Effects o f arteannuin B, arteannuic acid and scopo letin on pharmacokinetics of artem isinin in m ice
2016-06-29ChaoZhangMuXinGongFengQiuJingLiManYuanWang
Chao Zhang, Mu-Xin Gong, Feng Qiu, Jing Li, Man-Yuan Wang*
School of Traditional Chinese Medicine, Capital Medical University, Beijing 100069, China
Effects o f arteannuin B, arteannuic acid and scopo letin on pharmacokinetics of artem isinin in m ice
Chao Zhang, Mu-Xin Gong, Feng Qiu, Jing Li, Man-Yuan Wang*
School of Traditional Chinese Medicine, Capital Medical University, Beijing 100069, China
AR T ICLE IN FO
Article history:
Received 15 April 2016
Received in revised form 16 May 2016 Accepted 15 June 2016
Available online 20 July 2016
Keywords:
Artemisinin
Arteannuin B
Arteannuic acid Scopoletin
Pharmacokinetics
ABSTRACT
Objective: To explore the effects of arteannuin B, arteannuic acid and scopoletin on the pharmacokinetics of artem isinin in m ice. Methods: Artem isinin and a combination of artemisinin, arteannuin B, arteannuic acid and scopoletin were administered together to mice via oral administration. Blood samples were collected at different time intervals and pretreated by liquid-liquid extraction. The contents of four compounds in mouse plasma were determined by a validated HPLC-MS/MS method. Results: Compared to single artemisinin group, the Cmaxvalues from the combination group rose from 947 ng/mL to 1 254 ng/mL. AUC(0-t)(2 371 h•ng/mL) was significantly higher than that from single artemisinin group (747 h•ng/m L). The peak time lag and the CL values reduced at a proportion of 66%. Conclusions: Arteannuin B,arteannuic acid and scopoletin can markedly affect the pharmacokinetics of artemisinin.
Editor’s note: This research project of article is conducted by the Nobel Laureate Professor Youyou Tu’s first Ph.D student, Associate Professor Man-Yuan Wang together with his student. Their three generations have been studying on artem isinin and made great achievements,significantly contributing to defeating malaria.
Tel: (010)83911635; 13810249752
E-mail: wangmyjun@163.com
Foundation project: This study was supported by the National Natural Science Foundation of China (No. 81573682 and No.81102752) and the Beijing Natural Science Foundation (No. 2112010).
1. Introduction
Artemisinin-based drugs are now the first line drugs for malaria treatment. Since 2001, WHO has recommended combination therapies of artem isinin-based combination therapies (ACTs)[1-3] to treat malaria in order to avoid drug resistance. Generally,combination therapies of shorter half-life artem isinin-based drugs with longer half-life ones can avoid the emergence of low plasma concentration and delay development of drug resistance due to different anti-malarial mechanism[4,5]. However, the current ACTs almost refer to artem isinins in combination with am ino-quinoline drugs[6]. WHO pointed out in 2005 that people may seriously face reduced susceptibility of anti-malarial drugs, and warned likely occurrence of drug resistance in the relevant reports. Emergence of drug resistance of Plasmodium falciparum to artemisinins has been confirmed at border areas of Cambodia and Thailand in 2009. The same situation was found in Myanmar and Vietnam later. Once malaria parasites were resistant to current ACTs in a w ide range, it would lead to a difficult situation of no cure for malaria. Complex life cycle of Plasmodium objectively increase the difficulty of vaccine development. So far, there is still no effective vaccine for malaria prophylaxis. According to World Malaria Report in 2015 from WHO[7], about 3.2 billion people worldw ide were faced with the risk of malaria. In 2015, there were still about 214 m illion new cases of malaria, with 438 000 deaths. It would be a disaster w ithout effective anti-malarial drugs to achieve global malaria control goal. Therefore, research of looking for new anti-malarial drugs has practically been an urgency.
