Hepatoprotective properties of oleanolic and ursolic acids in antitubercular drug-induced liver damage
2016-06-29GabrielGutirrezRebolledoGeorginaSiordiaReyesMarianaMeckesFischerAdelinaJimnezArellanes
Gabriel A. Gutiérrez-Rebolledo, Georgina A. Siordia-Reyes, Mariana Meckes-Fischer,Adelina Jiménez-Arellanes*
1Unidad de Investigación Médica en Farmacología (UIMF), UMAE Hospital de Especialidades, Centro Médico Nacional-Siglo XXI (CMN-SXXI),Instituto Mexicano del Seguro Social (IMSS), México City, Mexico2Servicio de Patología, UMAE Hospital de Pediatría, CMN-SXXI, IMSS, Mexico City, Mexico3Centro de Diagnóstico en Metabolismo Energético y Medicina Mitocondrial, A.C. Mexico City, Mexico
Hepatoprotective properties of oleanolic and ursolic acids in antitubercular drug-induced liver damage
Gabriel A. Gutiérrez-Rebolledo1, Georgina A. Siordia-Reyes2, Mariana Meckes-Fischer3,Adelina Jiménez-Arellanes1*
1Unidad de Investigación Médica en Farmacología (UIMF), UMAE Hospital de Especialidades, Centro Médico Nacional-Siglo XXI (CMN-SXXI),Instituto Mexicano del Seguro Social (IMSS), México City, Mexico
2Servicio de Patología, UMAE Hospital de Pediatría, CMN-SXXI, IMSS, Mexico City, Mexico
3Centro de Diagnóstico en Metabolismo Energético y Medicina Mitocondrial, A.C. Mexico City, Mexico
AR T ICLE IN FO
Article history:
Received 15 April 2016
Received in revised form 16 May 2016 Accepted 15 June 2016
Available online 20 July 2016
Keywords:
Ursolic acid
Oleanolic acid
Hepatoprotector effect Antitubercular drugs Triterpenes
ABSTRACT
Objective: To estimate to what extent the m ixture of ursolic acid and oleanolic acid, in addition to the antitubercular standard regime, affects the hepatotoxicity profile. Methods: Liver injury was induced in male BALB/c mice by adm inistering, per os and daily for 11 weeks, a combination of anti-Tubercular (anti-TB) agents Rifampicin (10 mg/kg), Isoniazid (10 mg/kg),and Pyrazinamide (30 mg/kg). The ursolic acid and oleanolic acid mixture at doses of 100 or 200 μg/mouse/day was subcutaneously injected throughout the entire study period (11 weeks). Biochem ical and hematological analysis was supplemented by liver histological exam ination. Resu lts: Animals treated with the m ixture of triterpenic acids exhibited significantly decreased aspartate transam inase and alanine am inotransferase levels and amelioration of the histopathological alterations produced by the anti-TB drugs. Conclusions: The triterpene mixture is able to prevent the steatosis induced by the anti-TB drugs.
Tel: (+52)-55-56276900 (ext. 21367); (+)52-55-6395-0472.
E-mail: adelinajim08@prodigy.net.mx;
Foundation project: This study was partly supported by Grant from the Instituto Mexicano del Seguro Social (NO. FIS/IMSS/PROT/G12/1126; FIS/IMSS/PROT/ G14/1341).
1. Introduction
D rug-induced liver toxicity is a serious potential adverse effect produced by the currently used anti-Tubercular (anti-TB)chemotherapeutic regimen containing Isoniazid (INH), Rifampicin (RIF) and Pyrazinamide (PZA). All of these anti-TB drugs are potentially hepatotoxic, but when adm inistered in combination,their toxic effects are enhanced in a synergistic manner[1,2]. The precise mechanisms of INH and RIF hepatotoxicity are not fully understood, but hepatocyte injury and death are most likely due to
toxic hydrazine derivatives and free radicals that are responsible for oxidative stress, lipid peroxidation, and choline deficiency,leading to the lowering of phospholipid protein synthesis and the consequential alteration in cell wall configuration, reduced glutathione levels and the activation of Cytochrome P4502E1 (CYP2E1)[3,4].
