Influence on radiosensitivity of lung glandular cancer cells when ERCC1 gene silenced by targeted siRNA
2016-06-29YingJieRenXinQuanLvCaiXiaGuo
Ying-Jie Ren, Xin-Quan Lv, Cai-Xia Guo
Influence on radiosensitivity of lung glandular cancer cells when ERCC1 gene silenced by targeted siRNA
Ying-Jie Ren1, Xin-Quan Lv2*, Cai-Xia Guo3
1Department of Respiratory Medicine, Zhengzhou Central Hospital, Zhengzhou 450007, China
2Department of Pathology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China
3Department of Respiratory Medicine,Henan Chest Hospital,Zhengzhou450003, China
AR T ICLE IN FO
Article history:
Received 15 April 2016
Received in revised form 16 May 2016 Accepted 15 June 2016
Available online 20 July 2016
Keywords:
ERCC1
Lung glandular cancer
Radiation sensitivity
Transplanted tumor model G2/Mphase
ABSTRACT
Objective: To identify the influence on radiosensitivity of lung glandular cancer cells when excisions repair cross-complementing group1 (ERCC1) gene was silenced by targeted siRNA.
Methods: siRNA which targeting to ERCC1 and control siRNA was designed and synthesized. The human lung glandular cancer SPC-A-1 cells was transfected. A total of 56 nude mice were divided into two groups, and two kinds of SPC-A-1 cells were transplanted to armpit of right forelimb, to establish the nude m ice subcutaneous xenotransplanted tumor model of human lung glandular cancer cells. A fter the tumor was developed, the nude m ice were random ly divided into four groups and accepted different doses of X-Ray radiation, then the change of tumor volume, survival time of mice in every group were recorded and the average lifetime was calculated. Twenty-one days later of X-ray experiment, two mice were taken and sacrificed in each group and the tumors organizations were stripped. The cell apoptosis rate and cell cycle distributions were obtained by FCM (flow cytometry). Results: The volume of tumor which ERCC1 gene was silenced was less than single irradiation group after X-ray irradiation, and the grow th speed was slower and the lifetime of mice was lengthened as well (P<0.05). The cells apoptosis rate and the rate of G2/Mcells which ERCC1 gene was silenced were higher than the same dose control group and the rate of G1cells were lower, which indicated that the cells could be stopped at G2/Mpoint, the cell proliferation was inhibited, the cell apoptosis was promoted and the radiation sensitivity was improved after the ERCC1 was silenced. Conclusions: The radiation sensitivity of lung glandular tumor could be improved after the ERCC1 gene was silenced by siRNA.
E-mail:xinquan9712@163.com
Foundation project: The paper was supported by Foundation and Frontier Issues of Science and Technology Department of Henan Province (NO. 122300410066).
1. Introduction
Lung glandular cancer is a kind of non small cell lung cancer. In recent years, it has become a common subtype. Because of its unique pathological features, its recurrence rate of is higher
than other types of lung cancer. It is difficult to obtain ideal clinic treatment effect[1,2]. Radiotherapy and chemotherapy are two kinds of treatment for this malignant tumor. The main mechanism is to disturb and destroy DNA transcription and replication of cancer cells by radiation or drug. However, it is hard to achieve ideal treatment effect because of drug resistance or antagonism. Among them, the main mechanism is the pathway of nucleotide excision repair (NER)[3].
