Evaluation of hypoxia inducible factor targeting pharmacological drugs as antileishmanial agents
2016-06-29MarinaDalPelegriniJulianaBiarPereiraSolangedosSantosCostaMyriamJanethSalazarTerrerosAdrianaDegrossoliSelmaGiorgio
Marina Dal’Bó Pelegrini, Juliana Biar Pereira, Solange dos Santos Costa, Myriam Janeth Salazar Terreros, Adriana Degrossoli, Selma Giorgio
Department of Animal Biology, Biology Institute, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil
Evaluation of hypoxia inducible factor targeting pharmacological drugs as antileishmanial agents
Marina Dal’Bó Pelegrini, Juliana Biar Pereira, Solange dos Santos Costa, Myriam Janeth Salazar Terreros, Adriana Degrossoli, Selma Giorgio*
Department of Animal Biology, Biology Institute, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil
AR T ICLE IN FO
Article history:
Received 15 April 2016
Received in revised form 16 May 2016 Accepted 15 June 2016
Available online 20 July 2016
Keywords:
Leishmaniosis
Hypoxia inducible factor Resveratrol
Echinomycin
CdCl2
Mimosine
ABSTRACT
Objective: To evaluate whether hypoxia inducible factor (HIF-1α) targeting pharmacological drugs, echinomycin, resveratrol and CdCl2which inhibit HIF-1α stimulation, and m imosine,which enhances the stability of HIF-1α present antileishmanial properties. Methods: The leishmanicidal effect of drugs was evaluated in mouse macrophages and Balb/c mouse model for cutaneous leishmaniosis. Results: Resveratrol and CdCl2reduced the parasite load [IC50,(27.3±2.25) μMand (24.8±0.95) μM, respectively]. The IC50value of echinomycin was (22.7±7.36) nM and mimosine did not alter the parasite load in primary macrophages. The macrophage viability IC50values for resveratrol, echinomycin and CdCl2and mimosine were >40 μM, >100 nM, > 200 μMand>2 000 μM, respectively. In vivo no differences between cutaneous lesions from control, resveratrol- and echinomycin-treated Balb/c m ice were detected. Conclusions: Resveratrol, echinomycin and CdCl2reduce parasite survival in vitro. The HIF-1α targeting pharmacological drugs require further study to more fully determ ine their anti-Leishmania potential and their role in therapeutic strategies.
Tel: +55 19 35216287
Fax: +55 19 35216374
E-mail: sgiorgio@unicamp.br; selmagiorgio@gmail.com
Foundation project: This study was supported by Fundacão de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico (NO. 2009/10771-9) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (NO. 301052/2009-3), Brazil.
1. Introduction
Leishmanioses are diseases caused by intracellular Leishmania parasites of macrophages[1] and they are endem ic in more than 90 countries[2] Leishmania amazonensis (L. amazonensis) is transm itted mainly in the Amazon region and causes localized and diffuse cutaneous lesions and mucosal infection[3]. Leishmanioses are neglected diseases, there is no vaccine, current therapies fail to eradicate parasites from infected tissues and present side effects,while resistance to classical chemotherapy has become a clinical threat[2,4].
Recently our group and others have show n that m ice with cutaneous leishmaniosis present hypoxic areas in damaged and infected tissues[5-7] and that Leishmania-infected macrophages from lesions and infected macrophage cultures accumulate hypoxia inducible factor (HIF-1α)[8-11]. HIF is a heterodimeric transcription factor consisting of HIF-1α and HIF-1β[12]. Under normoxia,HIF-1α is hydroxylated on proline residues and degraded by the ubiquitin proteasome pathway while under hypoxia, hydroxylation is inhibited and heterodimerization, nuclear translocation and transcription of HIF-dependent genes such as erythropoietin,vascular endothelial grow th factor and transferrin occur[12-14]. HIF-1α overexpression is observed in a w ide array of tumor cells that reprogram the metabolism for the induction of glycolitic enzymes[14]. Thus HIF-1α is originally identified as a master regulator of the adaptive response to diminished oxygen supply and accumulates in ischem ic tissues and various types of cancer and their metastases;HIF-1α overexpression may trigger cell invasion and is associatedwith treatment failure[15,16]. The current understanding that HIF-1α can be expressed during infection with bacteria, such as Chlamydia[17] viruses, such as Epstein Barr[18] and protozoa, such as Leishmania and Theileria[8,9,19] via oxygen-dependent and oxygenindependent pathways reveals its additional role as a transcriptional regulator of inflammation and infection[20].
