Effect of partial splenic embolization on the immune function of cirrhosis patients with hypersplenism
2016-06-29GuiYunJinChuanZhuLvTangDengShaoenChengChaoQianLi
Gui-Yun Jin, Chuan-Zhu Lv, Tang Deng, Shao-W en Cheng, Chao-Qian Li*
1Guangxi Medical University2Hainan Medical University3the Affiliate Hospital of Hainan Medical College
Effect of partial splenic embolization on the immune function of cirrhosis patients with hypersplenism
Gui-Yun Jin1, Chuan-Zhu Lv2, Tang Deng3, Shao-W en Cheng3, Chao-Qian Li1*
1Guangxi Medical University
2Hainan Medical University
3the Affiliate Hospital of Hainan Medical College
AR T ICLE IN FO
Article history:
Received 15 April 2016
Received in revised form 16 May 2016 Accepted 15 June 2016
Available online 20 July 2016
Keywords:
Viral hepatitis type B
Cirrhosis
Hypersplenism
Partial splenic embolization Immune function
ABSTR ACT Objective: To discover the effect of partial splenic embolization on the immune function of cirrhotic patients with hypersplenism. Methods: Patients involved in the study were enrolled and divided into three groups, including control group, experimental group, and complication group. Numbers of CD3+, CD4+and CD8+T cells and CD4+CD25+CDl27low/-Treg cells in the peripheral blood of patients before surgery, 1 month, 6 months, 1 year, and 2 years after surgery were analyzed by fluorescence active cell sorting (FACS). Contents of immunoglobulins (IgA, IgG and IgM) were analyzed by auto immunoassay analyzer. Results: In the peripheral blood of patients from experimental group, numbers of CD3+, CD4+and CD8+T cells initially declined, but afterwards increased to normal level; in the peripheral blood of patients from complication group, CD3+and CD8+T cells showed the same trend, but the number of CD4+T cells was below normal level at all detection times. Furthermore, CD3+, CD4+and CD8+T cells in the peripheral blood of patients from complication group were initially less than those in experimental group, and afterwards were comparable between two groups. In patients from both experimental group and complication group, the number of CD4+CD25+CDl27low/-Treg cells increased 1 month and 6 months after surgery, and gradually restored to normal level. CD4+CD25+CDl27low/-Treg cell counts in patients from complication group were initially more than those in patients from experimental group 1 month and 6 months after surgery, but then they were comparable. Furthermore, contents of immunoglobulins (IgA, IgG and IgM) were comparable in three groups at all detection times. Conclusion: Partial splenic embolization influenced the immune function of cirrhotic patients with hypersplenism in the short term but the immune function could afterwards gradually restore to normal. Our results implicated that measures that prevent infection and improve immune function were necessary in early stage after undergoing PSE in order to reduce complications.
E-mail: Chuan-Zhu Lv, PhD supervisor, Hainan Medical College
TEL: 13907578989
1. Introduction
Chronic viral hepatitis B is a common disease in China, the morbidity of which is 8% to 10%. Patients with hepatitis B usually develop hypersplenism in the period of liver cirrhosis decompensation. Hypersplenism is clinically characterized as decline of hemocytoes, such as white blood cells, red blood cells,and platelet, which can result in gastrointestinal bleeding, anemia,and infection. As the development of interventional techniques,partial splenic embolization (PSE) with advantages of smaller trauma and partial spleen-conserving has been w idely applied in clinic to replace splenectomy.PSE, however, has been proved to cause some complications, such as peritoneal cavity infection, intractable abdominal cavity effusion,intestine flatulence, splenic abscess, and also portal vein and mesenteric arterial thrombosis, which can even lead to death [1-3]. Additionally, the effect of PSE on the immune function of patients has been a hot topic of academic research. However, there is still no unified evaluation criterion of spleen swelling and immune function in patients with PSE, and the mechanism of which has not been fully elucidated.
Here, to uncover the relevance between PSE, postoperative complications and immune function, CD 3+, CD4+and CD8+T cells, CD4+CD25+CDl27low/-Treg cells (regulatory T cell, Treg),and contents of immunoglobulins (including IgA, IgG and IgM) in the peripheral blood of cirrhotic patients with hypersplenism were analyzed.
