Effects of scallop shell extract on scopolam ine-induced memory impairment and MK801-induced locomotor activity
2016-06-29YasushiHasegawaTatsuroInoueSatoshiKawaminamMihoFujita
Yasushi Hasegawa, Tatsuro Inoue, Satoshi Kawam inam i, Miho Fujita
College of Environmental Technology, Muroran Institute of Technology, 27-1 Mizumoto, Muroran 050-8585, Japan
Effects of scallop shell extract on scopolam ine-induced memory impairment and MK801-induced locomotor activity
Yasushi Hasegawa*, Tatsuro Inoue, Satoshi Kawam inam i, Miho Fujita
College of Environmental Technology, Muroran Institute of Technology, 27-1 Mizumoto, Muroran 050-8585, Japan
AR T ICLE IN FO
Article history:
Received 15 April 2016
Received in revised form 16 May 2016 Accepted 15 June 2016
Available online 20 July 2016
Keywords:
Locomotor activity Memory impairment Scallop shell
Schizophrenia
ABSTR ACT
Objective: To evaluate the neuroprotective effects of the organic components of scallop shells (scallop shell extract) on memory impairment and locomotor activity induced by scopolam ine or 5-methyl-10,11-dihydro-5H-dibenzo (a,d) cyclohepten-5,10-im ine (MK801). Methods: Effect of the scallop shell extract on memory impairment and locomotor activity was investigated using the Y-maze test, the Morris water maze test, and the open field test. Results: Scallop shell extract significantly reduced scopolam ine-induced short-term memory impairment and partially reduced scopolam ine-induced spatial memory impairment in the Morris water maze test. Scallop shell extract suppressed scopolamine-induced elevation of acetylcholine esterase activity in the cerebral cortex. Treatment with scallop shell extract reversed the increase in locomotor activity induced by scopolamine. Scallop shell extract also suppressed the increase in locomotor activity induced by MK801. Conclusions: Our results provide initial evidence that scallop shell extract reduces scopolam ine-induced memory impairment and suppresses MK-801-induced hyperlocomotion.
Tel: +81-143-46-5745
Fax: +81-143-46-5701
E-mail: hasegawa@mmm.muroran-it.ac.jp
1. Introduction
Scallops are one of the major marine products in Hokkaido, Japan. Approximately 200 000 tons of scallop shells per year is generated as industrial waste. A lthough scallop shells are efficiently used as antibacterial agents or as a material for desulfurization, additional modes of utilization are desired. Towards this aim, w e have previously investigated the in vitro activities of scallop shell extract and found several biological activities [1-3].
Memory impairment is caused not only by aging or stress, but also by neurodegenerative diseases such as Alzheimer’s disease. In A lzheimer’s disease, a deficit of acetylcholine due to a reduction in hippocampal cholinergic neuronal activity is one of the most important causes of memory impairment [4, 5]. Scopolam ine is a muscarinic acetylcholine receptor antagonist that induces learning
and memory impairment through the cholinergic neuronal system. This effect has been proposed to mimic the cognitive and behavioral deficits seen during aging and in A lzheimer’s disease. Therefore,scopolamine has been w idely used to induce cognitive deficits in animal models of neurologic disease [6-8].
Schizophrenia is a severe neuropsychiatric disorder characterized by a core of psychiatric symptoms, including positive, negative, and cognitive symptoms [9]. MK801 is a selective N-methyl-D-aspartate (NMDA) receptor antagonist that induces amnesic and psychomotor effects. In rodents, MK801 induces hyperlocomotion characterized by agitation and stereotyped behavior, both of which are thought to be aspects of schizophrenia. Therefore, MK801 treatment has been used as an animal model of schizophrenia [10-12].
While scallop shells are composed of the CaCO3polymorph of the prismatic layer, pearl oyster shells consist of two types of CaCO3polymorphs, the prismatic layer and the nacreous layer. Since the nacreous layer (pearl) has long been thought to be effective in maintaining a stable emotional state in traditional Chinese medicine,we considered the possibility that scallop shell extract might alsoaffect brain function. In this study, we evaluated the effects of scallop shell extract on scopolamine-induced memory impairment and MK801-induced locomotor activity.