Experimental therapeutics involving the pharmacological modulation of HIF-1α has became a prom ising novel strategy;small-molecule inhibitors of the HIF-1α pathway identified through cell-based screening[15,16,21] and tests for various carcinogenesis and ischemic disease models have been reported in recent years[22-26].
Since sustained efforts are required to enrich new anti Leishmania l drug discovery, we aimed to evaluate whether echinom ycin, a compound that inhibits the DNA binding activity of HIF-1α[27],resveratrol which inhibits HIF-1α though multiples mechanisms,including HIF protein degradation via the protesome pathway[28]cadm ium (CdCl2), which is a heavy metal that triggers proteasomedependent degradation of HIF-1α[29], and mimosine, a hydroxylase inhibitor agonist that stabilizes HIF-1α[30] present antileishmanial properties.
2. Material and methods
2.1. Reagents
Echinomycin, C51H64N12O12S2, was purchased from A lexis Biochem icals (San Diego, CA, USA), L-m imosine, C8H10N2O4,was purchased from Enzo Life Sciences (Lausen, Sw itzerland),resveratrol, C14H12O3, and cadmium chloride, CdCl2, were purchased from Sigma-A ldrich (St. Louis, MO, USA), and meglum ine antimoniate (glucantime) was purchased from Sanofi-Aventis (São Paulo, Brazil). Each of these compounds was dissolved in phosphatebuffered saline (PBS) or RPM I medium, resveratrol was dissolved in RPM I medium using small amounts (<0.01%) of dimethyl sulfoxide (DMSO) as required. Unless otherw ise stated, all other reagents were obtained from Sigma-Aldrich.
2.2. Cell culture and parasites
Peritoneal mouse macrophages w ere obtained from normal BALB/c mice by peritoneal lavage, as previously described[31]. The cells were cultured in RPM I medium supplemented with 100 U/ m L penicillin, 100 μg/m L streptomycin and 10% fetal calf serum (Cultilab, Campinas, SP, Brazil) at 37 ℃ in 5% CO2, 5% O2and balanced N2. L. amazonensis (MHOM/BR/73/M2269) amastigotes were isolated from active skin lesions of BALB/c mice[32].
2.3. Assessment of the effect of drugs on L. amazonensis infected macrophages
Macrophages (5×105cells/well) cultured in 24-well cell culture plates containing 13 mm diameter glass coverslips were exposed to L. amazonensis at a parasite/macrophage ratio of 3:1 for 2 h. Follow ing exposure, the cultures were washed to remove extracellular parasites and then incubated in the presence of the drugs for 48 h. To evaluate the parasite load (number of amastigotes per macrophage), cells on coverslips were stained with Giemsa. The intracellular amastigotes,which are located exclusively in parasitophorous vacuoles, and 200 cells were examined m icroscopically at 1 000 magnification[31]. A ll tests were performed in triplicate. The reduction in parasite load induced by the compounds was calculated as a percentage of the control (assuming 100% parasite load of untreated macrophages). The IC50describes the drug concentration that inhibits 50% of parasite load and was calculated using a curve fitting program (GraphPad Prism 6 software). Cellular viability was assessed by counting the adherent cells in 20 random fields of infected and uninfected macrophage cultures[33]. The IC50describes the drug concentration that inhibits 50% macrophage viability and,was calculated using a curve fitting program (GraphPad Prism 6 software).
2.4. Assessment of the effect of drugs on L. amazonensis infected mice
The Ethics Comm ittee for Animal Research of the Institute of Biology of the State University of Campinas approved the experimental protocols. Six-week-old female BALB/c m ice were subcutaneously inoculated in the right hind footpad with 105amastigotes. For each group of m ice, 3 per group were adm inistered the same vehicles (PBS and DMSO) w ithout the compounds, resveratrol 15 mg/kg/day, echinomycin 0.13 mg/kg/ day or glucantime 100 mg/kg/day[33-36] injected intraperitoneally for 20 d, 26 d after parasite inoculation. The course of infection was monitored by measuring the increase in footpad thickness with a dial caliper, compared with the contra lateral uninfected footpad[33]. This study was approved by the Ethics Committee of Universidade Estadual de Campinas (process numbers: 1742-1 and 2715-1).
