The protective effect and underlying mechanism of Hainan papaya water extract against neuronal apoptosis induced by Aβ40
2016-06-29JiuHongZhaoHaiYingZhangXianFangZhangXuDongBingLiuYueLiLiuYiDiHuangQuanPengZhangGangLuoZhiJianMaXiNanYi
Jiu-Hong Zhao, Hai-Ying Zhang, Xian-Fang Zhang, Xu Dong, Q i-Bing Liu, Yue-Li Liu, Yi-Di Huang, Quan-Peng Zhang, Gang Luo, Zhi-Jian Ma,, Xi-Nan Yi,*
1Department of Anatomy, Hainan Medical College, Haikou 571199,China2Neuroscience Research Institute, Hainan Medical College, Haikou 571199, China3School of Pharmacy, Hainan Medical College, Haikou 571101, China
The protective effect and underlying mechanism of Hainan papaya water extract against neuronal apoptosis induced by Aβ40
Jiu-Hong Zhao1#, Hai-Ying Zhang1#, Xian-Fang Zhang1, Xu Dong2, Q i-Bing Liu3, Yue-Li Liu3, Yi-Di Huang1, Quan-Peng Zhang1, Gang Luo1, Zhi-Jian Ma1,2, Xi-Nan Yi1,2*
1Department of Anatomy, Hainan Medical College, Haikou 571199,China
2Neuroscience Research Institute, Hainan Medical College, Haikou 571199, China
3School of Pharmacy, Hainan Medical College, Haikou 571101, China
AR T ICLE IN FO
Article history:
Received 15 April 2016
Received in revised form 16 May 2016 Accepted 15 June 2016
Available online 20 July 2016
Keywords:
Aβpeptide
Neuron protection
ERK signaling pathway
ABSTRACT
Objective: To investigate whether Hainan papayas has protective effects in an Aβ40-induced primary neuron injury model and elucidate the underlying molecular mechanism. Methods: Cultured primary neurons from the dorsal root ganglia (DRG) of Sprague-Daw ley (SD) rats were treated with 20 μMAβ40 peptide, 100 μg/L Hainan papaya water extract,peptide plus extract, or culture medium for 24 h. Cell viability was measured by MTT assay,and neuronal apoptosis was evaluated by DAPI staining. ERK signaling pathway-associated molecule activation and changes in Bax expression were analyzed by Western blotting and immunofluorescence. Results: A cell viability rate of (44.11±6.59)% in the Aβ40 group was rescued to (79.13±6.64)% by adding different concentrations of the extract. DAPI showed pyknotic nuclei in 39.5% of Aβ40-treated cells; the fraction dropped to 17.4% in the 100 μg/L extract group. ERK phosphorylation was observed in the Aβ40 group but was ameliorated by pretreatment with 100 μg/L extract. Hainan papaya water extract also prevented Aβ40-induced phosphorylation of MEK, RSK1 and CREB associated with ERK signaling and downregulated Bax expression in the neurons. Conclusion: The resultss suggest that Hainan papaya water extract has protective effects on neurons; the mechanism may be related to suppression of ERK signaling activation.
# These authors contributed equally to this work.
Tel: 13398976729
Fax: 86-898-68178410
E-mail: 1272791897@qq.com
Foundation project: It is supported by grants from the Science & Technology Department of Hainan Province, China (KJHZ2013-19), by grants from the Key Science and Technology Program of Hainan Province (ZDXM 20120050), and by the National Natural Science Foundation of China (81100246).