Excision repair cross-complementing group1 (ERCC1), one of the DNA repair gene, plays an important role in the process ofNER pathway and cell apoptosis[4]. Many studies have showed that high expression of the gene can cause cancer cells produce drug resistance and reduce the effect of radiotherapy. It has been proved that the generation, development and prognosis of tumors are related closely to the expression of this gene, including colorectal cancer, ovarian cancer, non-small cell lung cancer, pancreatic cancer, breast cancer, bile duct cancer etc[5-10]. Furthermore,some studies have indicated that the expression of ERCC1 is related to the radiosensitivity of glioma and nasopharyngeal carcinoma[11,12]. In the study of lung glandular cancer, ERCC1 is regarded as poor prognosis of lung glandular cancer patients[13],and its expression plays a certain negative effect on clinical drug resistance of cisplatin chemotherapeutics for curing lung glandular cancer[14,15]. But so far, few research study the relationship between the gene and radiosensitivity of lung glandular cancer. Therefore,the author intends to study the effect of ERCC1 expression on the radiosensitivity of lung glandular cancer cells through the internal exposure transplant tumor model animal experiment, and to explore whether it can be gene targets to enhance the radiosensitivity of lung glandular cancer, so as to provide experimental and theoretical basis for the clinical treatment of lung glandular cancer.
2. Materials and methods
2.1. Materials
ERCC1 gene sequence is from GenBank, gene coding NM-001983. siRNA which targeting to ERCC1 was designed according to the reference[16], and synthesized by QIAGEN. The sequence of designed siRNA-ERCC1 is 5’-CAGGCGGCCCCTCAGACCTAC-3’;at the same time, a non specific siRNA target sequence 5’-GACTTCATAAGGCGCATGC-3’ was synthesized as negative control group. A fter Blast, the sequence has no homology with mRNA of human gene.
2.2. Cell culture and transfection
Human lung glandular cancer SPC-A-1 cells used in the study were from Shanghai Cell Bank of Chinese Academy of Sciences. A fter recover the cryopreserved SPC-A-1 cell line, it was conventionally cultured in RPM I solution containing 10% fetal bovine serum, 100 kU/L penicillin and 100 mg/L streptomycin (pH was adjusted to 7.2% with concentrated hydrochloric acid) at 37 ℃, 5% CO2and 90% relative humidity condition in incubator. The cultured cells were divided into two groups (marked as the first group and the second group), and transfected ERCC1-siRNA and negative control group respectively. When transfected, SPC-A-1 cells in logarithm ic phase were taken and routinely inoculated in 6-well plates, 2 m L per hole, density (1.5-2.5)×105/m L, then incubated for about 10 min in a constant-temperature incubator; 21×10-12mol/L siRNA, serumfree medium and HiperFect Transfection Reagent were blended according to 4: 100: 6 in EP tube w ithout RNA enzyme and then incubated for about (5-l0) min at room temperature to form complex. A fter that, every hole was added 110 μL m ixture, shook it to m ix well and cultured for 24 h at 37 ℃, 5% CO2condition in incubator.
2.3. Nude mice xenotransplanted tumor model
The nude m ice were purchased from the animal experimental center of Sun Yat-Sen University in China. The study was performed after the mice were allowed to acclimate for 1 week in the animal experimental center of Zhengzhou University. A total of 56 m ice were (4-6) weeks old, weighting (15-20) g with half male and half female. Mice were random ly divided into two groups, 28 in each group, inoculated with cultured cells of the first group and the second group respectively. Armpit of right forelimb of m ice which had been disinfected by alcohol were injected with 2×10-6/m L cell suspension drew in 1 m L sterile syringes, each only 0.2 m L. Then observe the tumor grow th in m ice. Average 7 d later, tumor can be found in the mice subcutaneous tissues.
2.4. Radiation experiment
Fourteen days after completing the xenotransplanted tumor model experiment, radiation experiment started. The m ice of the first group and the second group were divided into four groups (A1, B1, C1,D1 and A2, B2, C2, D2) and radiated according to different total radiation dose. The total radiation dose of A1, A2 group was 3 GY,the total radiation dose of B1, B2 group was 9 GY, the total radiation dose of C1, C2 was 15 GY, and the total radiation dose of D1, D2 group was 0 for receiving sham radiation. Radiation was operated three times, once a day for three days. The radiation source was 6MV linear accelerator with 200 cGy/m in dose rate and 10 cm×15 cm radiation area. Before radiation, nude m ice were anesthetized by 2% pentobarbital sodium injected in abdomens.