2. Materials and methods
2.1. Patients
The study was approved by the Ethics Committee of the authors' affiliation prior to initiation. Patients have been informed and signed the informed consent before blood draw ing. Patients involved in this study were enrolled and divided into three groups: control group (healthy population), experimental group (patients w ithout complications after PSE), and complication group (patients with complications after PSE). Symptoms of hepatitis B cirrhotic and hypersplenism were diagnosed according to clinic history, virus immunology examination, liver tests, peripheral hemogram test,and imageological exam ination. A total of 50 persons, including 33 males and 17 females, who were physically healthy, were enrolled as the control group (age range: 45-68 years, mean age: 56.2 years). The experimental group was consists of 57 cases, including 38 males and 19 females (age range: 41-64 years, mean age: 52.6 years). The complication group had 51 cases, including 37 males and 14 females (age range: 50-68 years, mean age: 57.3 years).
2.2. Partial splenic embolization
Partial splenic embolization (PSE) was operated as follow s. The participants were asked to fast for 12 hours and not to drink for 6 hours before undergoing the surgery. Seldinger puncture,combined with digital subtracting X-ray system, were applied to right arteriopuncture to reach carotid sheath. Catheter (Yashiro) was guided by guide w ire to spleen artery to monitor splenomegalia. We also measured the size of spleen during operation. A rea of embolism was subsequently calculated according to projected area and preoperative hemogram indexes. The area of embolism was accounted for about 1/3 to 1/2 of total spleen area. Spleen was perfused with gentam icin (1.6 105 units) and embolized using gelatin sponge particles (diameter: 150-350 μm) to retard bloodstream. Spleen artery radiography was operated to monitor range and degree of embolism. If necessary, re-embolism was conducted in case of insufficient embolism or excessive embolism.
2.3. Fluorescence active cell sorting (FACS) analysis
Draw peripheral blood from patients was conducted 1 day before PSE, 6 months, 1 year, and 2 year after PSE, respectively. CD3+,CD4+and CD8+T cells and CD4+CD25+CDl27low/-Treg cells in patients’ peripheral blood were analyzed by FACS. Three repeats of each peripheral blood sample were labeled with anti-CD4-PE, anti-CD4-PE/anti-CD25 FITC, anti-CDl27, and anti-CD3-PE/anti-CD8-FITC antibodies, respectively. Add 100 μL anticoagulant whole blood and incubate in dark for 25 min. Then, 2 m L lysis buffer was added in each samples and incubated in dark for 15 m in. After red blood cells hemolysis, samples were centrifuged at a speed of 200 g for 5 m in and washed with PBS three times in the same way. Pallets were resuspended in 200 μL 1% paraformaldehyde (PFA) and analyzed by FACS.
2.4. Analysis of immunoglobulins
Immunoglobulins (IgA, IgM and IgG) in patients’ peripheral blood,drawn 1 day before PSE, 6 months, 1 year and 2 years after PSE,were analyzed by auto immunoassay analyzer, respectively.
2.5. Statistical analysis
A ll statistical analyses were performed using SPSS 16.0 software. Data were presented as Mean ± SD. For each group, data from different detection times were tested by repetitive measurement ANOVA. For the same detection times, data from different groups w ere tested by one-way ANOVA. Dunnett-t test was used for analyzing variation between two groups. P<0.05 was considered statistically significant.
3. Results
3.1. FACS analysis
3.1.1. CD3+T cells in peripheral blood
CD3+T cells in the peripheral blood of patients from complication group was less than those in control group form pre-PSE to post-PSE(P<0.05). Numbers between two groups were comparable 2 years after PSE (P>0.05). 1 year and 2 years after PSE, numbers of CD3+T cells in patients from complication group were not significantly different from those before PSE (P>0.05).
CD3+T cells in the peripheral blood of patients from experimental group were less than that in control group from pre-PSE to 6 months after PSE (P<0.05). The numbers between two groups were comparable one years later (P>0.05). 1 month after PSE, the number of CD3+T cells dramatically declined (P<0.05). However, 6 months after PSE, it increased to normal level and maintained to 2 years after PSE.
1 month, 6 months and 1 year after PSE, CD3+T cells in complication group were less than those in experimental group (P<0.05). Then, they increased to the level of experimental group 2 years after PSE (P>0.05) (Table 1).
3.1.2. CD4+T cells in peripheral blood
CD4+T cells in the peripheral blood of patients from both complication group and experimental group were less than those in control group (P<0.05). 1 month, 6 months and 1 year after PSE, CD3+T cells of complication group and experimental group were less than those pre-PSE (P<0.05). The significant difference disappeared until 2 years after PSE. 1 month after PSE, the number of CD4+T cells in the peripheral blood of patients from experimental group was lower than preoperative level (P<0.05). However,it increased to the preoperative level 6 months after PSE, and maintained to 2 years after PSE. 6 months after PSE, CD4+T cells in the peripheral blood of patients from complication group were less than those in experimental group (P<0.05). However, there were no significant differences between two groups at other detection times after PSE (Table 1).