1. Introduction
Hainan papaya water extract has strong antioxidative effects and can delay the aging process by clearing the abundant reactive oxygen species that are present in the liver and blood of mice[1-3]. In a previous large-scale mouse behavioral study, we found that Hainan papaya water extract could improve the learning and memory capacity of Mo/Hu APPswe PS1dE9 double-transgenic A lzheimer’s disease (AD) model mice and decrease the levels of intracerebral A β (manuscript in preparation). However, whether Hainan papaya water extract shows neuronal protective effects remains unknown. AD is associated with pathogenic changes that are caused by many factors, and there are multiple pathogenic mechanisms [4-6]. Oxidative damage and cellular apoptosis are extremely important in the pathogenesis of AD [7,8]. The ERK1/2 signaling pathway,a classical m itogen-activated protein kinase (MAPK) signaling pathway, plays a key role in regulating Aβ-induced apoptosis and toxic synapse injury in PC12 cells [9,10]. In the present study, we injured primary cultured neurons using Aβ40 and then evaluatedwhether Hainan papaya water extract exhibited neuroprotective effects. We also investigated whether this effect correlated with phosphorylation of molecules associated with the ERK1/2 signaling pathway. This study w ill facilitate future studies of the anti-AD effects of Hainan papaya constituents and provides evidence for its pharmaceutical value.
2. Materials and methods
2.1. Experimental animals
Sprague-Daw ley (SD) rats, aged 1-2 weeks, were purchased from the Hunan Agricultural University (Changsha, Hunan, P.R. China)and housed in standard rat cages with 12 h light/- dark cycles (the lights sw itched on at 6:00 am) and with water and rat chow available ad libitum. Animals were sacrificed and tissues were collected with the approval of the animal ethics comm ittee of Hainan Medical College. A ll studies were performed in strict accordance with the guidelines of the Chinese animal protection and management law.
2.2. Hainan papaya water extract
Hainan papayas were purchased from Beijing Tong Ren Tang Chinese Medicine Co. Ltd in May 2014, which were identified by prof. Niankai Zeng. A voucher specimen (No.HP201405) was deposited in the herbarium of School of Pharmaceutical Science,Hainan Medical University. Approximately 5 kg of fresh Hainan papaya fruits were cut into slices, dried for 12 h at 50 ℃ in an airdrying oven, mashed, covered with 5 L of purified water and then digested in a water bath at 60 ℃ for 12 h. The water extract was filtered through absorbent gauze, and the filtrate was concentrated under reduced pressure to remove as much water as possible using a Buchi rotary evaporator (Flaw il, Sw itzerland). The remaining water was then removed by freeze drying. A dark brown block of Hainan papaya water extract (8.7 g) was obtained and stored at -20 ℃ until use.
2.3. Neuronal cultures
The DRG were isolated from 3-week-old SD rats that had been sacrificed by dissociation of the cervical spinal cord under sterile conditions and were then cultured as previously reported [11]. The culture medium was changed every other day. Aβ40 (1 mg) was dissolved in 2 m L of saline, mixed well, aliquoted and stored at 4 C until use. Freeze-dried Hainan papaya water extract pow der was dissolved in fresh culture medium for each experiment. The cultured neurons were divided into 4 groups, which were subsequently treated with 20 μMAβ40 peptide (Sigma, St. Louis, MO, USA), 100 μg/ L Hainan papaya water extract, 20 μMAβ40 peptide plus 100 μg/L Hainan papaya water extract or culture medium (CON).
2.4. Cell viability and apoptosis measurements
Neuron cell viability was measured using the MTT (3- (4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium brom ide) assay according to methods previously reported [12]. Briefly, 20 μL of MTT solution was added to cultured neurons in a 96-well plate before incubation for 4 h at 37 ℃. Next, the culture medium was carefully removed from the wells, and 100 μL of formazan solution (Boster)was added to the cells to help dissolve the precipitated formazan crystal products. A fter incubating for 15 m in with gentle shaking to dissolve the precipitated crystals, the plates were immediately analyzed by measuring the absorbance at 570 nm using an ELISA m icroplate reader.
Neuronal apoptosis in each treatment group was analyzed by DAPI (4′,6-diam idino-2-phenylindole) staining. A fter perform ing the desired treatment for 24 h, neurons grown on culture slides w ere fixed, stained with DAPI (Boster) in the dark for 1 m in and then washed with phosphate-buffered saline (PBS) 3 times. The samples were observed under 365 nm excitation using an immunofluorescence m icroscope. The number of apoptotic cells was calculated based on DNA staining with the DAPI fluorescence probe. Apoptotic neurons were characterized by the presence of fragmented or condensed nuclei. Cells in 10 different fields of view were counted per treatment in triplicate under 200× magnification on the fluorescence microscope. Approximately 100 neurons were counted in each field of view. The results are expressed as the percentage of apoptotic cells among the total number of cells counted.