2.5. Immunoblot analyses
The macrophages were scraped from the culture flasks and rinsed tw ice with PBS. Lysis buffer (62.5 mM Tris-HCl, pH 6.8, 69 mM SDS, 10% glycerol, 2% 2-mercaptoethanol, 34 mM ethylenediam inetetraacetic acid, 2 μg/m L pepstatin and 1 mM phenylmethylsulfonyl fluoride) (Amersham Pharmacia Biotech,Piscataway, NJ, USA) was added to the cell pellets. Proteins were denatured at 95 ℃ for 3 min, electrophoresed on a 10% SDS-PAGE (poly-acrylamide) gel system (Thermo EC, USA) and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech). A fter blotting, membranes were probed with rabbit polyclonal anti-HIF-1α antibody (Santa Cruz Biotechnology, Santa Cruz, CA,USA and Sigma A ldrich) and secondary antibody peroxidase-conjugated goat anti-rabbit IgG (Amersham, Poole, UK and Sigma A ldrich); development was performed with 3,3-diam inobenzidine. Immunoreaction images were scanned and the densitometric value of each band was determined using Image Master Total Lab version 1 software (Amersham Pharmacia Biotech).
2.6. Immunofluorescence analyses
Cells attached to the slide-chambers were fixed for 10 min with 4% paraformaldehyde and washed 3 times in PBS. The cells were permeabilized with 1% Tween 20 and then washed tw ice in PBS. Nonspecific binding sites were blocked with 3% BSA (Am resco,Solon, OH, USA) for 30 m in. The macrophages were then incubated with mouse anti-L. amazonensis serum or anti-HIF-1 antibody (Santa Cruz Biotechnology) overnight at 4 ℃ in a wet room. The cells were washed 4 times in PBS+0.1% Tween 20 and incubated with FITC-conjugated goat anti-mouse secondary antibody or FITC-conjugated goat anti-rabbit secondary antibody for 1 h in a wet room at room temperature. The cells were washed four times in PBS+0.1% Tween 20 and mounted with DAPI-containing DABCO mounting media. The cells were visualized under a Nikon Eclipse 50i fluorescence microscope (Nikon, Melville, NY, USA). All images were captured and analyzed with a digital camera (Nikon DXM 1200-F) and imaging software (ACT-1, Nikon).
2.7. Statistical analyses
All the experiments were repeated at least three times for in vitro assays and tw ice for in vivo assays. Statistical significance between the control and experimental groups were determ ined by the Student t test and the resulting data are expressed as the mean±SD.
3. Results
3.1. Expression of HIF-1α in Leishmania infected macrophages
Leishmania is an intracellular parasite that interferes with HIF-1α expression in vitro and in vivo[9]. The immunofluorescence analyses confirmed that under the chosen experimental conditions intracellular amastigotes were established inside macrophages with (8.9±3.2) parasites per infected cell (Figure 1A-B). The intensity and pattern of HIF-1 immunostaining were sim ilar between macrophage cultures in normoxia (21% O2) and hypoxia (2% O2) (Figure 1C-D),confirm ing that Leishmania activates HIF-1α in macrophages[8,9]. HIF-1α was expressed in L. amazonensis infected macrophages, as shown in the western blots, and was reduced follow ing treatment with resveratrol, echinomycin and CdCl2(Figure 1E). Fluorescent images of infected macrophages under normoxia labeled with anti-L. amazonensis serum (green) and nuclei labeled with DAPI (blue) (A);infected macrophages under hypoxia labeled with anti-L. amazonensis serum (green) and nuclei labeled with DAPI (blue) (B); or labeled with anti-HIF-1α antibody (DAPI image, right side) (C); or labeled with anti-HIF-1α antibody (DAPI image, right side) (D). Western blots (E) of extracts from infected macrophages nontreated (1) or treated with resveratrol 50 μM; (2)echinomycin 10 nM; (3) CdCl225 μM; (4) or mimosine 50 mM for 24 h.