The conversion of monoacetyl hydrazine (AcHz), a metabolite of INH, into a toxic metabolite via cytochrome P450leads to hepatotoxicity. On the other hand, RIF promotes the cytochrome P450enzyme, increasing the production of toxic metabolites from AcHz. The plasma half-life of AcHz is shortened and the compound is quickly converted into its active metabolites. Furthermore, RIF can increase the metabolism of INH to isonicotinic acid, which is hepatotoxic. PZA is responsible for severe hepatohypersensitivity reactions and, in combination with INH and RIF, increases the incidence of hepatotoxicity[4-7].
The search for hepatoprotectors to prevent risk situations in patients with tuberculosis (TB), who require treatment with anti-TB is an issue of grow ing importance. The literature points to the hepatoprotective effects of some synthetic compounds, such as N-acetylcysteine[8], reamberine, remaxol and ademethionine[9]. At the same time, a group of naturally occurring compounds has been reported as potential hepatoprotective agents against the toxic effects of anti-TB, with the effects of silymarin[10-12], curcum in and resveratrol[12-14], observed as remarkable. The search for hepatoprotective agents that avoid antitubercular drug-induced damage is mandatory, and this is reflected in the large number of medicinal plant extracts undergoing validation. The list of the latter is extensive and among these Silybum marianum, the wellresearched plant for the treatment of liver diseases is highlighted. The active components of the species (silybin A and B, isosilybin A and B silybin and other minor compounds) are found in the seed’s extract, denom inated silymarin, which possesses hepatoprotective effects against the toxic action of RIF[15] and which has been proposed as a dietary supplement for patients treated with anti-TB[11]. In a random ized, controlled clinical trial, Curcuma longa and Tinospora cordiflora, in addition to the standard anti-TB regime,very significantly prevented, in terms of incidence, the duration and severity of hepatotoxic episodes in patients with TB[3]. Some medicinal plants extracts are proposed as hepatoprotectors, such as garlic (A llium sativum)[16] and the root extracts from Punica granatum and Cassia auriculata[17,18], Cnidoscolus chayamansa[19],Vitex negundo[20], Hibiscus vitifolius[21], Pisonia aculeate[22], Mikania scandens[23], Moringa oleifera[24], Asteracantha longifolia[25] and others[26].
With regard to the mixture of triterpenes ursolic acid or oleanolic acid (UA/OA), the natural product-of-interest in the on-going study,in vivo anti-TB activity was already demonstrated in an experimental mouse model of progressive pulmonary TB, and a significant reduction of bacterial loads and pneumonia with a higher expression of Interferon gamma (IFN-γ) and Tumor necrosis factor alpha (TNF-α) in the lungs was described. The authors concluded that the antim icrobial activity of the m ixture is concom itant to an immunestimulatory effect[27].
The in vivo antitubercular activity of these natural compounds has been patented[28] and the results obtained here comprise a breakthrough in understanding the simultaneous role of these triterpene acids as both anti-TB and hepatoprotective agents.
On continuing the study of these compounds that offer potential as anti-TB drugs, the hepatoprotective effect was subjected to evaluation in an experimental model of liver damage induced with the commonly used anti-TB first-line drugs RIF/INH/PZA
2. Material and methods
2.1. Chemical compounds
The m ixture of UA/OA was obtained by chem ical fractionation of the methanolic extract of Bouvardia ternifolia aerial parts. The purification procedure was performed follow ing the methodology described previously[29] and was structurally characterized by mass spectra and protonic nuclear magnetic resonance spectrometric data by comparison with those previously reported by this author. The drugs INH, RIF, PZA, ultra-pure olive oil and sodium carboxymethyl cellulose were purchased from Sigma-Aldrich and the kits used for determ ination of blood chem istry parameters were purchased from Randox Co.
2.2. Animals’ treatment
In the present study, male Balb/C mice weighing (25±2) g were used and were obtained from CMN-SXXI Bioterium, Mexico City. The m ice were maintained in pathogen-free housing in plastic cages during a 7 d conditioning period prior to the performance of the experiments, under laboratory conditions [12 h/12 h light/dark cycles; temperature (25±2) ℃; hum idity 45%-55%], with rodent chow food and water ad libitum. The experiments were performed follow ing the Statutes of the International Comm ittee for the Care and Use of Laboratory Animals and Mexican Official Norm (NOM-062-ZOO-1999) revised in 2001. All experimental protocols complied with the Animal Care Comm ittee of the Hospital de Especialidades at the CMN-SXXI, IMSS.