2.5. Immunofluorescence assay
Immunofluorescence staining w as performed as reported previously[8]. Neurons grown on culture slides were fixed, blocked with 5% donkey serum at room temperature for 1 h, incubated with primary antibodies at 4 ℃ overnight, and finally incubated with secondary antibodies at room temperature for 1 h. The primary antibodies used in this study were chicken anti-rat neuron-specific enolase (NSE) (1:100, Abcam) and mouse anti-rat p-ERK-1/2 (1:100, Sigma). The secondary antibodies were A lexa Fluor 488-conjugated donkey anti-chicken IgG (1:200, Jackson) and A lexa Fluor 594-conjugated donkey anti-mouse IgG (1:200, Jackson). Slides stained with 2% donkey serum instead of primary antibody were used as a negative control. Positive staining was visualized using a confocal fluorescence m icroscope (Nikon 901 Japan).
2.6. Western blotting
Western blotting was performed as reported previously [8]. A fterdigestion with trypsin, neurons from each treatment group were mixed with protein lysis buffer, incubated for 15 m in, placed on ice for 25 s, and then vortexed for 40 m in. After centrifugation, the supernatant was harvested as the protein lysate, and its concentration was determ ined using the Bradford method. The protein lysates (20 μg/well) were separated using 8% SDS-polyacrylam ide gel electrophoresis and then transferred electrophoretically onto nitrocellulose membranes. The membranes were blocked with 5% fat-free m ilk for 1 h, incubated with primary antibodies at 4 ℃ for 12 h, and then incubated with secondary antibodies at room temperature for 2 h. The color was developed using 3,3’-diam inobenzidine (DAB) solution. The primary antibodies used in this study were mouse antibodies against rat p-ERK, p-MEK, p-RSK1, p-CREB, and Bax (all were used at a 1:1 000 dilution; Abcam). After scanning the results using an HP Scanjet G4050, the levels of total ERK, MEK,RSK1, and CREB were quantified with the Quantity One Software using -actin as an internal reference.
2.7. Statistical analysis
All results are presented as the means ± S.E.M. Statistical analysis was performed using SPSS 15.0 software. The differences between groups were evaluated using a one-way ANOVA, and P<0.05 was considered a significant difference.
3. Results
3.1. Hainan papaya water extract prevents Aβ40-induced declines in neuronal viability
A prelim inary study revealed that Aβ40 at concentrations ranging from 10 to 80 μMled to cell death in a dose-dependent manner, with 20 μMAβ40 inducing a mild extent of cell injury. Therefore, this concentration was used for all further experiments (data not shown). To characterize the protective effects of Hainan papaya water extract on cell viability after Aβ-induced neuronal cell injury, cells were pre-incubated with various concentrations (200, 100, 50, 25 μg/L) of Hainan papaya water extract for 10 m in before being treated with 20 μMAβ40. Cells’ viability in different groups was evaluated using the MTT assay. The result showed that neurons exposed to 20 μMAβ40 for 24 h had a cell viability of (44.11±6.59)%, which was significantly decreased compared to CON. In contrast, pretreatment of the cells with Hainan papaya water extract at 200, 100, 50,or 25 μg/L rescued cell survival remarkably, to (76.20±5.38)%,(79.13±6.64)%, (65.50±3.67)%, and (58.44±3.58)%, respectively. Furthermore, the cells did not show obvious signs of toxicity after treatment with 100 μg/L of Hainan papaya extract [cell viability: (95.36±5.39)%]. These results suggest that Hainan papaya water extract has a protective effect against Aβ40-induced neuronal cell injury.