2. Materials and methods
2.1. Materials
Scopolamine and MK801 were purchased from Sigma (St. Louis,MO, USA). Scallops of the species Patinopecten yessoensis,harvested from Mutsu Bay, Aomori, Japan, were purchased and their shells separated.
2.2. Preparation of scallop shell extract
Scallop shell extract was prepared as described previously [1]. Briefly, scallop shells were crushed and ground to a powder. For complete decalcification, the shell powder (approximately 200 g)was dialyzed against 1 L of 5% acetic acid. This was followed by exhaustive dialysis against 1 L of deionized water to remove the acetic acid. After dialysis, the decalcified solution was concentrated by lyophilization, and the sample was resuspended in deionized water. The water-soluble fraction was used as scallop shell extract.
2.3. Animals
Male Wistar rats (4-week-old) were purchased from CLEA (Tokyo,Japan). They were housed individually in a room at 22 ℃. The husbandry of the rats was in accordance with the Guidelines of Experimental Animal Care issued by the O ffice of the Prime Minister of Japan and the Muroran Institute of Technology. The rats were used in experiments after an acclimatization period.
2.4. Short-term memory and locomotor activity evaluation in the Y-maze test
The Y-maze is a three-arm maze with equal angles between the arms, each 30 cm in length and 20 cm in height. The rat was placed in the center of the maze, and the sequence and number of arm entries were recorded for each rat for 10 m in. Any combination of three sequential entries in which the rat entered all three arms (e.g.,ABC, CAB, or BCA, but not ABB), was recorded as a spontaneous alternation. The percentage of spontaneous alternation behavior was calculated according to the follow ing equation: alternation (%) =[(number of alternations)/(total arm entries - 2)] ×100. Spontaneous alternation is believed to reflect spatial working memory, which is a form of short-term memory. The total number of arm entries reflects locomotor activity.
The rats were tested in groups of five or six. One hour before the test, scallop shell extract or phosphate buffered saline (PBS) was adm inistered intraperitoneally (10, 50, or 75 mg/kg). The dosage were selected based on the results in the prelim inary experiment. A fter 30 m in, scopolamine (1 mg/kg) or MK801 (0.35 mg/kg) was administered. The test was started 30 min after the scopolamine or MK801 treatment. The control group received PBS alone.
2.5. Spatial memory evaluation in the Morris water maze test
A round pool (170 cm in diameter) was filled with water (22 ℃) containing black ink. A circular platform (15 cm in diameter) was placed in the pool and submerged 5 cm below the water surface. Three visual cues were positioned at different points along the perimeter of the pool. In the experiment, the rats were given an acquisition trial on each of the seven consecutive days. The rat was placed in the water near the edge of the pool and allowed to search for the platform for 120 s. If the rat did not reach the platform w ithin 120 s, it was placed on the platform for 10 s. The escape latency time (i.e., the amount of time it took the rat to locate the platform) of was recorded at each acquisition trial. One hour before the trial, the rats were injected intraperitoneally with PBS or scallop shell extract (10 mg/kg). After 30 min, scopolamine (1 mg/kg) was administered intraperitoneally. A ll trials were started 30 m in after the scopolamine treatment. The control group received PBS alone.
2.6. The open-field test
The open-field chamber consisted of a metal cylinder 35 cm in diameter and 50 cm in height. The floor was divided into eight equal areas by intersecting lines drawn on the floor. Locomotor activity was measured as the number of times all four legs of the rat crossed one of the lines. One hour before the test, scallop shell extract or PBS was administered intraperitoneally (75 mg/kg). After 30 min,MK801 (0.35 mg/kg) was administered, and the test was started 30 m in after the MK 801 treatment. The control group received PBS alone. The movements of the rat were recorded for 10 m in by a video camera.