2.5. Tumor growth record
Before the radiation experiment, the tumor volume of m ice was measured every 3 d. Specifically, tumor volume was measured with caliper including long diameter (a) and short diameter (b), then calculated according to the formula V=0.5×ab-2. The tumor grow th curve was drawn from the volume change of m ice in each group,and the tumor grow th inhibition rate was calculated according to the formula:
Tumor grow th inhibition rate = (tumor volume of the control grouptumor volume of the experimental group)/tumor volume of the control group 100%.
In this experiment, D2 group was the control group. Radiation sensitivity was calculated in accordance with the formula:
Radiation sensitivity = (the radiation effect of silenced ERCC1)/ (Single radiation effect) 100%.
Among them, the effect referred to suppression (decrease) degree of tumor volume compared with the control group (D2), such as the radiation sensitivity under 3 GY radiation = (VA2-VA1)/(VA2-VD2). Survival time of mice in every group was recorded and the average lifetime was calculated.
2.6. FCM experiment
Two m ice in each group were sacrificed 21 d after radiation experiment was performed by anesthetizing and dislocating the cervical vertebra; and the tumors organizations were stripped. The cell apoptosis rate and cell cycle distributions were obtained by FCM (model: EPICS-ELITE-ESP). The sacrificed mice were not included in the cumulative survival curve. A ll data is statistical analyzed by SPSS19.0. P<0.05 is considered to have significant difference.
2.7. Experimental ethics statement
All experiments in the study were conducted according to the guidelines on animal healthcare and use of animal experimental center of Zhengzhou University in Henan Province and approved by local ethics committee. Besides, experiments were conformed to the guidelines on healthcare and use of experimental animals made by National Institutes of Health (Publication No. 85-23, revised in 1985).
3. Results
3.1. Tumor growth record
The volume change and the inhibition rate of tumor grow th in each group were seen in Table 1, and the radiosensitivity of ERCC1 gene which was silenced was shown in Table 2. From Table 1, we could see that after expression and irradiation of ERCC1 gene which was silenced by targeted siRNA, the grow th of tumor tissue has been greatly inhibited (It showed significant difference between tumors volume in each group and D2 the control group, P<0.01),and the irradiation dose higher, inhibition effect was more obvious (PA1B1<0.05, PA1C1<0.01, PB1C1<0.05). It could also be seen that after 35d, the inhibition rate of tumor grow th in each group was more signficant than that of 23d, which means the inhibitory effect was accumulated over time. A fter the ERCC1 gene was silenced, the sensitivity of irradiation group was higher than single irradiation group, and the radiosensitivity effect was most obvious when the irradiation dose was 9 GY.
The average lifetime and cumulative survival time of mice in each group were shown in Table 3. From the cumulative survival time schedule, we could see after radiotherapy, the lifetime of m ice in each group were extended in some degree compared to the control group(P<0.05).

Table 1 Change of tumor volume of mice in each group (mm-3; d).
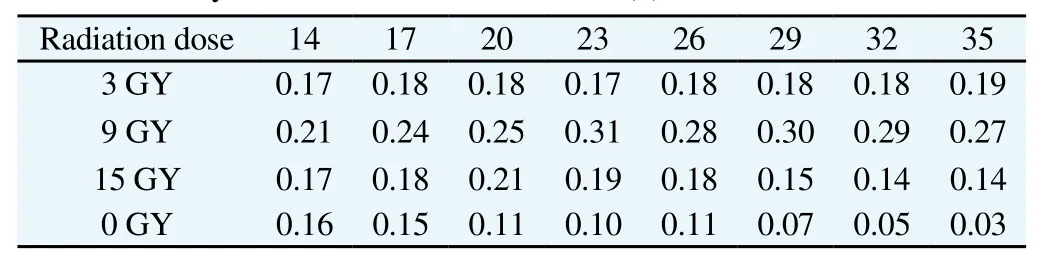
Table 2 Radiosensitivity with different radiation doses (d).