3.1.3. CD8 T cells in peripheral blood
From pre-PSE to 1 year after PSE, CD8+T cells in the peripheral blood of patients from complication group were less than those in control group (P<0.05), and it increased to normal level 2 years after PSE. 1 month and 2 years after PSE, numbers of CD8+T cells in complication group were lower than preoperative level (P<0.05), and showed no significance at other detection times after PSE.
One month and 1 year after PSE, CD8+T cells in experimental group were less than those in control group (P<0.05), but were comparable with those pre-PSE. However, 2 years after PSE, CD8+T cells in experimental group were much more than those pre-PSE (P<0.05).
One month after PSE, the number of CD8+T cells in complication group was lower than that in experimental group (P<0.05), but it was comparable between two groups at other detection times after PSE (Table 1).
3.1.4. CD4+CD25+CDl27low/-Treg cells in peripheral blood
One month and 6 months after PSE, CD4+CD25+CDl27low/-Treg cells in the peripheral blood of patients from complication group were much more than those pre-PSE and in control group (P<0.05). However, significant differences disappeared at follow ing postoperative visit.
One month after PSE, CD4+CD25+CD l27low/-Treg cells in experimental group were much more than those pre-PSE and in control group (P<0.05). However, numbers were comparable from 6 months and 2 years after PSE.
One month and 6 months after PSE, the number of CD4+CD25+CDl27low/-Treg cells in complication group was much higher than postoperative level of control group (P<0.05). From then on,numbers were comparable in two groups at other detection times after PSE (Table 2).

Table 1 CD3+, CD4+ and CD8+ T cells at different detection times in each group(%).
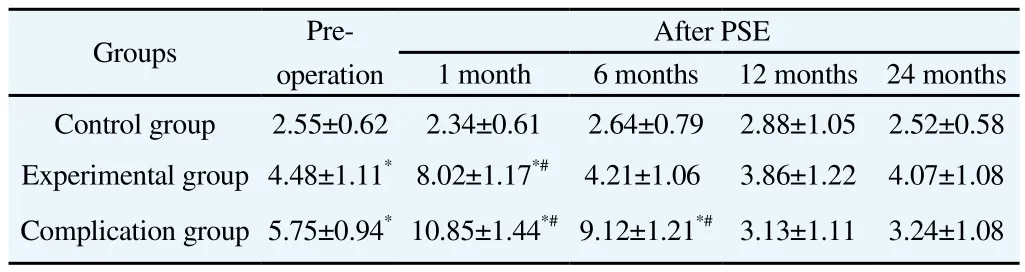
Table 2 CD4+CD25+CDl27low/- Treg cells at different detection times in each group.
3.2. Immunoglobulins analysis
Contents of IgA, IgG and IgM in the peripheral blood of patients from complication group were comparable with those pre-PSE and in control group (P>0.05).
From 1 year to 2 years after PSE, contents of IgA in experimental group were comparable with those pre-PSE and in control group (P>0.05).
Contents of IgA in complication group were also comparable with those in experimental group at all detection times after PSE (P>0.05)(Table 3).

Table 3 Contents of IgA, IgG and IgM at different detection times in each group (g/L).
4. Discussion
Pathological change of chronic viral hepatitis B cirrhosis is a complicated process, having a tremendous impact on the function of various systems of the body. Also, the immune function, particularly the cellular immune function, of patients with cirrhosis is badly affected. Cirrhosis can result in significant elevation of venous pressure, spleen ecchymosis, blood flow stasis, and cell hypoxia,leading to functional and morphological alterations of immunocytes,significant decrease of T and B cells, and decline of immune function [4]. Cirrhosis patients with hypersplenism suffer from numerous severe clinical conditions, such as immunodeficiency and coagulation defects, which result in infection, bleeding, even liver and multiple organ failure [5]. Meanwhile, increase of splenic vein flow volume (SVFV) in turn leads to an elevation of portal venous pressure. Thus, most of cirrhosis patients with hypersplenism suffer esophageal and gastric varices, and even rupture hemorrhage, which endanger their lives.
However, therapies of hepatitis B cirrhosis and hypersplenism are controversial about whether spleen can be conserved [6]. Spleen is a significant organ for body and 25% of the total lymphocytes are processed through it. Spleen with various functions, such as immunity, hemopoiesis, blood storage, and endocrine, is one of important components of immune-nerve-endocrine system. Among these, immune function is considered as one of most important functions of spleen [7-8].