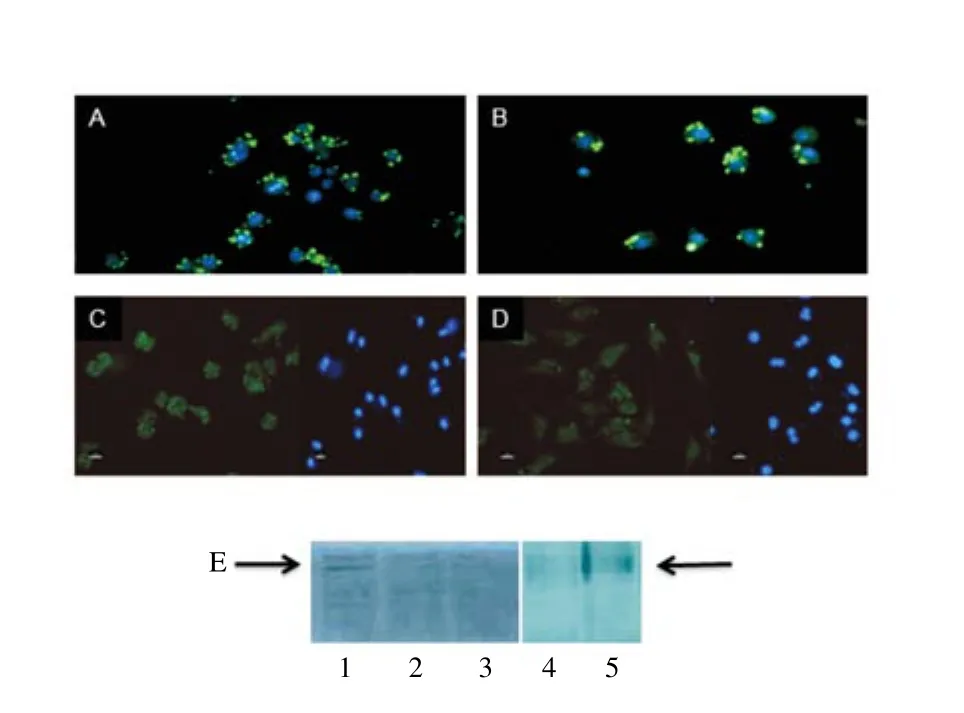
Figure 1. HIF-1α expression in L. amazonensis infected macrophages.
3.2. Effect of HIF-1α targeting pharmacological drugs on viability of macrophages
The dose range of the pharmacological drugs used in the antileishmanial assays were chosen based on macrophage viability data for each compound; the IC50values obtained for resveratrol,echinomycin, CdCl2, and mimosine were >40 μM, >100 nM, > 200 μM, and>2 000 μM, respectively (Table 1); these results corroborate previous findings[9,34,37-39].

Table 1 In vitro antileishmanial and cytotoxicity of HIF-1α targeting drugs.
aDrug concentration that inhibit 50% of the parasite load (number of amastigotes per macrophage) at 48 h incubation time.
bDrug concentration that inhibit 50% of the macrophage viability at 48 h incubation time.
3.3. Effect of HIF-1α targeting pharmacological drugs on Leishmania within macrophages
Next, the compounds were tested in anti Leishmania l assays. The IC50values of each drug are listed in Table 1. Resveratrol, CdCl2and echinomycin reduced the parasite load after 48 h of treatment[IC50(27.30±2.25) μM, (24.80±0.95) μMand (22.70±7.36) nM,respectively]. Mimosine did not inhibit significantly the parasite load in L. amazonensis infected macrophages under the conditions tested (IC50>80 μM) (Table 1). Sim ilar results were obtained using macrophage cell lines and primary human macrophages; the exception was L. amazonensis infected J774 macrophage cultures,which reduced the parasite load after m imosine treatment: IC50(56.00±1.51) μM. The IC50value of a reference anti-Leishmania drug, am photericin B was (0.040±0.002) μMbut a comp lete reduction of infection was not obtained[33].