As was previously reported, subcutaneous (s.c.) administration of the UA/OA mixture in models of acute toxicity, median Lethal dose (LD50) was >2 g/kg in mice and rats and sub-acute toxicity (for 28 d)did not cause lethality or alterations in blood chem istry parameters or to histological changes in liver and kidney[29]. Taking this data into account, doses of 100 and 200 μg (s.c.) were adm inistered daily to the mice.
Hepatotoxic damage was induced with a combination of anti-TB drugs composed of INH (10 mg/kg), RIF (10 mg/kg) and PZA (30 mg/kg) dissolved in Isotonic saline solution (ISS), which was intragastrically (i.g.) adm inistered daily for 11 weeks. Doses of anti-TB drugs were the same as those reported for the in vivo assay[27]. The UA/OA mixture was dissolved in ultra-pure olive oil (Sigma) and was adm inistered s.c. together with the anti-TB drugs for 11 weeks. A special #2 cannula was utilized to facilitate i.g. adm inistration of anti-TB drugs. Assessment of the hepatoprotective potential of UA/OA was guided by the methodology reported in references 21 and 48, with some modifications.
Healthy m ice were random ly assigned to six groups of eight animals each as follow s: GroupⅠ(negative control) with vehicle (ISS i.g. and ultra-pure olive oil s.c.); Group Ⅱ (positive control)w ere treated with anti-TB drugs (RIF/INH/PZA) adm inistered i.g. via; Groups Ⅲ and Ⅳ received UA/OA in doses of 100 and 200 μg/mouse/day by s.c. via, respectively; Group V animals were administered with anti-TB drugs and UA/OA at a dose of 100 μg/ mouse/daily and Group Ⅵ received anti-TB drugs and UA/OA at a dose of 200 μg/mouse/day.
During the experimental period, the animals were observed for signs of morbidity and mortality. The weight of all m ice wasrecorded from day zero and every 7 d thereafter throughout the experiment until week 11. Animals that died during the experimental period were dissected and the organs were obtained, weighed and observed macroscopically.
2.3. Hematology and serum biochemistry
After the last treatment, the animals were left to fast for 12 h and blood samples were collected by means of retro-orbital sinus puncture w ithout the use of anesthesia. Tubes with EDTA were used for hematological testing and w ithout anticoagulant for biochemical analysis. Hematological analysis was performed using a Beckman Coulter Cell Counter and the follow ing parameters were determined: Total Red blood cell count; Hemoglobin, Hematocrit;Mean corpuscular vo lume; Mean corpuscu lar hemoglobin concentration; Mean corpuscular hemoglobin; Total platelet count,Total White blood cell count and a white-cell differential study was also performed to evaluate lymphocytes, segmented neutrophils,eosinophils, monocytes, and basophils.
Biochem ical parameters with the commercially RANDOX kits were obtained with Selectra Analyser (Vitalab 2 model) automated equipment. In accordance with the manufacturer’s instructions, the follow ing parameters were determ ined: glucose; creatinine; urea;the liver marker enzymes Serum glutam ic oxaloacetic transam inase or Aspartate am inotransferase (AST), Serum glutam ic pyruvic transam inase or A lanine am inotransferase (ALT) and A lkaline phosphatase (ALP).
2.4. Histopathological evaluation
The m ice were sacrificed by cervical dislocation and necropsy was carried out soon after death for macroscopic examination of liver, kidneys and spleen. Tissue biopsies from these organs were fixed in 10% formalin, processed and embedded in paraffin. The paraffin block was cut into (4-5) μm slices with a rotary microtome and stained with Hematoxylin and Eosin (H&E), follow ing the procedure described previously in 27. The samples were exam ined under a light microscope with particular attention paid to organs exhibiting: microscopic findings in liver such as steatosis, necrosis,m icroabscesses, fibrosis, portal linfoide inflamm ation and centrolobulillar hydropic degeneration; in spleen was hematopoiesis and kidney tubular necrosis and hydropic tubular changes.