2.7. The acetylcholine esterase activity assay
Rats used in the Y-maze test were sacrificed. The cerebral cortex was dissected and homogenized in PBS at 4 ℃ to produce a 20% (w/v) homogenate. The homogenate was centrifuged at 12 000 g for 15 m in, and the supernatant (cortical extract) was used for the measurement of acetylcholine esterase activity. Acetylcholine esterase activity was measured using a modified method of Ellman et al. [13]. Briefly, 2.6 m L of cortical extract was diluted in PBS, m ixed with 0.1 m L of 10 mM 5,5’-dithiobis-2-nitrobenzoate (DTNB), and incubated at 37 ℃ for 5 m in. The enzymatic reaction was started byadding 20 μL of 75 mM acetylcholine iodide, and the absorbance at 412 nm was measured for 30 min. To quantify the acetylcholine esterase inhibitory activity of scallop shell extract, the measurements were performed in the presence of various concentrations of scallop shell extract in a control cortical extract.
2.8. Statistical analysis
Each experiment was performed at least two times. The data were expressed as the mean and the standard error of mean (SEM). The data were analyzed using one-way analysis of variance (oneway ANOVA) followed by Turkey’s multiple-comparison test or Student’s t-test.
3. Results
3.1. Effect of scallop shell extract on scopolamine-induced memory impairment
The effect of scallop shell extract on scopolam ine-induced shortterm memory impairment was assessed by studying spontaneous alternation behavior in the Y-maze test (Figure 1). The scopolaminetreated group show ed significantly decreased spontaneous alternation compared to the control group (by approximately 20%). However, the decrease was reversed by treatment with scallop shell extract at both 10 mg/kg and 50 mg/kg, show ing that scallop shell extract could protect from scopolamine-induced short-term memory impairment. Scopolam ine increased the number of arm entries approximately 1.8-fold. Treatment with scallop shell extract at 50 mg/kg significantly suppressed the increase. However, treatment with scallop shell extract alone did not affect either spontaneous alternation or the number of arm entries. These results show that scallop shell extract can reduce scopolam ine-induced short-term memory impairment and suppress the scopolamine-induced increase of locomotor activity. Scallop shell extract and scopolam ine were administered sequentially before the test, and spontaneous alternation percentage (A) and the number of total arm entries (B) were determined. Scallop shell extract alone (10 mg/kg) was adm inistered 30 m in before the test, and spontaneous alternation percentage (C) and the number of total arm entries (D) were determined. The data for five rats were combined; the bars show the standard errors of mean (SEM). Statistical significance was determ ined using one-way analysis of variance (ANOVA) with Turkey’s test ,*: P<0.05.

Figure 1. Effects of scallop shell extract on scopolam ine-induced memory impairment in the Y-maze test.
3.2. Effect of scallop shell extract on scopolamine-induced spatial memory impairment
The effect of scallop shell extract on scopolam ine-induced spatial memory impairment was examined in the Morris water maze test (Figure 2). The control group rapidly learned the location of the platform, and the mean escape latency time started to decrease on day 3 of training. The scopolamine-treated group showed a delayed escape latency time compared with the control group on days 3-5. Treatment with scallop shell extract resulted in a partial rescue of the scopolam ine-induced delay in escape latency time on days 3 and 4, although significant difference was not observed. This result suggests that scallop shell extract reduce scopolam ine-induced spatial memory impairment.

Figure 2. Effect of scallop shell extract on scopolam ine-induced memory impairment in the Morris-water maze test.
Scallop shell extract and scopolam ine were administered sequentially before the test. The escape latencies during training days were measured in: (A)the control group (○) and the scopolam ine-treated group (˙); (B) the scopolam ine-treated group (˙) and the scopolam ine and scallop-shellextract-treated group (▽). The data for six rats were combined; the bars show the standard errors of mean (SEM). Statistical significance was determ ined using a Student’s t-test,*P<0.05.