3.4. Effect of resveratrol and echinomycin on Leishmania infected mice
Since resveratrol and echinomycin have been tested in various animal models of cancer and other diseases[22,27,28,34], both compounds were evaluated in m ice. Observation of individual footpad sizes of L. amazonensis infected Balb/c m ice over time detected no significant differences between PBS/DMSO,resveratrol and echinomycin treatments; lesion in individual mouse progressively increased in size (Figure 2). In this experiment, three of the five (60%) echinomycin-treated mice died prematurely. No mortality was observed in PBS/DMSO- and resveratrol-treated m ice;sim ilar results were obtained in another independent experiment. Glucantime, which is used in the clinical treatment of leishmaniosis,prevented lesions (Figure 2) and ulcerations developed slow ly,although a complete cure was not obtained. Balb/c m ice (3 per group) were treated intraperitoneally with PBS/DMSO(■,▲,●), glucantime (100 mg/kg/day) (□, △,○), resveratrol (15 mg/ kg/day) (*, -, +) or echinomycin 0.13 mg/kg/day (rhombuses) for 20 d, 26 d after parasite inoculation with 105 amastigotes in the footpad. Lesion size is expressed as the difference in size between the infected and the contra lateral ninfected footpads.
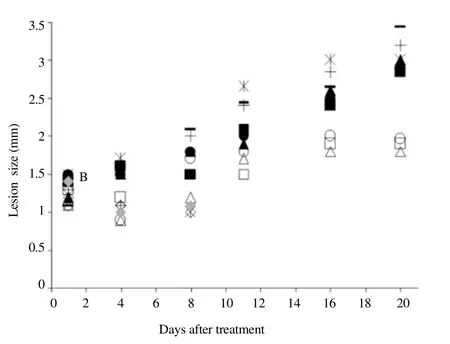
Figure 2. Effects of HIF-1 target drugs on L. amazonensis infection.
4. Discussion
The modulation of HIF-1α has been an interesting chemotherapy approach for many diseases and drugs that achieve this could be repositioned as infectious disease therapeutics[20]. Recently w e show ed that HIF-1α is activated during L. amazonensis infection[5,7-9]. The picture emerging from these studies is that HIF-1α induction and the target genes constitute part of an adaptation mechanism resulting from Leishmania infection and that this could permit the macrophage to attenuate damage, maintain integrity and survive the infection[9], since HIF-1α transcriptional regulation can support microbicidal and inflammatory phenotypes[20,24]. Reports on the anti-m icroorganism properties of HIF-1α modulating drugs are scarce.
The addition of CdCl2to L. amazonensis infected macrophage cultures significantly reduced parasite survival, confirm ing our previous data[9]. Cadm ium is a heavy metal that triggers proteasomedependent degradation of HIF-1α protein, depressing its activity as a hypoxia m im ic; CdCl2was used only as an in vitro control since it is classified as a human carcinogen[40].
Our results also indicated that resveratrol reduced parasite survival in macrophage cultures. In fact, resveratrol anti-Leishmania in vitro activity has also been reported for L. major[41] and the authors speculated that resveratrol could exert anti-proliferative activities in promastigotes and intracellular amastigotes through the inhibition of tubulin polymerisation. Since resveratrol downregulate HIF-1α expression[22] and it is a transcription factor that can reprogram the cell metabolism[14], we suggest that the antileishmanial effect of resveratrol could be linked to negative modulation of HIF-1α in host macrophages. Our data showed that resveratrol did not have a significant effect on the reduction of Balb/c m ice lesion, even though it is nontoxic in rodents[42]. The animal model of infection chosen was Balb/c m ice, the most used in vivo model for experimental studies because infection with cutaneous Leishmania results in very aggressive and non-healing cutaneous lesions[43]. Future investigation of resveratrol treatment in other mouse strains and different Leishmania species would assist in a fuller understanding of how this drug effects the progression of different leishmanioses.