2.5. Statistical analysis
SigmaPlot ver. 12.0 software (20112012) was employed for analysis of results and graphic elaboration. Data are presented as mean and Standard error of the mean±SEM. Values of body weight (BW)gain values were subm itted to a bifactorial Analysis of variance (ANOVA) and to a post hoc Student-Newman-Keuls (SNK) test. Results of P<0.05 were considered significant. For hematological and biochem ical data analysis, one-way ANOVA was employed with a post hoc SNK test, in which P<0.05 was considered significant. Finally, relative organ weights in treated mice and Hematocrit data and the Kruskal-Wallis test (ANOVA on ranks) were carried out with a post hoc SNK test, in which relevant outcomes were those with a value of P<0.05.
3. Results
3.1. Body weight of the animals
In the course of 11 experimental weeks, a gradual increase in BW gain in all groups was observed from day 28; however, up the end of the study and in animals treated with the anti-TB (GroupⅡ),this parameter was significantly lower than that presented by the remaining groups, determining one half of those values registered for control-group animals, which received only the vehicle (3.54 g vs 6.00 g). BW gain in Groups Ⅲ and Ⅳ m ice, which were injected with UA/OA in 100 and 200 μg doses, exhibited behavior close to that of the control-group animals. With respect to Group Ⅴ and Ⅵm ice treated with UA/OA at 100 and 200 μg doses in addition to anti-TB drugs, BW gain with the 200 μg dose demonstrated slightly lower values than the controls (6.08 g), although at the end of the study, BW gain was close to that of the control group (6.33 and 5.95 g, respectively) (Figure 1).
Data are mean±SEM. Bifactorial statistical ANOVA of repeated measures,post hoc SNK test (P<0.05);avs. Vehicles;bvs. Anti-TB;cvs. 100 μg UA/OA;dvs. 200 μg UA/OA;evs anti-TB + 100 μg UA/OA;fvs. Anti-TB + 200 μg UA/ OA; Anti-TB (RIF, INH, PZA); n = 8.
3.2. Relative organ weights
Significant differences in the percentage of organ weight/BW ratios were found only in the case of liver; therefore, we refer to the particular case of this organ. Compared with the control group (relative liver weight, 4.05%), an increase to 4.78% was calculated for the group treated with anti-TB drugs. Relative liver weights in m ice treated only with 100 or 200 μg UA/OA were similar to those of the control groups (4.06% and 4.12%, respectively). Mice treated with anti-TB drugs in addition to 100 μg UA/OA exhibited a relative liver weight of 4.12%, sim ilar to that of the control group; in contrast, a different behavior was observed when the dose of UA/OA to 200 μg. In this latter case, relative liver weight increased to 4.74%,a value similar to that of the group administered anti-TB drugs only (Figure 2).
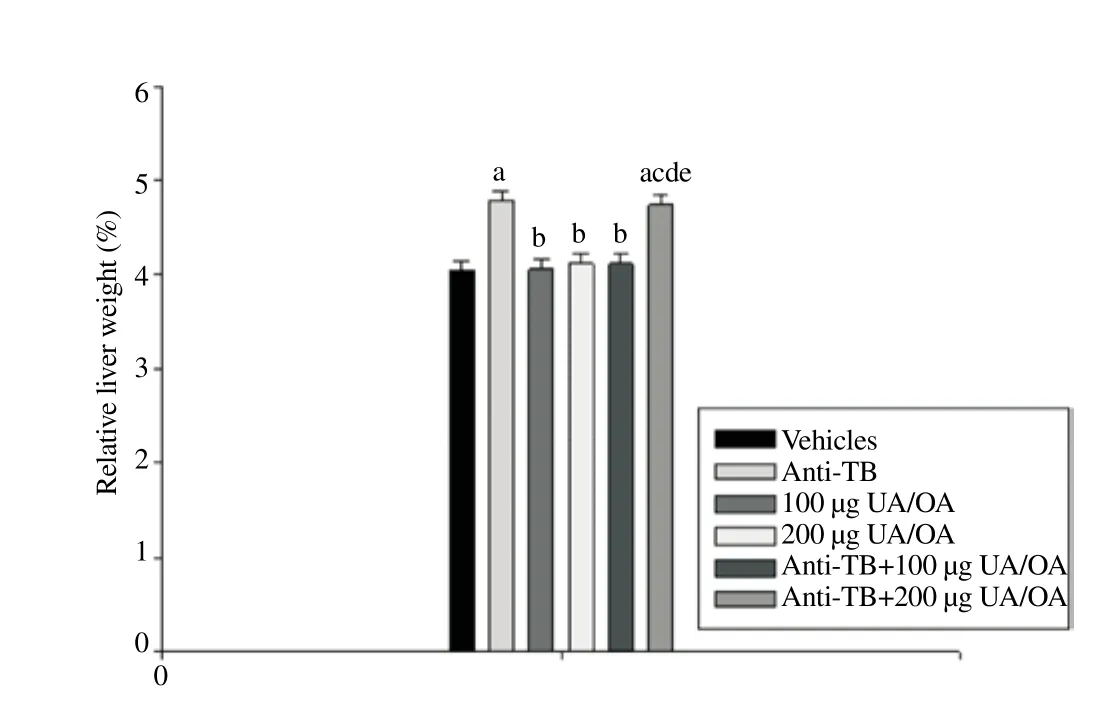
Figure 2. Effect of the UA/OA mixture on % relative weight in anti-TB-induced hepatotoxicity mouse model.