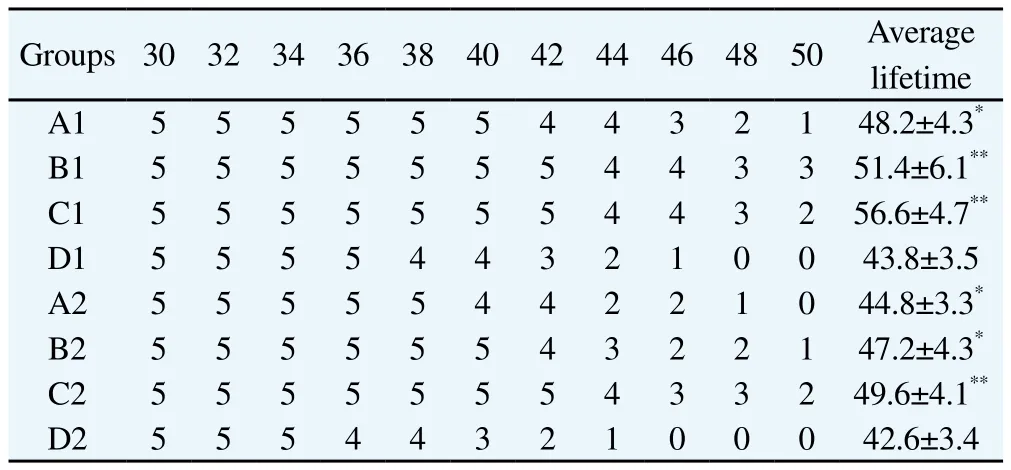
Table 3 Change of survival mice numbers in each group (n; d).
3.2. FCM experiment
The results of cell apoptosis rate obtained by FCM were as follows: A1: (23.21% +4.32%); B1: (30.47% + 4.12%); C1: (36.60% + 5.21%); D1: (10.81% + 3.55%); A2: (13.86% + 2.33%); B2: (20.21% + 4.23%); C2: (26.82% + 4.11%); D2: (5.24% + 1.41%). The results of cell cycle distribution were shown in Table 4. We could see that after ERCC1 gene was silenced by targeted siRNA (A2, A3 group), the apoptosis rate of them was higher than the control group, and the proportion of cells decreased at G2/Mpoint.
4. Discussion
The basic principle of radiotherapy is destructing nucleotide replication of tumor cells by ionizing radiation, and ERCC1 is one of the most important genes involved in nucleotide damage repair. Many studies show that ERCC1 can reduce the chemotherapy and radiotherapy effect of many tumors because of its impact on the nucleotide repair pathway[17]. In this study, the author inhibited the expression of ERCC1 gene in lung glandular cancer cells by small interfering RNA technology, and then irradiated xenotransplanted tumor model of lung glandular cancer cells to study the relationship between ERCC1 gene and the radiosensitivity of lung glandular cancer. From the experimental results, we could see that after transfection siRNA-ERCC1 cells and independent sequence control group cells were transplanted into subcutaneous tissue of nude m ice and radiated under different doses, the grow th status had a significant statistical difference. The inhibition rate of tumor volume and grow th of transfection siRNA-ERCC1was significantly lower than the control group, which meant siRNA-ERCC1 could promote the apoptosis of lung glandular cancer cells under ray radiation,inhibits tumor grow th and have certain radiosensetivity effect. When irradiation dose was 15 GY, radiosensetivity of it was lower than that of 9 GY radiation dose which proved that higher radiation dose didn’t mean higher radiosensetivity. It provided new ideas and methods for the molecular targeted radiation of lung glandular cancer.