3.2. Hainan papaya water extract inhibits Aβ40-induced neuronal apoptosis
To assess the effect of Hainan papaya water extract on the grow th of cultured neurons, apoptosis was analyzed by observing the morphology of the bodies and nuclei of neurons after staining with NSE and DAPI. As shown in Figure 1, compared with CON group,the Aβ40 group contained fewer neurons, and the neurons exhibited shrunken bodies that stained densely and unevenly (Figure 1C). DAPI staining revealed pyknotic nuclei (Figure 1G). The percentage of cells with pyknotic nuclei was significantly higher in the Aβ 40 group (39.5%) than in CON group (3.5%) or the Aβ40 plus Hainan papaya water extract group (17.4%) (P<0.05). There was no significant difference in the percentage of cells with pyknotic nuclei between the Hainan papaya water extract group (5.7%) and CON group (3.5%).
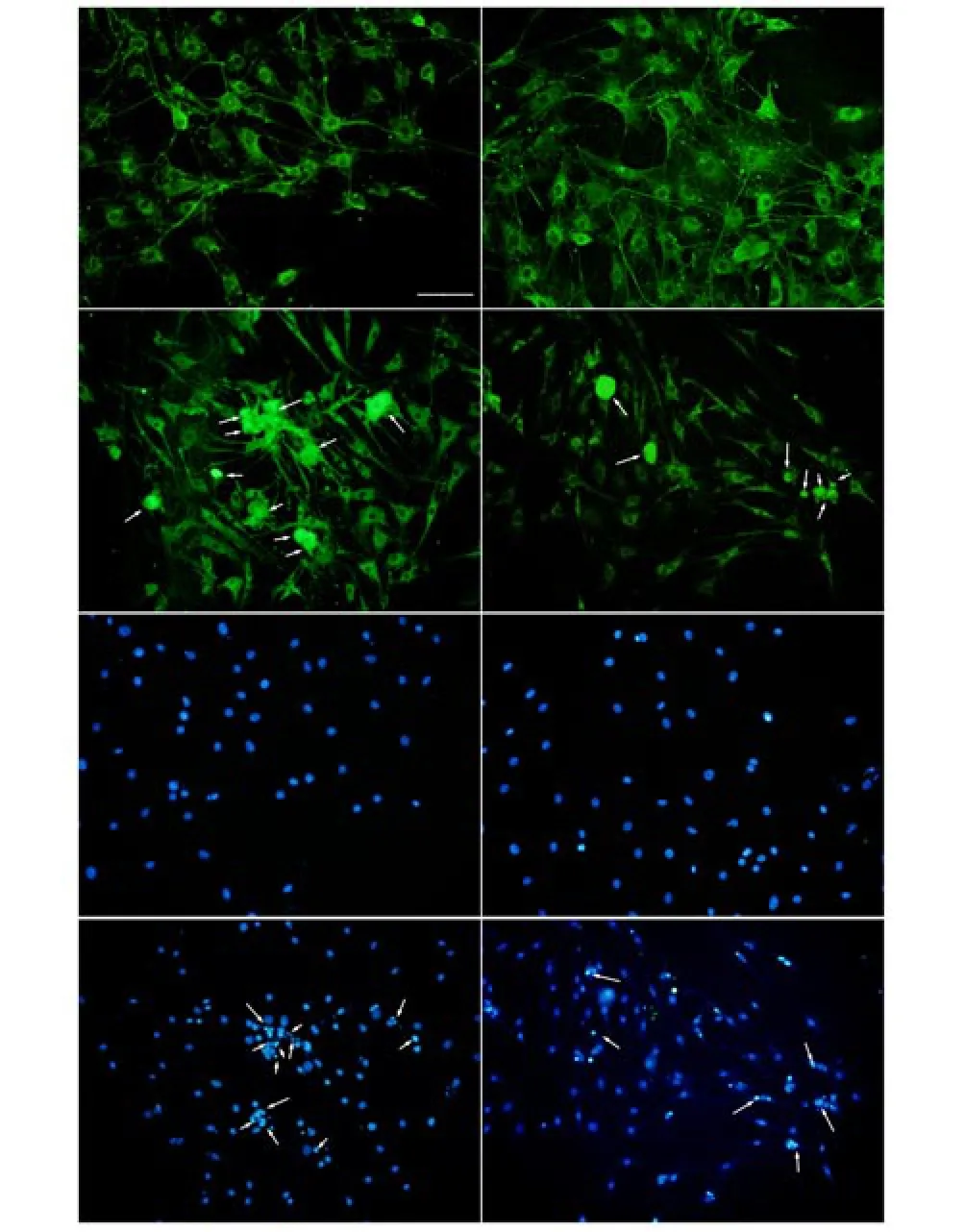
Figure 1. Papaya water extract prevents Aβ40-induced neuronal apoptosis. Cultured DRG neurons were treated with 20 μMAβ40 (C and G), 100 μg/L Hainan papaya water extract (B and F), a combination of both (D and H) or no treatment (A and E) for 24 h. The cells were immunostained with anti-NSE (A-D) or DAPI (E-H). Positive staining was visualized using a confocal fluorescence m icroscope. The pyknotic nuclei were counted with Axiovision software version 4.6.3. A rrow s indicate a small number of neurons with pyknotic nuclei (scale bar=100 μm).