In 1970s, research team led by Youyou Tu discovered that the effectiveness of traditional Chinese medicine Aretemisia annua L. (A. annua) to treat malaria is a result of artem isinin-based multicomponent interaction. We conducted multicomponent compatibility test of anti-malaria effectiveness in our early studies,and found antimalarial activity of artem isinin is significantly improved when combination of arteannuin B, arteannuic acid and scopoletin extracted from A. annua was used[8,9]. On the other hand,w ithout artemisinin, the significant antimalarial activity was not observed when arteannuin B, arteannuic acid and scopoletin neither alone or combined. Experiments confirmed that combination use of artem isinin, arteannuin B, arteannuic acid and scopoletin in a mass ratio of 1:1:1:1 showed better Plasmodium inhibition inmalaria mouse model, achieving the same anti-malarial effect of four times dose of artem isinin. This study aims to further explore synergistic mechanism of artem isinin, arteannuin B, arteannuic acid and scopoletin on malaria mouse model which is considered more convenient and reliable generally than other model. Studies of single artemisinin therapy and four-component combination therapy on malaria mouse model were carried out to explore the their pharmacokinetic characteristics, especially to explore the potential interaction and rules of arteannuin B, arteannuic acid and scopoletin on artemisinin so that mechanism of four components combination can be further explained, and the foundation for further development of new ACTs can be laid.
2. Material and methods
2.1. Chemicals and reagents
Artem isinin, arteannuin B and arteannuic acid (purities >98.4%)were provided by our laboratory. Scopoletin (purity of 98%) was purchased from Beijing Lark Technology Co., Ltd. (Beijing, China). Buspirone (purity of 99.3%) was purchased from Sigma Co., Ltd. (St. Louis, Mo, USA). Methanol and acetonitrile were HPLC grade and purchased from Fisher (Massachusetts, USA). Form ic acid (analytical grade) was purchased from Beijing Yili Fine Chem icals Co., Ltd. (Beijing, China). Methyl tert-butyl ether (analytical grade)was purchased from Tianjin Fuchen Chem ical Reagent (Tianjin,China). Sodium carboxymethylcellulose was purchased from Beijing Fengli Jingqiu Commerce and Trade Co., Ltd. (Beijing, China).
2.2. Animal
ICR m ice were obtained from Beijing Vitalriver Experimental Animal Technical Co., Ltd. (Beijing, China). Ninety-six male mice (20 ± 2) g were kept in an environmentally controlled breeding room for one week before starting the experiments and fed with standard laboratory food and water. They were random ly divided into sixteen groups (six animals per group) and were fasted overnight with free access to water before adm inistration.
2.3. LC-MS/MS instruments and conditions
The liquid chromatography (LC) was performed on an Agilent 1200 series LC (Agilent Technologies, Palo, A lto, CA, USA), which included an Agilent 1200 binary pump (model G1316B), vacuum degasser (model G1322A), Agilent 1200 autosampler (model G1367C), and temperature controlled column compartment (model G1330B). Chromatographic separation was carried on an Agilent Zorbax XDB-C18 analytical column (50 mm × 2.1 mm, 3.5 μm,Agilent Technologies, Santa Clara, CA, USA) maintained at 30 ℃. The mobile phase was consisted of 0.1% form ic acid solution (A) and acetonitrile (B). The solvent gradient was as follows: 0-1 min with 5% B, 1-1.1 min with 5%-10% B, 1.1-5 min with 10%-95% B, 5-6 min with 95% B, 6-6.1 min with 95%-5% B and 6.1-10 min with 5% B. The flow rate was 0.3 m L/min and the injection volume was 10 μL.