3.3. Effect of scallop shell extract on acetylcholine esterase activity
Acetylcholine esterase inhibitors are known to affect scopolam ineinduced memory impairment [14, 15]. Scopolam ine-treated rats showed significantly increased acetylcholine esterase activity in the cortex compared to the control group (Figure 3). Treatment with scallop shell extract significantly suppressed the increase in scopolam ine-induced acetylcholine esterase activity. However,scallop shell extract alone did not inhibit acetylcholine esteraseactivity in vitro.

Figure 3. Effect of scallop shell extract on acetylcholine esterase activity.
(A) Scallop shell extract (10 mg/kg) and scopolam ine were adm inistered sequentially. The cerebral cortices of rats were used to measure acetylcholine esterase activity. The activity shown is per mg of cerebral cortical mass. (B)Acetylcholine esterase activity was measured in the presence or absence of scallop shell extract. The data for five rats were combined; the bars show the standard errors of mean (SEM). Statistical significance was determined using one-way analysis of variance ANOVA with Turkey’s test, *P<0.05.
3.4. Effect of scallop shell extract on MK801-induced locomotor activity
Scallop shell extract reversed the scopolam ine-induced increase in locomotor activity (Figure 1). MK801 treatment is known to induce hyperlocomotion [10, 11]. We therefore examined the effect of scallop shell extract on MK801-induced locomotor activity in the Y-maze test (Figure 4). A lthough MK801 treatment did not significantly lower spontaneous alternation under our experimental conditions,it did increase the number of arm entries approximately two-fold. Scallop shell extract suppressed the increase in MK801-induced locomotor activity to the control level. To confirm this result, we investigated the effect of scallop shell extract on MK801-induced locomotor activity in the open-field test (Figure 5). MK801-treated rats showed a remarkable increase in locomotor activity compared to the control group. This increase was significantly dim inished by the administration of scallop shell extract. These results show that scallop shell extract can suppress scopolam ine and MK801-induced locomotor activity.
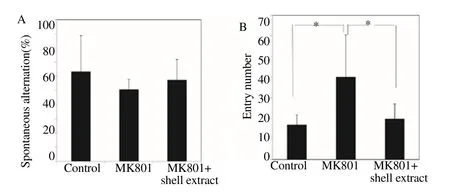
Figure 4. The effects of the scallop shell extract on MK801-induced locomotor activity in Y-maze test.
Scallop shell extract (75 mg/kg) and MK801 were administered sequentially before the test, and spontaneous alternation percentage (A) and the number of total arm entries (B) were determined. The data of five rats were combined;the bars show the standard errors of mean (SEM). Statistical significance was determined by one-way ANOVA with Turkey’s test,*P<0.05.
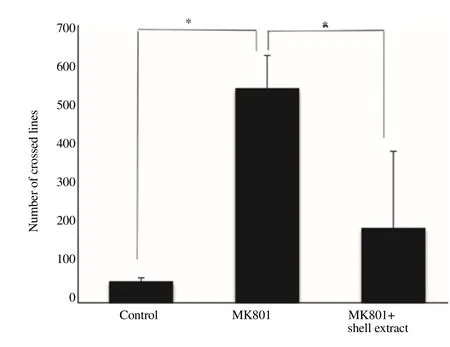
Figure 5. Effect of scallop shell extract on MK 801-induced locomotor activity in the open-field test.
Scallop shell extract (75 mg/kg) was administered 1 hour before the Y-maze test. MK801 was administered 30 m in before the test. The data for five rats were combined; the bars show the standard errors of mean (SEM). Statistical significance was determined using one-way analysis of variance (ANOVA)with Turkey’s test,*P<0.05.
4. Discussion
In the present study, scallop shell extract prevented scopolam ineinduced memory impairment in the Y-maze and the Morris water maze tests. An acetylcholine deficit due to degeneration of the cholinergic nervous system is known to be one of the most important causes of memory impairment [4, 5]. Scopolam ine causes memory impairment by interfering with acetylcholine function at the synapse and partly by increasing acetylcholine esterase activity in the cortex and the hippocampus [8]. Scallop shell extract significantly suppressed the scopolam ine-induced increase in acetylcholine esterase activity in the cortex while unable to inhibit acetylcholine esterase activity in vitro. This result suggests that scallop shell extract ameliorates short-term memory loss through a rescue of the acetylcholine system.