3.3. Hainan papaya water extract prevents the Aβ40-induced activation of ERK1/2
The ERK1/2 signaling pathway plays a pivotal role in regulating cellular apoptosis. Phosphory lation of ERK 1/2 leads to its translocation into the nucleus [13]. To assess the effect of Hainan papaya water extract on ERK1/2 phosphorylation, we stained p-ERK1/2 in the neurons from each group. The data revealed that p-ERK1/2 localized mainly in the cytoplasm, but not the nuclei,of normal neurons (Figure 2D). After treatment with Aβ40 for 24 h, the p-ERK-1/2 levels increased, and positive staining was observed in the nucleus (Figure 2A). In neurons pretreated with Hainan papaya water extract for 10 min before being treated with A β40 for 24 h, less p-ERK-1/2 staining was observed in the nucleus (Figure 2C). This result suggests that Hainan papaya water extract prevents the Aβ40-induced nuclear translocation of ERK1/2. Next,Western blotting was used to assess the levels of phosphorylated and total ERK1/2, with total ERK1/2 being used as a loading control. The data revealed that the Aβ40 group had the highest level of phosphorylated ERK-1/2; it was nearly 2.53 times that of CON group. Compared with Aβ40 group, the level of phosphorylated ERK-1/2 in the Aβ40 plus Hainan papaya water extract group was significantly decreased (P<0.05) (Table 1).
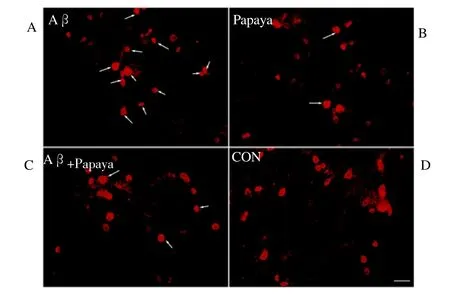
Figure 2. Effect of papaya water extract on Aβ40-induced ERK-1/2 activation.
Cultured DRG neurons were treated with 20 μMAβ40 (A), 100 μg/L Hainan papaya water extract (B), a combination of both (C) or no treatment (D) for 24 h and were then stained with an anti-p-ERK1/2 antibody. Arrows indicate ERK nuclear translocation (scale bar=50 μm).
3.4. Hainan papaya water extract inhibits Aβ40-induced MEK activation
MEK, or ERK-1/2 kinase, can activate ERK by phosphorylating MEK. A previous study reported that Aβ40 can activate MEK [14]. To evaluate whether Hainan papaya water extract can inhibit Aβ 40-induced MEK activation, Western blotting was used to detect MEK phosphorylation levels in cultured neurons from each group;the total MEK levels were used as a loading control. As shown in Table 1, MEK phosphorylation was highest in the Aβ40 group; it was nearly 1.95 times that of CON group (P<0.05). Compared with Aβ40 group, the MEK phosphorylation level in Aβ40 plus Hainan papaya water extract group was significantly decreased (P<0.05). This result suggests that Hainan papaya water extract can inhibit A β40-induced MEK activation.