The reason why silenced ERCC1 can improve the radiosensitivity of lung glandular cancer cells is worth exploring. Studies have shown that ERCC1 can rapidly repair damaged DNA at G2/Mpoint and enter a state of normal proliferation[18]. Therefore, it can be seen that after silenced ERCC1 expressed, cells were stopped at G2/ Mpoint, could not be normally proliferated and induced apoptosis. The researchers believed that ERCC1 played a dual role in tumor tissue. In some tumor tissues, when the cancer cells appeared, cell proliferation became abnormal. At this time, ERCC1 played repair function of nucleotide to resist malignant proliferation of tumor[19];On the other hand, in the course of treatment of tumors, platinum based chemotherapy drugs or radiation destroyed the replication of nucleotide in tumor cells, and repair function played by ERCC1 also had an antagonistic effect[20,21]. That was because whether chemotherapy or radiotherapy, cancer cells were killed in nonnormal means which caused harm to normal tissues and cells while inhibiting tumor grow th. Physiological reactions not only determined organisms taking certain measures to repair these damages, but also produced drug resistance or radiation antagonism. When silenced ERCC1 was expressed and radiation dose was 9 GY (not 15 GY),m ice cumulative survival curve area was the largest, which might because high radiation dose increased the radiosensitivity, but damage the other organs of mice, thus the cumulative survival time of m ice was reduced. Therefore, the detailed mechanism of ERCC1 in different tumor tissues has not been clearly defined[22], so it still needs further experiment and research.
This study inhibited the expression of ERCC1 gene in lung glandular cancer cells through small interfering RNA technology,establish m ice xenotransplanted tumor model of lung glandular cancer cells, and evaluate the grow th condition of xenotransplanted tumor model. A fter irradiation, the proliferation activity and apoptosis rate were measured and found that com pared with the control group, siRNA-Bcl-2 cells could effectively improve the apoptosis rate of gastric cancer cells, which proved that the expression of silenced Bcl-2 gene could enhance the radiosensitivity of gastric cancer cells.

Table 4 The proportion of cell cycle distribution in each group (%).
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Burns DM. Changing rates of adenocarcinoma of the lung. Chem Res Toxicol 2014; 27(8): 1330-1335.
[2] D’Amico AG, Maugeri G, Magro G, Salvatorelli L, Drago F, D’Agata V. Expression pattern of parkin isoforms in lung adenocarcinomas. Tumour Biol 2015; 36(7): 5133-5141.
[3] Spivak G. Nucleotide excision repair in humans. DNA Repair (Amst) 2015;36: 13-18.
[4] Takayama K, Kawakam i Y, Lee S, Greco N, Lavasani M, Mifune Y, et al. Involvement of ERCC1 in the pathogenesis of osteoarthritis through the modulation of apoptosis and cellular senescence. J Orthop Res 2014;32(10): 1326-1332.
[5] Dai Q, Luo H, Li XP, Huang J, Zhou TJ, Yang ZH. XRCC1 and ERCC1 polymorphisms are related to susceptibility and survival of colorectal cancer in the Chinese population. Mutagenesis 2015; 30(3): 441-449.
[6] Uemura S, Kuramochi H, Higuchi R, Nakajima G, Yamamoto M. ERCC1 mRNA expression as a postoperative prognostic marker in extrahepatic bile duct cancer. Ann Surg Oncol 2014; 21(Suppl 4): S627-633.
[7] Muallem MZ, Braicu I, Nassir M, Richter R, Sehouli J, Arsenic R. ERCC1 expression as a predictor of resistance to platinum-based chemotherapy in primary ovarian cancer. Anticancer Res 2014; 34(1): 393-399.
[8] Fuereder T, Stift J, Kuehrer I, Stranzl N, Hoeflmayer D, Kornek G, et al. Response to GEMOX plus erlotinib in pancreatic cancer is associated with ERCC1 over expression. Eur J Clin Invest 2014; 44(10): 958-964.
[9] Gerhard R1, Carvalho A, Carneiro V, Bento RS, Uemura G, Gomes M, et al. Clinicopathological significance of ERCC1 expression in breast cancer. Pathol Res Pract 2013; 209(6): 331-336.
[10] Li C1, Liu M, Yan A, Liu W, Hou J, Cai L, et al. ERCC1 and the efficacy of cisplatin in patients with resected non-small cell lung cancer. Tumour Biol 2014; 35(12): 12707-12712.