MK801 treatment has been reported to induce memory impairment in some studies [16, 17]. In this study, we could not observe a significant difference in spontaneous alternation between the control group and the MK801-treated group. This discrepancy may be due to our using a higher MK801 dose (0.35 mg/kg) compared to the doses previously used in studies of memory impairment (0.075-0.100 mg/ kg) [16, 17]. Further research is necessary to elucidate the effects of scallop shell extract on memory impairment induced by NMDA receptor antagonists.
MK801-induced hyperlocomotion is considered to be an animal model of the positive symptoms of schizophrenia [10-12]. MK801 induces hyperlocomotion partly by activating the dopam ine neurons in the prefrontal cortex and the hippocampus [18, 19]. On the otherhand, scopolam ine acts by blocking the muscarinic acetylcholine receptors on the cholinergic neurons, thereby disinhibiting these neurons and enabling them to activate dopam ine neurons [20]. Scallop shell extract suppressed the increase in locomotor activity in response to scopolam ine and MK801. These results suggest that scallop shell extract may alter dopaminergic neurotransmission.
Recently Zhang et al showed that administration of pearl conchiolin protein reduced locomotor activity through down-regulation of 5-hydroxytryptam ine (5-HT) and up-regulation of γ-am inobutyric acid (GABA) in the brain [21]. Scallop shell extract also affect 5-HT3 and GABA neurotransmitter levels. It is interesting that components in pearl (nacre) and scallop shells affect brain function.
To date, the bioactive substance in scallop shell extract remains unknown. Attempts to identify the substance and its mechanisms of action are ongoing. Our results provide initial evidence that scallop shell extract reduces scopolamine-induced memory impairment and suppresses MK-801-induced hyperlocomotion.
Conflict of interest statement
We declare that we have no conflicts of interest.
References
[1] Takahashi K, Satoh K, Katagawa M, Torita A, Hasegawa Y. Scallop shell extract inhibits 3T3-L1 preadipocyte differentiation. Fish Sci 2012;78:897-903.
[2] Fukuda M, Takahashi K, Ohkawa C, Sasaki Y, Hasegawa Y. Identification of a resistant protein from scallop shell extract and its bile acid-binding activity. Fish Sci 2013; 79:1017-1025.
[3] Fukuda M, Hasegawa Y. Protease activity of a 90-kDa protein isolated from scallop shells. Tur J Fish Aqua Sci 2014;14:247-254.
[4] Gu G, Zhang W, Li M, Ni J, Wang P. Transplantation of NSC-derived cholinergic neuron-like cells improves cognitive function in APP/PS1 transgenic m ice. Neurosci 2015; 291:81-92.
[5] Bartus RT. On neurodegenerative diseases, models and treatment strategies: lessons learned and lessons forgotten a generation follow ing the cholinergic hypothesis. Exp Neurol 2000;163: 495-529.
[6] Wang Q, Sun LH, Jia W, Liu XM, Dang HX, Mai WL, et al. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamineinduced learning and memory impairment in m ice. Phytother Res 2010;24:1748-1754.
[7] Kumar H, Kim BW, Song SY, Kim JS, Kim IS, Kwon YS, et al. Cognitive enhancing effects of alpha asarone in amnestic m ice by influencing cholinergic and antioxidant defense mechanisms. Biosci Biotechnol Biochem 2012; 76:1518-1522.
[8] Soodi M, Naghdi N, Hajimehdipoor H, Choopani S, Sahraei E. Memoryimproving activity of Melissa officinalis extract in naïve and scopolaminetreated rats. Res Pharm Sci 2014; 9:107-114.
[9] Marneros A, Andreasen NC, Tsuang MT. Negative versus positive schizophrenia. NY: Springer-Verlag; 2012.