Data are mean±SEM. Statistical analysis, Kruskal-Wallis test, ANOVA, on ranks, post hoc SNK test (P<0.05);avs. Vehicles;bvs. Anti-TB;cvs. 100 μg UA/ OA;dvs. 200 μg UA/OA;evs. Anti-TB+100 μg UA/OA;fvs. Anti-TB + 200 μg UA/OA; Anti-TB (RIF, INH, PZA); n = 8.
3.3. Hematological and biochemical parameters
Hem atological param eters fell w ithin the range of those determ ined for control m ice and mean values among groups were not statistically significant (data not shown). Regarding evaluation of biochemical parameters, in none of the groups tested were statistical differences established in serum glucose and creatinine (parameters determining renal function), although urea concentration in controls (62.140±1.319) mg/dL increased slightly (16.33%) in the anti-TB group (75.29±2.19) mg/dL. In contrast,urea levels in m ice treated with both anti-TB and UA/OA at the previously mentioned doses showed values of (56.800±1.151)mg/dL and (64.90±2.06) mg/dL, respectively, whereas the group treated only with UA/OA at 100 and 200 μg doses, show ing values of (65.51±2.97) and (68.42±1.86) mg/dL respectively had plasma concentrations close to those of the controls (Figure

Figure 3. Effect of UA/OA m ixture on serum urea concentration in anti-TB-induced hepatotoxicity mouse model.
Data are mean±SEM. Statistical analysis, one-way ANOVA, post hoc SNK test (P<0.05);avs. Vehicles;bvs. Anti-TB;cvs. 100 μg UA/OA;dvs. 200 μg UA/ OA;evs. Anti-TB + 100 μg UA/OA;fvs. Anti-TB + 200 μg UA/OA; Anti-TB (RIF, INH, PZA); n = 8.
Transaminase values in the control group was AST (155.91±2.67)UI/L and ALP (45.27±2.59) UI/L. Evaluation of the liver function of the anti-TB group showed an increase in both serum transam inase levels: AST (180.67±3.85) UI/L and ALT (86.33±3.79) UI/L; this increase diminished significantly when the animals received anti-TB drugs and 100 μg of UA/OA (AST 100.33±2.86 UI/L; ALT 55.42±4.25 UI/L), although the group that received anti-TB plus 200 μg of UA/OA showed a high values with respect to the control: AST(188.87±1.87) UI/L and ALT (98.00±3.17) UI/L, these values was sim ilar to those of the anti-TB group. Results are illustrated in Figures 4 and 5. Furthermore, the serum concentration of ALP in the anti-TB group showed the highest value with respect to negative controls (182.33 vs. 161.09) UI/L. On comparison with the controls,all treated groups of animals had higher ALP concentrations: UA/OA treatment at doses of 100 and 200 μg yielded values of 174.67 and 177.91 UI/L, respectively, close to those of the groups with UA/OA at 100 and 200 μg and the anti-TB challenge (172.25 and 176.75)UI/L (Figure 6).Data are mean±SEM. Statistical analysis, one-way ANOVA post hoc SNK test (P<0.05);avs. Vehicles;bvs. Anti-TB;cvs. 100 μg UA/OA;dvs. 200 μg UA/ OA;evs. Anti-TB + 100 μg UA/OA;fvs. Anti-TB + 200 μg UA/OA; Anti-TB (RIF, INH, PZA); n = 8.