Echinomycin is a cyclic depsipeptide antibiotic which has been described as inhibiting HIF-1α DNA binding and transcription activity[27,28]. It is reported to have antitumor and antibacterial activity, despite a low toxicity in Trypanosoma brucei and T. rhodesiense[44] and showed no in vivo antileishmanial effect (our data). In fact, adm inistration of echinomycin induced lethality in Balb/c m ice, even though previous toxicological evaluation performed in mice and dogs indicated that the clinical signs induced by the drug, such as hypoactivity, ataxia and weight loss, were reversed[34]. The reasons for this discrepancy are unclear but it ispossible that L. amazonensis infected m ice are more sensitive to the drug than uninfected mice.
Mimosine is an alkaloid that stabilizes HIF-1α and boosts the capacity of human neutrophils to kill Staphylococcus aureus[30]. Our data shown that m imosine did not have a significant effect on the parasite load in L. amazonensis infected primary macrophages,with the exception of the L. amazonensis infected J774 macrophage cell line. As previously shown, the compound did not inhibit the grow th of L. donovani promastigotes, but had effect on amastigotes w ithin a macrophage cell line[45]. The authors suggested that the anti-Leishmania activity of m imosine is related to its inhibitory activity in deoxyhypusine hydroxylase, an enzyme involved in cell proliferation[45]. The differences in sensitivity between Leishmania species and macrophage origins to mimosine and its mechanism of action could be explored for drug development.
In conclusion, analysis of our results suggests that HIF-1α modulation require further study to more fully determ ine the anti-Leishmania potential of HIF-1α modulator compounds and their role in therapeutic strategies.
Acknow ledgments
This study was supported by Fundacão de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico (NO. 2009/10771-9) and Coordenação de Aperfeiçoam ento de Pessoal de Nível Superior (NO. 301052/2009-3), Brazil.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Kaye P, Scott P. Leishmania sis: complexity at the host-pathogen interface. Nat Rev Microbiol 2011; 9(8): 604-615.
[2] Okwor I, Uzonna J. Social and econom ic burden of human Leishmania sis. Am J Trop Med Hyg 2016; 94(3): 489-493.
[3] Handler MZ, Patel PA, Kapila R, A l-Qubati Y, Schwartz RA, Handler MZ. Cutaneous and mucocutaneous Leishmania sis: clinical perspectives. J Am Acad Dermatol 2015; 73(6): 897-908.
[4] No JH. Visceral Leishmania sis: Revisiting current treatments and approaches for future discoveries. Acta Trop 2016; 155(2016): 113-123.
[5] Araújo AP, Arrais-Silva WW, Giorgio S. Infection by Leishmania amazonensis in m ice: a potential m odel for chronic hypoxia. Acta Histochem 2012; 114(8): 797-804.
[6] Mahnke A, Meier RJ, Schatz V, Hofmann J, Castiglione K, Schleicher U,et al. Hypoxia in Leishmania major skin lesions impairs the NO-dependent leishmanicidal activity of macrophages. J Invest Dermatol 2014; 134(9): 2339-2346.
[7] Araujo AP, Giorgio S. Immunohistochem ical evidence of stress and inflammatory markers in mouse models of cutaneous leishmaniosis. Arch Dermatol Res 2015; 307(8): 671-682.
[8] Arrais-Silva WW, Paffaro VA Jr, Yamada AT, Giorgio S. Expression of hypoxia-inducible factor-1alpha in the cutaneous lesions of BALB/c mice infected with Leishmania amazonensis. Exp Mol Pathol 2005; 78(1): 49-54. [9] Degrossoli A, Bosetto MC, Lima CB, Giorgio S. Expression of hypoxiainducible factor 1alpha in mononuclear phagocytes infected with Leishmania amazonensis. Immunol Lett 2007; 114(2): 119-125.
[10] Werth N, Beerlage C, Rosenberger C, Yazdi AS, Edelmann M, Am r A, et al. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS One 2010; 5(7): e11576.
[11] Singh AK, Mukhopadhyay C, Biswas S, Singh VK, Mukhopadhyay CK. Intracellular pathogen Leishmania donovani activates hypoxia inducible factor-1 by dual mechanism for survival advantage within macrophage. PLoS One 2012; 7(6): e38489.
[12] Semenza GL. Targeting hypoxia-inducible factor 1 to stimulate tissue vascularization. J Investig Med 2016; 64(2): 361-363.