As the development of interventional medicine, partial splenic embolization (PSE) has been w idely applied in clinical treatment of hypersplenism. Compared with traditional therapy, PSE has fewer surgical risks, smaller trauma, shorter hospital stays, and better clinical effects. Moreover, PSE can conserve partial spleen, which is critical to the basic immune function of patients. Thus, it is also referred to as functional splenectomy and is accepted by doctors and patients.
Cluster differentiation (CD) molecules are specific biomarkers on surface of lymphocytes [11-12]. CD+cells play important roles in immune response. Nowadays, studies of immune function of spleen in patients with hypersplenism mainly focus on the number of CD+T cells and immune factors secreted by lymphocytes. The relevance between subsets of T cells (e.g. Treg cells) and immune function of spleen in patients with hypersplenism has been well studied [13]. CD4+CD25+, Treg cells are characteristic of immune anergy and immunosuppression. Immunosuppression is defined as repression of activation and proliferation of CD4+and CD8+T cells when CD4+CD25+Treg cells are activated by some signal pathways [14-15]. It has been reported that activated CD4+CD25+Treg cells highly expressed CD127 (CD4+CD25+CD l27 high), while CD4+CD25+Treg cells with active effect slightly expressed CD127 (CD4+CD25+CDl27low/-). Therefore, CD4+CD25+CDl27low/-can be used as the definitive biomarker of natural Treg cells [16-18]. Researchers have demonstrated that patients with hypersplenism have more CD4+CD25+CDl27low/-Treg cells than normal people [19]. Moreover, the number of CD4+CD25+CDl27low/-Treg cells has a negative relation with that of CD3+and CD4+T cells in patients [19], which may be a result of T cells proliferation repressed by CD4+CD25+CDl27low/-Treg cells.
In this study, CD3+, CD4+and CD8+T cells, and CD4+CD25+Treg cells, as well as expression of IgA, IgG and IgM, in peripheral bloodof patients were analyzed. We discovered that numbers of CD3 and CD8+T cells in patients from complication group was initially lower than that in control group, and afterwards increased to normal level. However, CD4+T cells in complication group were less than those in control group all the time. After PSE, numbers of CD3+, CD4+and CD8+T cells in patients from complication group initially decreased,but afterwards increased to normal level compared with those pre-PSE. In the peripheral blood of patients from experimental group,numbers of CD3+, CD4+and CD8+T cells were initially lower than normal level at early stage of post-PSE, and afterwards increased to normal level. CD3+and CD4+T cells after PSE had a similar trend compared with pre-operation. However, the number of CD8+T cells after PSE was comparable with that pre-PSE. Moreover, in the peripheral blood of patients from complication group, numbers of CD3+, CD4+and CD8+T cells in early stage of post-PSE were lower than those in experimental group, but they were similar in late stage. In patients form both experimental group and complication group, CD4+CD25+CDl27low/-Treg cells initially increased 1 month and 6 months after surgery, and then gradually restored to normal level. However, CD4+CD25+CDl27low/-Treg cells in complication group were initially more than those in experimental group 1 month and 6 months after PSE, but they were afterwards comparable. Furthermore, contents of immunoglobulins (IgA, IgG and IgM) in the peripheral blood of patients from three groups were comparable at all detection times. Our results indicated that PSE had little impact on immune function of cirrhosis patients with hypersplenism, which was consist with previous studies [20].
In conclusion, PSE only has a short-term impact on the immune function of cirrhosis patients with hypersplenism, whose immune function can restore to the normal level in the long term, even patients with complications. We also suggest that in early stage of post-PSE, preventing infection and improving immune function of patients are necessary so as to reduce complication incidence.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Cai M, Huang W, Lin C, Li Z, Qian J, Huang M, et al. Partial splenic embolization for thrombocytopenia in liver cirrhosis: predictive factors for platelet increment and risk factors for major complications. Eur Radiol 2016;26(2):370-380.
[2] Hadduck TA, McW illiams JP. Partial splenic artery embolization in cirrhotic patients . World J Radiol 2014;6(5):160-168.
[3] Sakai T, Shiraki K, Inoue H, Sugimoto K, Ohmori S, Murata K, et a1. Complication of partial splenic em rbolization in cirrhotic patients. Dig Dis Sci 2002;47(2):388-391.
[4] Golsaz-Shirazi F, Shokri F. Hepatitis B immunopathogenesis and immunotherapy. Immunotherapy 2016;8(4): 461-477.
[5] Okazaki T, Hifumi T, Manabe A, Matsumura H, Egawa S, Hamaya HN,et al. Invasive group B streptococcal infection in a patient with post splenectomy for hypersplenismsecondary to liver cirrhosis and portal hypertension. World J Emerg Med 2016;7(1):68-70.