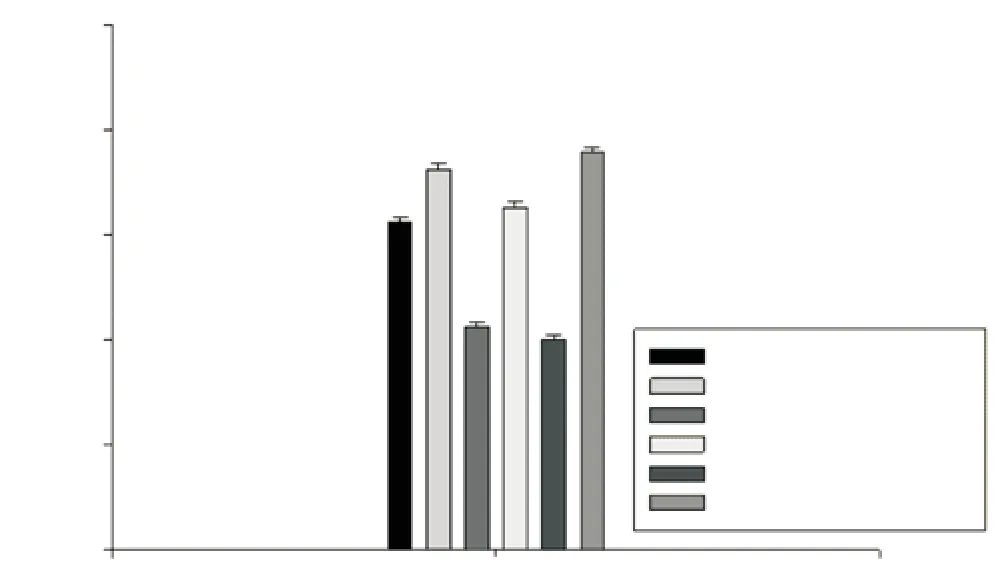
Figure 4. Effect of UA/OA mixture on serum AST concentration in anti-TB-induced hepatotoxicity mouse model.

Figure 5. Effect of UA/OA m ixture on serum ALT concentration in anti-TB-induced hepatotoxicity mouse model.
Data are mean±SEM. Statistical analysis, one-way ANOVA, post hoc SNK test (P<0.05);avs. Vehicles;bvs. Anti-TB;cvs. 100 μg UA/OA;dvs. 200 μg UA/ OA;evs. Anti-TB + 100 μg UA/OA;fvs. Anti-TB + 200 μg UA/OA; Anti-TB (RIF, INH, PZA); n = 8.

Figure 6. Effect of UA/OA mixture on serum ALP concentration in anti-TB-induced hepatotoxicity mouse model.
Evidences both in vitro and in vivo suggest that UA possesses multi-fold biological properties, including anti-inflammatory, antioxidative, hipolipidem ic and a hepatic metallothionein-inducer property, hepatoprotector effect, as well as other significant effects[29-32]. The same profile can be noted regarding OA, because this triterpene possesses promising pharmacological activities, such as an anti-inflammatory agent, an antioxidant, a cardioprotector, an antidiabetic and as a hepatoprotector[33-39].
Since the report of Jeong[39] concerning the protective effect of OA against Carbon tetrachloride (CCl4)-induced hepatotoxicity in m ice, studies on the hepatoprotector effect of both terpenoids against various drugs that induce hepatotoxicity have appeared in the scientific literature. Studies on in vivo experimental models have shown the effects of OA and UA as protectors of acute liver injury induced by CCl4, acetam inophen, paracetamol, ethanol and D-galactosamine, being UA the most active drug[32,40-44]. Clinical trials in China showed that oral administration of OA for 3 months or more in patients with acute and chronic liver diseases decreased serum aminotransferase levels, symptoms and the occurrence of cirrhosis in cases of patients with chronic hepatitis[33,45].