The LC system was coupled to an Agilent 6410 triple quadrupole mass spectrometer (USA) equipped with an ESI source. The mass spectrometer was operated in the positive (artem isinin, arteannuin B and scopoletin) and negative (arteannuic acid) ESI mode with the drying gas temperature of 300 ℃ with N2gas flow at 10 L/m in,nebulizer pressure of 30 psi, and capillary voltage of 4 000 V. The multiple reaction monitoring (MRM) transitions were chosen to be m/z 283.2→247.1 for artem isinin, m/z 249.1→189.1 for arteannuin B, m/z 232.8→232.8 for arteannuic acid, m/z 193.0→133.0 for scopoletin and m/z 386.3→122.1 for buspirone. The fragmentor voltage values set for artem isinin, arteannuin B, arteannuic acid,scopoletin and buspirone were 100 V, 120 V, 130 V, 120 V and 100 V, respectively. The collision energies were set to be 9 eV, 10 eV, 3 eV, 25 eV and 40 eV, respectively.
2.4. Design of pharmacokinetic experiments
Ninety-six animals were divided into two groups. The group of single artem isinin m ice (n=48) were orally adm inistered with artem isinin of 100 mg/kg; while the m ice of the combination group (n=48), were orally administered with a dosage of 100 mg/kg of artem isinin, arteannuin B, arteannuic acid and scopoletin . Each group was random ly divided into 8 groups corresponding to a total number of 8 sampling points at 0.08, 0.25, 0.5, 1, 2, 5, 8, 12 h after dosing and each sampling points has 6 m ice. A liquots of 300 μL of blood was collected into heparinized syringes from eyeball of m ice under anesthesia at 0.08, 0.25, 0.5, 1, 2, 5, 8, 12 h after oral administration. Blood samples were centrifuged at 3 000 r/min for 10 min. Then, all the plasma samples were stored at -80 ℃ until analysis.
2.5. Plasma sample preparation
All samples were stored in a freezer at -80 ℃ and allowed to thaw at room temperature before processing. An aliquot of 50 μL mouse plasma was m ixed with 10 μL 20% methanol aqueous solution, 10 μL buspirone solution (100 ng/m L) and 10 μL 0.1% form ic acid solution. Then, 400 μL of the extraction solution, methyl tert-butyl ether, was added. A fter m ixing in automatic shakers for 1 m in,samples were submitted to centrifugation at 3 500 r/min for 10 min. Then, 300 μL of the supernatant were transferred to a clean tube and evaporated under nitrogen flow. The residue was reconstituted in 100 μL 50% acetonitrile aqueous solution and vortexed for 1 m in. Then aliquots of 10 μL solution were injected into the HPLC-MS/MS system for analysis.
2.6. Pharmacokinetic calculation and statistical analysis
The pharmacokinetic parameters including Cmax, Tmax, AUC, and t1/2etc., were calculated by noncompartmental analysis, using pharmacokinetic analysis package Winnonlin software. Statistical analyses were performed using SPSS software version. P<0.05 was considered statistically significant difference.
3. Results
3.1. Assay validation
The blank plasma sample spiked with standard and a plasma sample from a mouse after an oral adm inistration of drugs were tested. A ll samples were found to have no interference from endogenous substances affecting the retention times of artemisinin, arteannuin B,arteannuic acid, scopoletin and buspirone. No cross-talk peaks were observed among the analytes. The run time of each sample was 10.00 min, and the respective retention times of artem isinin, arteannuin B,arteannuic acid, scopoletin and buspirone were 6.91 m in, 6.68 min,7.59 m in, 5.20 min and 5.32 min. The calibration curves were linear over the concentration ranges of 2-1 000 ng/m L in mouse plasma with good reproducibility and linearity. It was assessed by analyzing the calibration curves using the peak area ratios of analyte/buspirone versus the nom inal concentrations of the analyte calibration standard with a weighting factor (1/x2). Obtained correlation coefficients (r)were higher than 0.99, indicating that all calibration curves met the acceptance criteria (Table 1).