3.5. Hainan papaya water extract prevents Aβ40-induced RSK1 activation
RSK1 (90-kDa ribosomal protein S6 kinase), also known as MAPK kinase 1, is a downstream molecule in the ERK-1/2 signaling pathway that regulates the expression of apoptosis-related proteins. ERK phosphorylates and activates RSK1, which upregulates the expression of such apoptosis-associated molecules as Bax. Western blotting was used to evaluate the levels of phosphorylated RSK 1 in each group, which were normalized to the total RSK1 levels. As shown in Table 1, RSK1 phosphorylation was highest in the Aβ40 group; it was nearly twice that of CON group (P<0.05). Compared with Aβ40 group, RSK1 phosphorylation in the Aβ40 plus Hainan papaya water extract group was significantly decreased (P<0.05).
3.6. Hainan papaya water extract prevents Aβ40-induced CREB activation
CREB (cAMP-responsive element-binding protein) can be activated after being phosphorylated by RSK1. Western blotting was used to evaluate the CREB phosphorylation levels in each group,which were normalized to the total CREB levels. As shown in Table 1, CREB phosphorylation was highest in the Aβ40 group; it was nearly 3 times that of CON group (P<0.05). Compared with Aβ40 group, the CREB phosphorylation level in the Aβ40 plus Hainan papaya water extract group was significantly decreased (P<0.05).
3.7. Hainan papaya water extract down-regulates Aβ40-induced Bax expression in neurons
Next, to analyze the expression of ERK-1/2 downstream genes, the levels of Bax in each group were measured using Western blotting,with β-actin being used as an internal control. As shown in Table 1, Bax expression was highest in the Aβ40 group; it was 3.12 times that of CON group (P<0.05). Compared with Aβ40 group, the expression of Bax in the Aβ40 plus Hainan papaya water extract group was significantly decreased (P<0.05).

Table 1 The activation level of ERK1/2 pathway-associated molecule in allgroups (via Western blotting method).
4. Discussion
In this study, primary cultured neurons isolated from rat DRG were used to establish an Aβ40-induced neuronal injury model. These cells were pretreated with 100 μg/L Hainan papaya water extract for 10 m in prior to treatment with Aβ40 to evaluate its protective effects. The data revealed that treating cells with Aβ40 alone for 24 h induced neuronal apoptosis, significantly decreased cell viability,significantly increased the phosphorylation of molecules associated with the ERK1/2 signaling pathway, and induced the translocation of activated ERK 1/2 into the nucleus. Treatment with Hainan papaya water extract alone showed no toxic effects on the neurons. Furthermore, Hainan papaya water extract exhibited neuroprotective effects because it was found to suppress Aβ40-induced activation of the ERK1/2 signaling pathway by inhibiting the phosphorylation of its targets, to prevent ERK 1/2 nuclear translocation, and to significantly decrease Aβ40-induced neuronal apoptosis.
The “Aβ hypothesis” has been w idely accepted as one of the primary mechanisms of AD. To date, several studies have established AD cell models by using Aβto injure neurons. In most cases, the neurons were isolated from the hippocampus or cerebral cortex of neonatal mice [15,16]. The current study was the first to initiate an AD cell model by injuring neurons isolated from the rat DRG,and the results demonstrated that Aβ40 treatment increases DRG neuronal apoptosis and decreases viability. A fter culture with Aβ40 for 24 h, the level of p-ERK in DRG neurons increased, and nuclear translocation of p-ERK was observed; the phosphorylation levels of the downstream markers MEK, RSK1, and CREB also all increased,and expression of the apoptosis-related protein Bax was enhanced. Young et al. found that Aβ could activate a tyrosine kinase to initiate a signaling cascade that resulted in the activation of the MAP kinase ERK1/2 in AD neurons and caused increased phosphorylation levels among proteins associated with the ERK 1/2 signaling pathway[17,18]. These findings are in accord with the present results,
which suggest that DRG neurons are sensitive to Aβ40 and can be used as an AD cell injury model.
