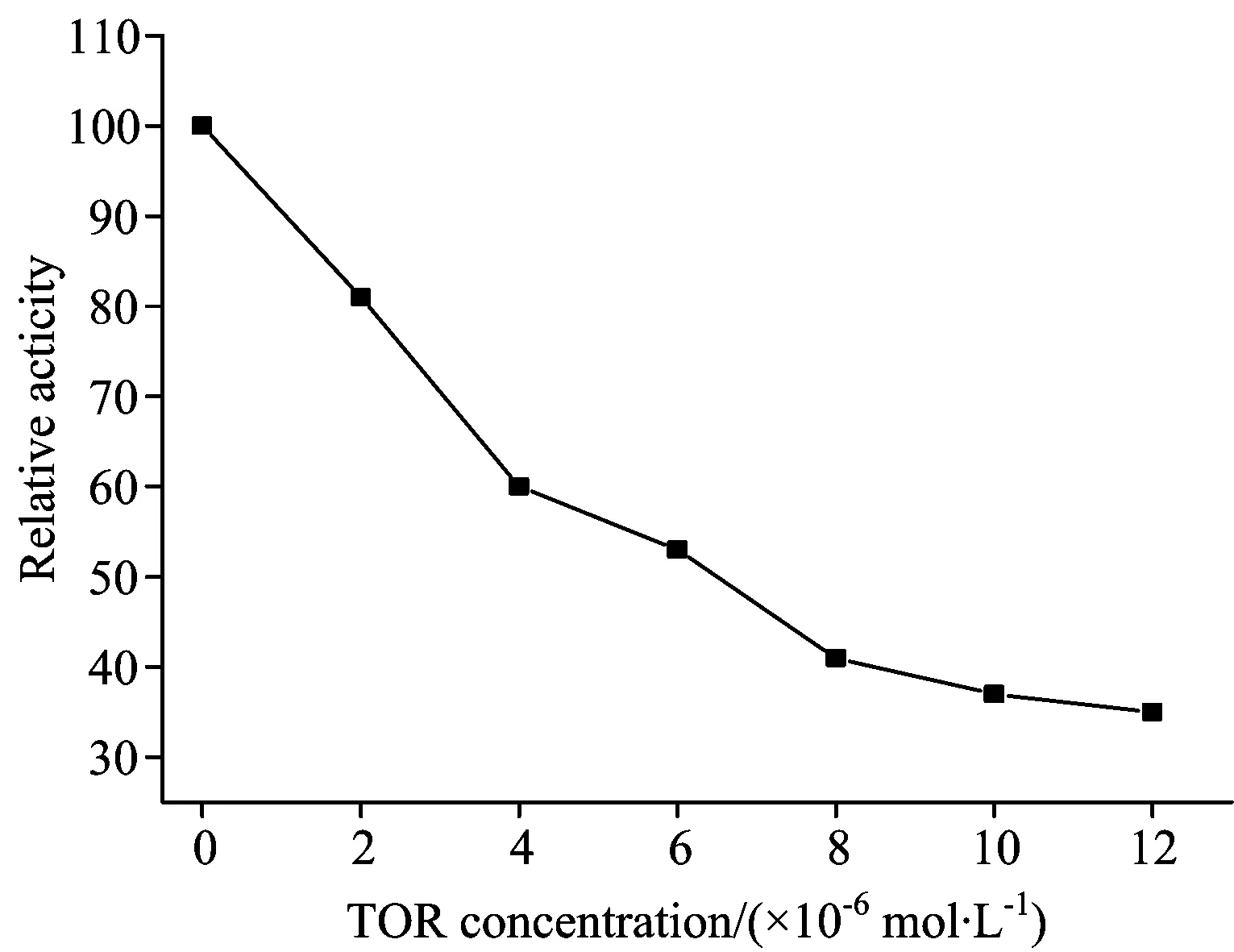
Fig.11 Effect of TOR concentration on the activity of pepsin
cPepsin=1×10-5mol·L-1;cTOR(×10-6mol·L-1)=0.0, 2.0, 4.0, 6.0, 8.0, 10.0, 12.0
3 Conclusions
After elimination of the inner filter effects, the fluorescence of pepsin and trypsin were quenched by TOR through a static quenching mechanism due to the formation of ground state complex. As demonstrated by the synchronous fluorescence spectroscopy, UV-vis absorption and three dimensional (3D) fluorescence, the molecular conformation of pepsin and tryspin were changed and the polarity around Tyr residues of pepsin or trypsin changed more obviously than that around Trp residues in the presence of TOR. The results of CD suggested that TOR induces the changes of the natural secondary structure of pepsin and trypsin by increasing the content of α-helix and decreasing the content of β-sheet. Molecular docking results showed that the TOR bound to pepsin at the active site, which influenced the micro-environment of the pepsin activity site and caused the inhibition of pepsin activity. Besides, the hydrophobic forces played a major role in the biding of TOR to the primary substrate-binding pocket (S1 binding pocket) of trypsin, which help the substrate bind into the enzyme activity center, leading to the more exposure of the catalytic activity and expression of the trypsin activity increased finally. This study raises critical concerns regarding the transport, distribution and toxicity effects of TOR in the human body.