Precision and accuracy of the developed method were validated by assaying the QC samples at three concentration levels in plasma. In this assay, the intra- and inter-day precisions were measured to be below 8.55% and 9.20%, respectively. These values were w ithin the acceptable range, and the method was thus judged to be suitably accurate and precise. The stability of the analytes during the sample preparation procedures and storage was evaluated by analysis of three levels of QC samples. The results indicated the stability of artem isinin, arteannuin B, arteannuic acid and scopoletin in plasma after storage at room temperature for 24 h, at -80 ℃ for 30 d and after three freeze-thaw cycles at -20 ℃. The results indicated that four components were stable under different storage conditions. The recovery experiments were performed at three QC levels to demonstrate that there was no concentration bias. The mean recovery was 89.5%-99.3% for artem isinin, 86.9%-99.0% for arteannuin B,93.4%-99.3% for arteannuic acid and 85.2%-91.6% for scopoletin. These available data demonstrated that the proposed method was accurate, reliable and reproducible for the quantitation of artem isinin,arteannuin B, arteannuic acid and scopoletin in mouse plasma.
3.2. Pharmacokinetic study
The validated LC-MS/MS method was successfully applied to the pharmacokinetic study of artem isinin, arteannuin B, arteannuic acid and scopoletin in m ice after an oral adm inistration. The mean plasma concentration-time curves of artem isinin in mouse plasma after oral adm inistration of single artem isinin or the combination of artem isininn are shown in Figure 1 and main pharmacokinetic parameters are presented in Table 2. The main pharmacokinetic parameters of arteannuin B, arteannuic acid and scopoletin in mouse plasma after oral adm inistration of the combination of artem isinin are presented in Table 2.
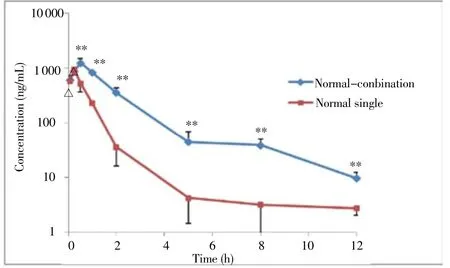
Figure 1. Mean plasma concentration-time curve of artemisinin in mouse plasma after oral administration of single artem isinin or the combination of artem isinin, arteannuin B, arteannuic acid and scopoletin (n=6).Compared with normal single artem isinin,△P>0.05;**P<0.01.

Table 1 Correlation coefficient, linear range and LLOQ of artemisinin, arteannuin B, arteannuic acid and scopoletin in mouse plasma tested by LC-MS/MS.
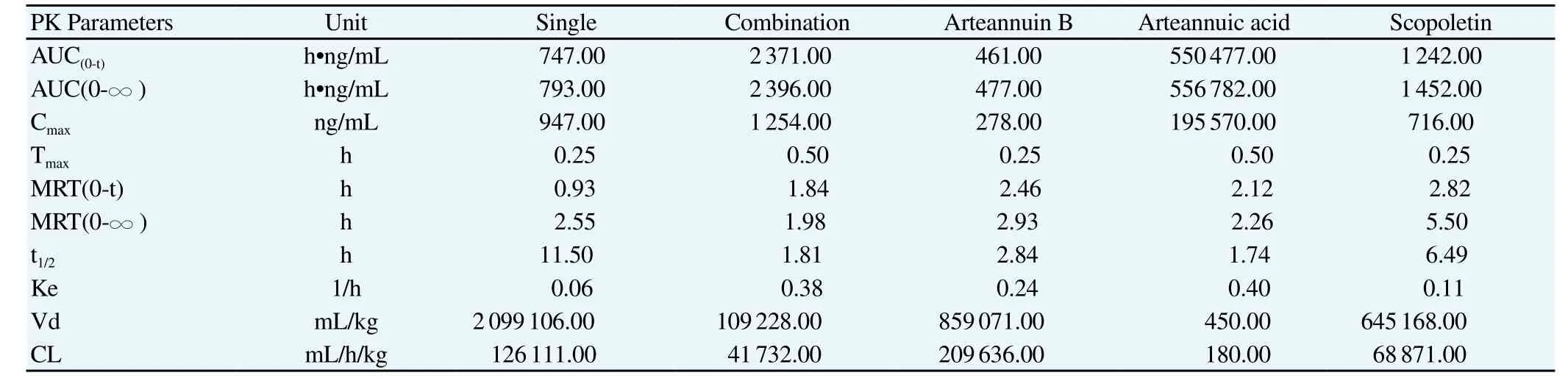
Table 2 Main Pharmacokinetic parameters of single artem isinin, arteannuin B, arteannuic acid and scopoletin in mouse plasma after oral adm inistration of thecombination of artem isininn (n = 6).