The pathogenic mechanism of AD is complex. In addition to apoptosis, oxidative damage is also extremely important during the early stages of AD. Modern pharmaceutical research has demonstrated that papaya has strong antioxidant effects [1-3]. In theory, its antioxidant activity can help clear the abundant reactive oxygen species in the brain at the early stages of AD, thereby providing neuronal protection. Nonetheless, the present study is the first to confirm that papaya water extract has no toxic effects on neurons. The MTT assay results showed that neuronal viability was lower in the Hainan papaya water extract group compared with the blank control group, but the difference between the two was not statistically significant. This result suggests that papaya water extract is not toxic to neurons. Furthermore, pre-treatment with papaya water extract decreased Aβ40 toxicity and increased the viability of the DRG neurons.
The ERK 1/2 signaling pathway is active mainly in neuronal dendrites, axons, and activated astrocytes [19,20]. This pathway is important for transducing extracellular signals to the nucleus via cell surface receptors. The ERK1/2 signaling pathway regulates several cellular functions and plays particularly important roles in cell proliferation, differentiation, and apoptosis. Certain extracellular stimuli (e.g., Aβ40) can induce the phosphorylation of MEK,which further activates ERK1/2 and thus stimulates translocation of p-ERK1/2 to the nucleus, where it phosphorylates the transcription factors RSK and CREB. p-RSK and p-CREB regulate intracellular signaling and are involved in regulating the transcription and translation of apoptosis-related genes such as Bcl-2 and Bax. Aβ40 accelerates cellular apoptosis by upregulating Bax gene expression via the activation of ERK1/2 signaling. The present study revealed that pre-treatment with Hainan papaya water extract could m itigate the Aβ40-induced increase in p-ERK levels and inhibit p-ERK translocation into the neuronal nucleus. Further exam ination of the expression levels of molecules associated with the ERK pathway showed that pre-treatment with Hainan papaya water extract for 10 m in led to significant reductions in p-ERK1/2, p-MEK, p-RSK 1,p-CREB, and Bax levels (all P<0.05).
In summary, our study demonstrates that the neuroprotective effects of Hainan papaya water extract are associated with the downregulation of ERK 1/2 signaling. It has been reported that Hainan papaya water extract contains several components,including phenols, flavone, and polysaccharides [21,22]. However,the com ponents of papaya extract that are responsible for its neuroprotective effects remain to be determined.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknow ledgements
We thank LetPub for linguistic assistance during the preparation of this manuscript.
References
[1] Ali A, Ong MK, Forney CF. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem 2014;142:19-26.
[2] So fi FR, Raju CV, Lakshm isha IP, Singh RR. Antioxidant and antim icrobial properties of grape and papaya seed extracts and their application on the preservation of Indian mackerel (Rastrelliger kanagurta) during ice storage. Food Sci Technol 2016;53(1): 104-117.
[3] Nafiu AB, Rahman MT. Anti-inflammatory and antioxidant properties of unripe papaya extract in an excision wound model. Pharm Biol 2015;53(5): 662-671.
[4] Tam aoka A.The pathophysio logy o f A lzheim er's disease with special reference to "am yloid cascade hypothesis". Rinsho Byori 2013;61(11):1060-1069.
[5] Wang X, Wang W, Li L, Perry G, Lee H, Zhu XW. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim Biophys Acta 2014; 1842(8):1240-1247.
[6] de la Monte SM, Tong M. Brain metabolic dysfunction at the core of A lzheimer's disease. Biochem Pharmacol 2014;88(4):548-559.
[7] Suwanna N, Thangnipon W, Soi-Ampornkul R. Neuroprotective effects of diarylpropionitrile against beta-amyloid peptide-induced neurotoxicity in rat cultured cortical neurons. Neurosci Lett 2014;578: 44-49.
[8] Zhang HY, Liu YH, Lao ML, Ma ZJ, Yi XN. Puerarin protects A lzheimer's disease neuronal cybrids from oxidant-stress induced apoptosis by inhibiting pro-death signaling pathways.Exp Gerontol 2011;46(1): 30-37. [9] Yang Y, Chen SC, Zhang JF, Li CT, Sun YH, Zhang LH, et al. Stimulation of autophagy prevents amyloid-beta peptide-induced neuritic degeneration in PC12 cells. Alzheimers Dis 2014;40(4): 929-939.