[11] Liu ZG, Chen HY, Cheng JJ, Chen ZP, L XN, Xia YF. Relationship between methylation status of ERCC1 promoter and radiosensitivity in glioma cell lines. Cell Biol Int 2009; 33(10): 1111-1117.
[12] Zhou X, Gao Y, Chen X. Research status of sensitive molecular markers related to radiation therapy for esophageal cancer. Med Rev 2013; 19(22): 4093-4096. doi:10.3969/j.issn.1006-2084.2013.22.021.
[13] Jiang H, Wang H, Wang S, Pei Z , Fu Z , Fang C, et al. Expression of ERCC1, TYMS, RRM 1, TUBB3, non-muscle myosin II, myoglobin and MyoD1 in lung adenocarcinoma pleural effusions predicts survival in patients receiving platinum-based chemotherapy. Mol Med Rep 2015;11(5): 3523-3532.
[14] Podmaniczky E, Fábián K, Pápay J, Puskás R, Gyulai M, Furák J, et al. Decreased ERCC1 expression after platinum-based neoadjuvant chemotherapy in non-small cell lung cancer. Pathol&Oncol Res 2015;21(2): 423-431.
[15] Lee SH, Noh KB, Lee JS. Thym idylate synthase and ERCC1 as predictive markers in patients with pulmonary adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer 2013; 81(1): P102-108.
[16] Chang IY, Kim MH, Kim HB, Lee DY, Kim SH, Kim HY, et al. Small interfering RNA-induced suppression of ERCC1 enhances sensitivity of human cancer cells to cisplatin. Biochem Biophys Res Commun 2005; 327(1): 225-233.
[17] Gentile F, Tuszynski JA, Barakat KH. New design of nucleotide excision repair (NER) inhibitors for combination cancer therapy. J Mol Graph Model 2016; 65: 71-82.
[18] Orelli B, McClendon TB, Tsodikov OV, Ellenberger T, Niedernhofer LJ, Schärer OD. The XPA-binding domain of ERCC1 is required for nucleotide excision repair but not other DNA repair pathways. Biol Chem 2010; 285(6): 3705-3712.
[19] Zhou W, Liu G, Park S, Wang Z, Wain JC, Lynch TJ, et al. Gene-smoking interaction associations for the ERCC1 polymorphisms in the risk of lung cancer. Cancer Epidermal Biomarkers Prey 2005; 1(2): 491-496.
[20] Torii Y, Kato R, Minami Y, Hasegawa K, Fujii T, Udagawa Y. ERCC1 expression and chemosensitivity in uterine cervical Adenocarcinoma cells. Anticancer Res 2014; 34(1): 107-115.
[21] Doll CM. High ERCC1 expression is associated with worse survival in patients with locally advanced cervical cancer treated with radiotherapy (RT): an evaluation of AQUA® versus conventional ihc methods. Int J Radiat Oncol Biol Physic 2008; 72(Suppl1): S18-S19.
[22] Durrington HJ. Is ERCC1 a reliable prognostic protein biomarker in nonsmall-cell lung cancer? Thorax 2014; 69(4): P345.
doi:Document heading 10.1016/j.apjtm.2016.05.002
*Corresponding author:Xin-Quan Lv, Department of Pathology, the First A ffiliated Hospital of Zhengzhou University, NO.1 Eastern Jianshe Road, Zhengzhou, 450052,China.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effects of scallop shell extract on scopolam ine-induced memory impairment and MK801-induced locomotor activity
- Predicted pattern of Zika virus infection distribution with reference to rainfall in Thailand
- H igh seroprevalence of asymptomatic viral haemoparasites among prospective blood donors in N igeria
- Evaluation of hypoxia inducible factor targeting pharmacological drugs as antileishmanial agents
- Molecular epidem iology and phylogeny of N ipah virus infection: A m ini review
- Green coffee bean extract improves obesity by decreasing body fat in high-fat diet-induced obese m ice