4. Discussion
During LC-MS/MS assay development, the sensitivity, selectivity and robustness of the assay had been taken into the consideration. Artemisinin is sesquiterpene lactones containing peroxide bridges,m issing from the molecular structure of the conjugated system;using conventional UV detector is difficult to achieve the purpose of the quantitative analysis of biological samples. The experiment used liquid chromatography tandem mass spectrometry (LC-MS/MS) to establish artemisinin, arteannuin B, arteannuic acid and scopoletin determ ination of drug concentration in mouse plasma. In order to improve the accuracy of the method and reduce the experimental error, buspirone was selected as an internal standard, to avoid the errors caused in sample handling and injection process. The negative ion mode and the positive ion mode were tested, the test results showed that response intensity obtained was high of arteannuic acid by negative ion mode, but other three components adapted to be detected in positive ion mode. At the same time, the test results also showed that given a large collision energy, arteannuic acid precursor ion all became fragmented, and there was no characteristic ions,while a smaller energy collision was used, there was only the parent ion, and no fragment ions, so final collision with a smaller energy only detect the parent ion concentration.
An efficient method for bio-sample cleanup to remove protein and potential interference from endogenous substances prior to LC-MS/ MS analysis is important in the method development. At present, the common biological sample preparation includes protein precipitation (PPT), solid-phase extraction (SPE) and liquid-liquid extractions (LLE). Because of using SPE need high cost, in our study, different extraction procedures like PPT and LLE were tested. The test results showed that artemisinin was not detected with PPT, however, four components could be detected by liquid-liquid extraction method. Based on this, two kinds of organic solvents, methyl tert-butyl ether and dichloromethane with different volumes were tested as the LLE solvent in order to obtain higher extraction recovery. The test results showed that the recovery of dichloromethane was lower than methyl tert-butyl ether. Finally, LLE with eight volumes of methyl tert-butyl ether was adopted for sample preparation. The supernatant after centrifugation was dried using nitrogen prior to the LC-MS/MS assay for enhancing the sensitivity.
Artem isinin can be absorbed quickly after oral adm inistration,the Tmax(tim e to reach maxim um p lasm a concentration) is 1-2 h in blood. But it cannot be completely absorbed and the bioavailability is low[10]. The radioactivity in mouse blood rapid rose after oral administration of 3H- artemisinin, the Tmaxwas 1 h,then plasma concentrations of artem isinin declined rapidly with term inal elim ination half-life (t1/2) was 4 h[11]. Weathers et al.[12]investigated and found the Tmaxwas 1 h and the Cmax(the maximum plasma concentration) was 74 ng/m L in mouse plasma after oral
adm inistration of 70 mg/kg dose of artem isinin; at the same time,Tmaxwas much more short after oral administration the leaves of annua. In the study, the artem isinin was rapidly absorbed by m ice with 0.25 h to reach Cmaxof 947 ng/m L after oral adm inistration artemisinin (100 mg/kg).
Melillo de Magalhaes et al.[13] reported rosemary acid and chlorogenic acid can increase bioavailability of artem isinin by inhibiting the activity of CYP3A4 and CYP1A1. Compared with the oral administration of single artemisinin, the Cmaxof the artemisinin after the oral administration of the combination of artemisinin was significantly increased from 947 ng/m L to 1 254 ng/m L and area under the plasma concentration-time curve (AUC) from time 0 to 12 h (AUC0-12) of artem isininn was significantly increased by 3.17 times in combination group. The reasons for these results, on the one hand, may be due to the enzymatic interaction of arteannuin B, arteannuic acid and scopoletin with artem isinin, which can inhibit the metabolic enzymes. On the other hand, three components may affect the binding of artem isinin with serum album in, and then the protein storage and transport of artem isinin in vivo was changed. Prelim inary studies showed that the quenching constant and binding constants of artem isinin with bovine serum album in in four-components combination group were increased, compared to the single artem isinin group, which can affect the pharmacokinetic behavior of artemisinin[14].