[6] Zhu J, Chen XJ, Hu XT, Zhu H, He H. A comparative study of surgical splenectomy, partial splenic embolization, and high-intensity focused ultrasound for hypersplenism. Ultrasound Med 2016;35(3): 467-474.
[7] Huang CM, Wang Y, Wang JB. Laparoscopic spleen-preserving splenic hilar lymph node dissection for advanced upper gastric cancer in patients with high body mass index. Hepatogastroenterology 2015;62(139): 742-747.
[8] Nayak SB, Shetty P, Deepthinath R, Sirasanagandla SR, Shetty SD. A lobulated spleen with multiple fissures and hila. Clin Diagn Res 2014 ;8(9):1-2.
[9] Pravisani R, Baccarani U, Adani G, Lorenzin D, Vit A, Cherchi V, et al. splenic artery syndrome as a possible cause of late onset refractory ascites after liver transplantation: management with proximal splenic artery embolization. Transplant Proc 2016;48(2):377-379.
[10] Matsuoka S, Ishii T, Miyazawa S, Mizutani T, Ito K, Kamimura S, et al. Utility of partial splenic embolization for hypersplenism using guglielm i detachable coils. Hepatogastroenterology 2015;62(139):683-687.
[11] Okamura T, Sum itomo S, Morita K, Yukiko Iwasaki, Mariko Inoue,Shinichiro Nakachi. TGF-β3-expressing CD 4+CD 25(-)LAG3+regulatory T cells control humoral immune responses. Nat Commun 2015;19(6):6329.
[12] Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W, et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res 2015;3(2):149-160.
[13] Garcia Santana CA, Tung JW, Gulnik S. Human treg cells are characterized by low/negative CD6 expression. Cytometry A 2014;85(10): 901-908.
[14] Yun X, Shang Y, Li M. Effect of Lactobacillus salivarius on Th1/Th2 cytokines and the number of spleen CD4+CD25+Foxp3+Treg in asthma Balb/c mouse. Int J Clin Exp Patho 2015;8(7):7661-7674.
[15] Lu WP, Lin ZH, Liu H, Wang XL. Apoptosis and its mechanisms of spleen CD4+CD25+regulatory T cells in severe aplastic anemia mouse model. Zhonghua Xue Ye Xue Za Zhi 2013;34(11):931-935.
[16] Wang H, Li L, Zhang Y. Expression and significance of CD4(+)CD25(+)CD127(-) regulatory T cells in peripheral blood of patients with different phenotypes of Guillain-Barré syndrome. Int J Clin Exp Med 2015;8(10):19126-19131.
[17] Daniel V, Trojan K, Opelz G. Immunosuppressive drugs affect induction of IFNy+ Treg in vitro. Hum Immunol 2016;77(1):146-152.
[18] Liu J, Wang H, Yu Q, Zheng S, Jiang Y, Liu Y, et a1. Aberrant frequency of IL-10-producing B cells and its association with Treg and MDSC cells in non non small cell lung carcinoma patients. Hum Immunol 2016;77(1):84-89.
[19] Mohammadnia-A frouzi M, Zavaran Hosseini A, Khalili A,Abediankenarib S, Hosseinic V, Malekic I. Decrease of CD4(+) CD25(+)CD127(low) FoxP3(+) regulatory T cells with impaired suppressive function in untreated u lcerative co litis patients. Autoimmunity 2015;48(8):556-561.
[20] Walusimbi MS, Dominguez KM, Sands JM, Markert RJ, McCarthy MC. Circulating cellular and humoral elements of immune function following splenic arterial embolisation or splenectomy in trauma patients. Injury 2012;43(2):180-183.
doi:Document heading 10.1016/j.apjtm.2016.05.005
*Corresponding author:Chao-Qian Li, PhD supervisor, Guangxi Medical University. Tel:13807887867
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Predicted pattern of Zika virus infection distribution with reference to rainfall in Thailand
- Perfusion of gastrodin in abdom inal aorta for alleviating spinal cord ischem ia reperfusion injury
- Study on the effect and mechanism of the dysfunction of CD4+T cells in the disease process of chronic cardiac failure
- Influence on radiosensitivity of lung glandular cancer cells when ERCC1 gene silenced by targeted siRNA
- Experimental study on the inhibition effect of m iR-106a inhibitor on tumor grow th of ovarian cancer xenografts m ice
- Study on the therapeutic mechanisms of pseudolaric acid in m ice with allergic contact dermatitis