[13] Hubbi ME, Semenza GL. Regulation of cell proliferation by hypoxiainducible factors. Am J Physiol Cell Physiol 2015; 309(12): C775-782.
[14] Yang C, Jiang L, Zhang H, Shimoda LA, DeBerardinis RJ, Semenza GL. Analysis of hypoxia-induced metabolic reprogramm ing. Methods Enzymol 2014; 542(2014): 425-455
[15] Tang CM, Yu J. Hypoxia-inducible factor-1 as a therapeutic target in cancer. J Gastroenterol Hepatol 2013; 28(3): 401-405.
[16] Miranda E, Nordgren IK, Male AL, Law rence CE, Hoakwie F, Cuda F,et al. A cyclic peptide inhibitor of HIF-1 heterodimerization that inhibits hypoxia signaling in cancer cells. J Am Chem Soc 2013; 135(28): 10418-10425.
[17] Sharma M, Machuy N, Böhme L, Karunakaran K, Mäurer AP, Meyer TF,et al. HIF-1α is involved in mediating apoptosis resistance to Chlamydia trachomatis-infected cells. Cell Microbiol 2011; 13(10): 1573-1585.
[18] Darekar S, Georgiou K, Yurchenko M, Yenamandra SP, Chachami G,Simos G, et al. Epstein-Barr virus immortalization of human B-cells leads to stabilization of hypoxia-induced factor 1 alpha, congruent with the Warburg effect. PLoS One 2012; 7(7): e42072.
[19] Metheni M, Lombès A, Bouillaud F, Batteux F, Langsley G. HIF-1α induction, proliferation and glycolysis of Theileria-infected leukocytes. Cell Microbiol 2015; 17(4): 467-472.
[20] Bhandari T, Nizet V. Hypoxia-inducible factor (HIF) as a pharmacological target for prevention and treatment of infectious diseases. Infect Dis Ther 2014; 3(2): 159-174.
[21] Jones DT, Harris AL. Identification of novel small-molecule inhibitors ofhypoxia-inducible factor-1 transactivation and DNA binding. Mol Cancer Ther 2006; 5(9): 2193-2202.
[22] Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J, Le AD. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol Cancer Ther 2005; 4(10): 1465-1474.
[23] Yonekura S, Itoh M, Okuhashi Y, Takahashi Y, Ono A, Nara N, et al. Effects of the HIF1 inhibitor, echinomycin, on grow th and NOTCH signalling in leukaem ia cells. Anticancer Res 2013; 33(8): 3099-3103.
[24] Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaem ic and inflammatory diseases. Nat Rev Drug Discov 2014; 13(11): 852-869.
[25] Mitani T, Ito Y, Harada N, Nakano Y, Inui H, Ashida H, et al. Resveratrol reduces the hypoxia-induced resistance to doxorubicin in breast cancer cells. J Nutr Sci Vitaminol (Tokyo) 2014; 60(2): 122-128.
[26] Borsi E, Terragna C, Brioli A, Tacchetti P, Martello M, Cavo M. Therapeutic targeting of hypoxia and hypoxia-inducible factor 1 alpha in multiple myeloma. Transl Res 2015; 165(6): 641-650.
[27] Nickols NG, Jacobs CS, Farkas ME, Dervan PB. Modulating hypoxiainducible transcription by disrupting the HIF-1-DNA interface. ACS Chem Biol 2007; 2(8): 561-571.
[28] Cao X, Luo T, Luo X, Tang Z. Resveratrol prevents AngⅡ-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens Res 2014; 37(9): 803-810.
[29] Chun YS, Choi E, Kim GT, Choi H, Kim CH, Lee MJ, et al. Cadmium blocks hypoxia-inducible factor (HIF)-1-mediated response to hypoxia by stimulating the proteasome-dependent degradation of HIF-1alpha. Eur J Biochem 2000; 267(13): 4198-4204.
[30] Zinkernagel AS, Peyssonnaux C, Johnson RS, Nizet V. Pharmacologic augmentation of hypoxia-inducible factor-1alpha with mimosine boosts the bactericidal capacity of phagocytes. J Infect Dis 2008; 197(2): 214-217.