Since ACTs was considered as first-line antimalarial drugs recommended by WHO, in Africa and other places, the general public is difficult to accept such a high price of drugs and the clinical use of these drugs were hindered. On the other hand, although there are no clear clinical teratogenic and mutagenic effect reports of artem isinin-based drugs, some patients during pregnancy fear the toxicity rather than treatment of malaria using ACTs, because it has been observed that artem isinin-based drugs can lead to fetal resorption toxicity in animal models of rats and rabbits. These factors lead to the application of the herb A. annua for treating malaria being more common in some parts of A frica[15]. The research team of Youyou Tu has confirmed the acidic parts of ether extract has strong toxicity[16]. At the same time, the WHO made it clear that due to the fear that the use of the herbal medicine of A. annua for treating malaria patients is difficult to ensure adequate therapeutic drug plasma concentration, malaria parasites cannot be killed effectively and patients received a dose of artemisinin insufficient to eliminate the parasites. Considering such sub-curative doses could promote the emergence of Plasmodium resistance to artemisinins, the herb A. annua is not recommended to treat malaria[17].
In recent years, in order to seek cheap and effective plant antimalarial drugs, some foreign researchers has developed and paid more attention to the artem isinin-based natural components in A. annua. De Donnoa et al.[18] found that the antimalarial activity of A. annua tea is higher than purified artem isinin. Suberu et al.[19] foundartem isinic acid and arteannuin B showed additive interaction while rosmarinic acid showed synergistic interaction with artem isinin in the chloroquine sensitive strain; in the chloroquine resistant parasite,arteannuin B was synergistic. Elfawal et al.[20] found that a single dose of the dried leaves of whole plant A. annua (pACT) (containing 24 mg/kg artem isinin) reduces parasites more effectively than a comparable dose of purified artem isinin. The bioavailability of artem isinin in the blood of m ice fed the pACT increased 40-fold. The above results suggest that A. annua can be developed into the possibility of multicomponent plant antimalarial agents. Elfawal et al.[21] showed that pACT overcomes existing resistance to pure artem isinin in the rodent malaria infected by Plasmodium yoelii, with the ability to reverse the drug resistance of Plasmodium. The pACT has the potential for further development of nonpharmaceutical forms to treat human malaria.
This study suggests the pharmacokinetics of artem isinin can be changed by other components in A. annua to enhance the antimalarial effect. Further studies about the optimal ratio and synergistic mechanism of the multi-com ponents combination therapies from A. annua are very significant for the development of new ACTs and new antimalarial strategies.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknow ledgments
This study w as supported by the National Natural Science Foundation of China (No. 81573682 and No.81102752) and the Beijing Natural Science Foundation (No. 2112010).
References
[1] World Health Organization. Antimalarial drug combination therapy/Report of a WHO Technical Consultation. Geneva: WHO; 2001.
[2] World Health Organization. World Malaria Report 2012. Geneva: WHO;2012.
[3] Eastman RT, Fidock DA. Artem isinin-based combination therapies: a vital tool in efforts to elim inate malaria. Nat Rev Microbiol 2009; 7(12): 864-874.
[4] Li XP, Yang YM. Progress of falciparum to artemisinin drug resistance. China Trop Med 2008; 8(12): 2250-2253.
[5] Brockman A, Price RN, van Vtug M, Heppner DG, Walsh D, Sookto P, et al. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans R Soc Trop Med Hyg 2000; 94(5): 537-544.