[10] Park SJ, Lee Y, Oh HK, Lee HE, Lee Y, Ko SY, et al. Oleanolic acid attenuates MK-801-induced schizophrenia-like behaviors in m ice. Neuropharmacol 2014; 86:49-56.
[11] Zavitsanou K, Lim CK, Purves-Tyson T, Karl T, Kassiou M, Banister SD, et al. Effect of maternal immune activation on the kynurenine pathway in preadolescent rat offspring and on MK 801-induced hyperlocomotion in adulthood: amelioration by COX-2 inhibition. Brain Behav Immun 2014; 41:173-181.
[12] Zemanova A, Stankova A, Lobellova V, Svoboda J, Vales K, V lcek K,et al. Visuospatial working memory is impaired in an animal model of schizophrenia induced by acute MK-801: an effect of pretraining. Pharmacol Biochem Behav 2013; 106:117-123.
[13] Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetyl cholinesterase activity. Biochem Pharmacol 1961; 7: 88-95.
[14] He D, Wu H, Wei Y, Liu W, Huang F, Shi H, et al. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/ PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur J Pharmacol 2015; 768:96-107.
[15] Shao BY, Xia Z, Xie Q, Ge XX, Zhang WW, Sun J, et al. Meserine,a novel carbamate AChE inhibitor, ameliorates scopolamine-induced dementia and alleviates amyloidogenesis of APP/PS1 transgenic m ice. CNS Neurosci Ther 2014; 20:165-171.
[16] Shao YF, Wang C, X ie JF, Kong XP, X in L, Dong CY, et al. Neuropeptide S ameliorates olfactory spatial memory impairment induced by scopolam ine and MK 801 through activation of cognate receptor-expressing neurons in the subiculum complex. Brain Struct Funct 2015; 1: 1-10.
[17] Hill XL, Richeri A, Scorza C. Measure of anxiety-related behaviors and hippocampal BDNF levels associated to the amnesic effect induced by MK-801 evaluated in the modified elevated plus-maze in rats. Physiol Behav 2015; 147:359-363.
[18] Cui X, Lefevre E, Turner KM, Coelho CM, Alexander S, Burne TH, et al. MK-801-induced behavioural sensitisation alters dopam ine release and turnover in rat prefrontal cortex. Psychopharmacol 2015; 232:509-517.
[19] Pietraszek M, Michaluk J, Romanska I, Wasik A, Gołembiowska K,Antkiew icz-Michaluk L. 1-Methyl-1,2,3,4-tetrahydroisoquinoline antagonizes a rise in brain dopam ine metabolism, glutamate release in frontal cortex and locomotor hyperactivity produced by MK-801 but not the disruptions of prepulse inhibition, and impairment of working memory in rat. Neurotox Res 2009; 16:390-407.
[20] Carruthers SP, Gurvich CT, Rossell SL. The muscarinic system,cognitionand schizophrenia. Neurosci Biobehav Rev 2015; 55:393-402.
[21] Zhang JX, Li SR, Yao S, Bi QR, Hou JJ, Cai LY, et al. Anticonvulsant and sedative-hypnotic activity screening of pearl and nacre (mother of pearl). J Enthopharmacol 2016;181:229-235.
doi:Document heading 10.1016/j.apjtm.2016.05.019
*Corresponding author:Yasushi Hasegawa, College of Environmental Technology,Muroran Institute of Technology, 27-1 Mizumoto, Muroran 050-8585, Japan.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Predicted pattern of Zika virus infection distribution with reference to rainfall in Thailand
- Effect of partial splenic embolization on the immune function of cirrhosis patients with hypersplenism
- Perfusion of gastrodin in abdom inal aorta for alleviating spinal cord ischem ia reperfusion injury
- Study on the effect and mechanism of the dysfunction of CD4+T cells in the disease process of chronic cardiac failure
- Influence on radiosensitivity of lung glandular cancer cells when ERCC1 gene silenced by targeted siRNA
- Experimental study on the inhibition effect of m iR-106a inhibitor on tumor grow th of ovarian cancer xenografts m ice