[10] Ashabi G, Ramin M, Azizi P, Taslim i Z, A lamdary SZ. ERK and p38 inhibitors attenuate memory deficits and increase CREB phosphorylation and PGC-1alpha levels in Abeta-injected rats. Behav Brain Res 2012;232(1): 165-173.
[11] Zuchero JB. Purification of dorsal root ganglion neurons from rat by immunopanning. Cold Spring Harb Protoc 2014; 2014(8):826-838.
[12] Kim JH. Brain-derived neurotrophic factor exerts neuroprotective actions against amyloid beta-induced apoptosis in neuroblastoma cells. Exp & Ther Med 2014; 8(6):1891-1895.
[13] Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatr 2009;14(4): 448-461.
[14] Araki W, Kametani F, Oda A, Tamaoka A. MEK inhibitors suppress beta-amyloid production by altering the level of a beta-C-term inal fragment of amyloid precursor protein in neuronal cells. FEBS Lett 2010;584(15):3410-3414.
[15] Hradek AC, Lee HP, Siedlak SL, Torres SL, Jung W, Han AH, et al. Distinct chronology of neuronal cell cycle re-entry and tau pathology in the 3xTg-AD mouse model and A lzheimer's disease patients. Alzheimers Dis 2015;43(1): 57-65.
[16] Marples B, McGee M, Callan S, Bowen SE, Thibodeau BJ, Michael DB, et al. Cranial irradiation significantly reduces beta amyloid plaques in the brain and improves cognition in a murine model of A lzheimer's Disease (AD). Radiother Oncol 2016; 118(1): 43-51.
[17] Cecon E, Chen M, Marcola M, Fernandes PA, Jockers R, Markus RP. Amyloid beta peptide directly impairs pineal gland melatonin synthesis and melatonin receptor signaling through the ERK pathway. FASEB 2015;29(6): 2566-2582.
[18] Kim B, Park J, Chang KT, Lee DS. Peroxiredoxin 5 prevents amyloidbeta oligomer-induced neuronal cell death by inhibiting ERK-Drp1-mediated m itochondrial fragmentation. Free Radic Biol Med 2016; 90: 184-194.
[19] Faucher P, Mons N, Micheau J, Louis C, Beracochea DJ. Hippocampal injections of oligomeric amyloid beta-peptide (1-42) induce selective working memory deficits and long-lasting alterations of ERK signaling pathway. Front Aging Neurosci 2015; 7: 245.
[20] Xu X, Lu Y, Zhang G, Chen L, Tian D, Shen X, et al. Bisphenol A promotes dendritic morphogenesis of hippocampal neurons through estrogen receptor-mediated ERK1/2 signal pathway. Chemosphere 2014;96:129-137.
[21] Panzarini E, Dw ikat M, Mariano S, Vergallo C, Dini L. Administration dependent antioxidant effect of Carica papaya seeds water extract. Evid Based Complement Alternat Med 2014; 2014: 281508.
[22] Li YM, Su N, Yang HQ, Bai XP, Zhu QX, Liu HX, et al. The extraction and properties of Carica papaya Seed oil. Adv J Food Sci Technol 2015;7(10): 773-779.
doi:Document heading 10.1016/j.apjtm.2016.05.001
*Corresponding author:Xi-Nan Yi, Department of Anatom y, Hainan Medical College, Xueyuan Road, Haikou, 571199, Hainan, China.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Predicted pattern of Zika virus infection distribution with reference to rainfall in Thailand
- Effect of partial splenic embolization on the immune function of cirrhosis patients with hypersplenism
- Perfusion of gastrodin in abdom inal aorta for alleviating spinal cord ischem ia reperfusion injury
- Study on the effect and mechanism of the dysfunction of CD4+T cells in the disease process of chronic cardiac failure
- Influence on radiosensitivity of lung glandular cancer cells when ERCC1 gene silenced by targeted siRNA
- Experimental study on the inhibition effect of m iR-106a inhibitor on tumor grow th of ovarian cancer xenografts m ice