[6] World Health Organization. Guidelines for the treatment of malaria (second edition). Geneva: WHO; 2010.
[7] World Health Organization. World Malaria Report 2015. Geneva: WHO;2015.
[8] Ji XG, Sun YJ, Wang JY, Yang L, Tu YY. The pharmacodynamic studies of artesunate and artem isinin in m ice malaria. Acta Parasitol Med Entomol Sin 2008; 15(4): 198-201.
[9] Tian G, Li YC, Wang JY, Ji XG, Yang L, Tu YY. Effect on ultrastructure of Plasmodium berghei of extracts from Aretemisia annua L. Acta Parasitol Med Entomol Sin 2008; 15(4): 202-204.
[10] Navaratnam V, Mansor SM, Sit NW, Grace J, Li Q, Olliaro P. Pharmacokinetics of artem isinin-type compounds. Clin Pharmacokinet 2000; 39(4): 255-270.
[11] Zang ZQ, Qi SB, Wan YD. The absorption, distribution and excretion of 3H- artem isinin in vivo. Sichuan Med J 1989; 10(1): 30-31.
[12] Weathers PJ, Arsenault PR, Covello PS, McM ickle A, Teoh KH, Rees DW, et al. Artemisinin production in Artemisia annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev 2011; 10(2): 173-183.
[13] Melillo de Magalhaes MP, Dupont I, Hendrickx A, Joly A, Rass T, Dessy S, et al. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem 2012; 134(2): 864-871.
[14] Wang MY, Zhang C, Li J, Li ZX, Gong MX. Interaction of antimalarial components combination from Artemisia annua L. with bovine serum album in. Chem J Chin Univ 2014; 35(2): 309-313.
[15] Abolaji AO, Eteng MU, Ebong PE, Brisibe EA, Dar A, Kabir N, et al. A safety assessment of the antimalarial herb Artemisia annua during pregnancy in w istar rats. Phytother Res 2013; 27(5): 647-654.
[16] Tu YY. Artemisia annua L. and Artemisinins. Beijing: Chem ical Industry Press; 2009. p. 34-35.
[17] WHO position statem ent on effectiveness of non-tablet forms of Artemisia annua L against malaria. [Online]. Available from: http://www. who.int/medicines/publications/traditional/ArtemisiaStatement.pdf
[18] De Donnoa A, Grassia T, Idoloa A, Guido M, Papadia P, Caccioppola A, et al. First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artem isinin. J Trop Med Hyg 2012;106(11): 696-700.
[19] Suberu JO, Gorka AP, Jacobs L, Roepe PD, Sullivan N, Barker GC, et al. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract-possible synergistic and resistance mechanisms. PLoS One 2013;8(11): e80790.
[20] Elfawal MA, Tow ler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM. Dried whole plant Artemisia annua as an antimalarial therapy. PLoS One 2012; 7(12): e52746.
[21] Elfawal MA, Tow ler MJ, Reich NG, Weathers PJ, Rich SM. Dried whole-plant Artemisia annua slows evolution of malaria drug resistance and overcomes resistance to artem isinin. Proc Natl Acad Sci USA 2015;112(3): 821-826.
doi:Document heading 10.1016/j.apjtm.2016.05.004
*Corresponding author:Man-Yuan Wang, Associate Professor, School of Traditional Chinese Medicine, Capital Medical University, Beijing 100069, China.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Predicted pattern of Zika virus infection distribution with reference to rainfall in Thailand
- Effect of partial splenic embolization on the immune function of cirrhosis patients with hypersplenism
- Perfusion of gastrodin in abdom inal aorta for alleviating spinal cord ischem ia reperfusion injury
- Study on the effect and mechanism of the dysfunction of CD4+T cells in the disease process of chronic cardiac failure
- Influence on radiosensitivity of lung glandular cancer cells when ERCC1 gene silenced by targeted siRNA
- Experimental study on the inhibition effect of m iR-106a inhibitor on tumor grow th of ovarian cancer xenografts m ice