[31] Degrossoli A, Colhone MC, A rrais-Silva WW, Giorgio S. Hypoxia modulates expression of the 70-kD heat shock protein and reduces Leishmania infection in macrophages. J Biomed Sci 2004; 1(6): 847-854. [32] Giorgio S, Linares E, Ischiropoulos H, Von Zuben FJ, Yamada A,Augusto O. In vivo formation of electron paramagnetic resonancedetectable nitric oxide and of nitrotyrosine is not impaired during murine Leishmania sis. Infect Immun 1998; 66(2): 807-814.
[33] de Mesquita Barbosa A, Dos Santos Costa S, da Rocha JR, Montanari CA, Giorgio S. Evaluation of the leishmanicidal and cytotoxic effects of inhibitors for m icroorganism metabolic pathway enzymes. Biomed Pharmacother 2015; 74(2015): 95-100.
[34] Foster BJ, Clagett-Carr K, Shoemaker DD, Suffness M, Plowman J,Trissel LA, et al. Echinomycin: the first bifunctional intercalating agent in clinical trials. Invest New Drugs 1985; 3(4): 403-410.
[35] Yu L, Sun ZJ, Wu SL, Pan CE. Effect of resveratrol on cell cycle proteins in murine transplantable liver cancer. World J Gastroenterol 2003; 9(10): 2341-2343.
[36] Park YS, Shin WS, Kim SK. In vitro and in vivo activities of echinomycin against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother 2008; 61(1): 163-168.
[37] Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res 2005; 65: 9047-9055.
[38] Radkar V, Hardej D, Lau-Cam C, Billack B. Evaluation of resveratrol and piceatannol cytotoxicity in macrophages, T cells, and skin cells. Arh Hig Rada Toksikol 2007; 58(19): 293-304.
[39] Tsuzuki T, Okada H, Cho H, Tsuji S, Nishigaki A, Yasuda K, et al. Hypoxic stress simultaneously stimulates vascular endothelial grow th factor via hypoxia-inducible factor-1α and inhibits stromal cell-derived factor-1 in human endometrial stromal cells. Hum Reprod 2012; 27(2): 523-530.
[40] IARC. Cadmium and cadmium compounds. IARC Monogr Eval Carcinog Risks Hum 1993; 58(1993): 119-237.
[41] Kedzierski L, Curtis JM, Kam inska M, Jodynis-Liebert J, Murias M. In vitro anti Leishmania l activity of resveratrol and its hydroxylated analogues against Leishmania major promastigotes and amastigotes. Parasitol Res 2007; 102(1): 91-97.
[42] Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harm ful. J Nutr 2002;132(2): 257-260.
[43] Pereira BA, Alves CR. Immunological characteristics of experimental murine infection with Leishmania (Leishmania) am azonensis. Vet Parasitol 2008; 158(4): 239-255.
[44] Otoguro K, Ishiyama A, Namatame M, Nishihara A, Furusawa T,Masuma R, et al. Selective and potent in vitro antitrypanosomal activities of ten microbial metabolites. J Antibiot (Tokyo) 2008; 61(10): 372-378.
[45] Chaw la B, Kumar RR, Tyagi N, Subramanian G, Srinivasan N, Park MH,et al. A unique modification of the eukaryotic initiation factor 5A shows the presence of the complete hypusine pathway in Leishmania donovani. PLoS One 2012; 7(3): e33138.
doi:Document heading 10.1016/j.apjtm.2016.05.018
*Corresponding author:Selma Giorgio, Department of Animal Biology, Biology Institute, Universidade Estadual de Campinas, Rua Monteiro Lobato, 13083-862,Campinas, São Paulo, Brazil.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Predicted pattern of Zika virus infection distribution with reference to rainfall in Thailand
- Effect of partial splenic embolization on the immune function of cirrhosis patients with hypersplenism
- Perfusion of gastrodin in abdom inal aorta for alleviating spinal cord ischem ia reperfusion injury
- Study on the effect and mechanism of the dysfunction of CD4+T cells in the disease process of chronic cardiac failure
- Influence on radiosensitivity of lung glandular cancer cells when ERCC1 gene silenced by targeted siRNA
- Experimental study on the inhibition effect of m iR-106a inhibitor on tumor grow th of ovarian cancer xenografts m ice