[1] Shen L L, Xu H, He Z D, et al. Spectrochim. Acta A,2015, 135: 256.
[2] Zeng H J, Liang H L, You J, et al. Luminescence,2014, 29: 715.
[3] Liu Y Y, Zhang G W, Liao Y J, et al. Spectrochim. Acta A,2015, 151: 498.
[4] Arumugam S, Remya S, Miyashita S, et al. Exp. Mol. Pathol.,2014, 97: 137.
[5] Li C X, Liu S P, Hu X L, et al. Acta Chimica Sinica,2011, 69: 1408.
[6] Hu T Y, Liu Y. J. Pharm. Biomed. Anal.,2015, 107: 325.
[7] Zeng H J, Yang R, Liang H L, et al. Spectrochim. Acta A,2015, 151: 576.
[8] Chai J, Xu Q F, Liu R T, et al. Spectrochim. Acta A,2013, 105: 200.
[9] Hu X X, Yu Z H, Liu R T. Spectrochim. Acta A,2013, 108: 50.
[10] Wang Y Q, Zhang H M, Kang Y J, et al. J. Mol. Struct.,2016, 1107: 91.
[11] Song H, Chen C Y, Zhao S L, et al. J. Food. Drug. Anal.,2015, 23: 234.
[12] Song W, Yu Z H, Liu R T, et al. Spectrochim. Acta A,2015, 137: 286.
*通讯联系人
R446.1
A
光谱法和分子对接模拟技术研究托拉塞米与胃蛋白酶和胰蛋白酶的相互作用
王艺润1,方 庆1,郭晨辉1,刘 颖1,2*
1. 中央民族大学生命与环境科学学院,北京 100081
2. 中央民族大学北京市食品环境与健康工程技术研究中心,北京 100081
托拉塞米(TOR)属于吡啶磺酰脲类袢利尿剂,被广泛有效地用于高血压,心脏衰竭,慢性肾功能衰竭和肝脏疾病的治疗。TOR在治疗过程中易引起的不良反应之一为轻微肠胃不适。然而,TOR与消化蛋白酶(胰蛋白酶和胃蛋白酶)分子间的相互作用鲜有报道。在模拟生理条件下,采用荧光光谱、紫外-可见吸收光谱、圆二色谱和分子对接技术研究了不同温度下托拉塞米(Torasemide, TOR)与胃蛋白酶(Pepsin)和胰蛋白酶(Trypsin)间的相互作用。所有荧光数据均进行了内滤光校正以获得更准确的结合参数。结果表明,TOR-Pepsin和TOR-Trypsin体系的猝灭常数(KSV)均与温度呈负相关,说明TOR与Pepsin及Trypsin之间的作用机制均为静态荧光猝灭。利用紫外-可见吸收光谱、同步荧光光谱、3D荧光光谱和圆二色光谱法考查了TOR对Trypsin和Pepsin构象的影响。结果发现胃蛋白酶或胰蛋白酶中酪氨酸残基的极性改变较色氨基更明显,TOR可改变色氨酸残基的微环境并降低Trypsin和Pepsin中β-折叠结构,进而可能影响其生理功能。分子对接结果表明,TOR与Pepsin的结合位点位于由Asp-32和Asp-215组成的活性中心周围,从而抑制Pepsin活性。而TOR通过疏水作用力结合在Trypsin的口袋型底物结合位点(S1口袋),促进底物进入酶活性中心,最终表现为Trypsin活性升高。该研究探讨了TOR与胃蛋白酶和胰蛋白酶的结合作用和毒性机制,为TOR的安全使用提供重要依据。
托拉塞米;胃蛋白酶;胰蛋白酶;多光谱法;分子对接
2016-02-29,
2016-05-30)
Foundation item:The National Natural Science Foundation of China (21177163), 111 Project B08044, First-Class University First Class Academic Program of Minzu University of China (YLDX01013), Special Guidance Fund of Building World First-Class Universities (Disciplines) and Characteristic Development of Minzu Unviersity of China (2016), Coordinate Development of First-Class and First-Class University Discipline Construction Funds(10301-0150200604), The Academic Team Construction Project of Minzu University of China (2015MDTD25C&13C), The Fundamental Research Funds for the Central Universities”(10301-01404031,2015), First-Class Universities and First-Class Discipline Construction Transitional Funds under Special Funding (2016. ph.D), 2015MDTD08C
10.3964/j.issn.1000-0593(2016)10-3414-08
Received:2016-02-29; accepted:2016-05-30
Biography:WANG Yi-run, (1989—), Doctor of College of Life and Environmental Sciences, Minzu University of China e-mail: yrwang8@126.com *Corresponding author e-mail: liuying4300@163.com