The hepatoprotective effect of OA and UA against liver damage induced by anti-TB drugs lengthens the list of the multiple biological activities possessed by these triterpenes, highlighting the already reported antimycobacterial activity and antitubercular effect of the m ixture[27,46]. The majority of endo- and exogenous substances are biotransformed in the liver and when reactive products are generated,and they alter the functional and structural integrity of the organ[4,5]. Drug use is a major cause of hepatotoxicity and severe conditions such as liver cirrhosis or hepatocellular carcinoma may occur, a wellknown example comprising the hepatotoxicity caused by anti-TB
Data are mean±SEM. Statistical analysis, one-way ANOVA, post hoc SNK test (P<0.05);avs. Vehicles;bvs. Anti-TB;cvs. 100 μg UA/OA;dvs. 200 μg UA/ OA;evs. Anti-TB + 100 μg UA/OA;fvs. Anti-TB + 200 μg UA/OA; Anti-TB (RIF, INH, PZA); n = 8.
3.4. Histopathogical analysis
The liver histological analysis revealed steatosis and increased apoptosis only in the group submitted to anti-TB drugs for 11 weeks (Figure 7A). Fatty accumulation was not observed in the livers of control animals (Figure 7B), nor were liver alterations detected in groups treated with 100 and 200 μg UA/OA (Figure 7C), nor in animals receiving the anti-TB challenge in addition to 100 or 200 μg UA/OA (Figure 7D). No changes in the kidney m icroscopic exam ination were identified. Splenic hematopoiesis was sim ilar in mice with and w ithout treatment.
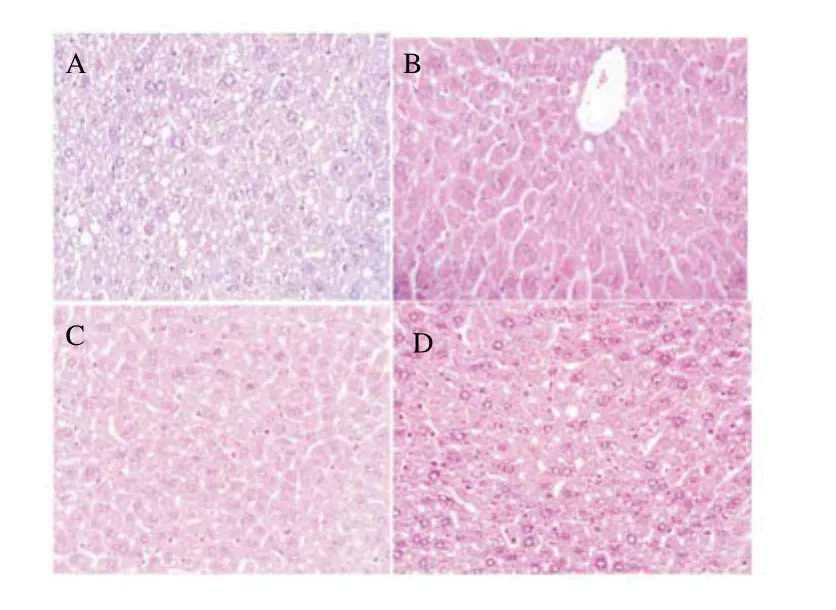
Figure 7. Liver histology, m ice groups with anti-TB (A), control (B), m ixture of UA/OA at 100 mg/m ice (C) and anti-TB plus m ixture of UA/OA at 100 mg/m ice (D).
4. Discussion
agents[13,19]. Hepatotoxic effects in anti-TB therapy with first-line agents are considered unique among liver problems because all these show dependence on the toxicity of the drug used and the regime established[12,47].
The therapeutic value, efficacy, and toxicity of drugs are parameters that are evaluated as a first step in animals with experimentally induced liver damage. The animal model employed in the present study induced hepatotoxicity in Balb/C mice by means of anti-TB agents commonly used in humans, a combination of RIF, INH and PZA[48]. It is well known that alterations in anti-TB-induced BW usually reflect physiological changes in liver function. Throughout the experiment (11 weeks), the gradual increase of BW gain in mice treated with anti-TB drugs was significantly lower than those registered in the other evaluated groups (a 58% reduction at the end of the study). This behavior was not reproduced in mice treated with anti-TB drugs plus 100 or 200 μg of UA/OA, meaning that the triterpene m ixture supported the normal grow th of animals. On the other hand, significant differences in the relative organ weights were found only in liver samples. In this case, the group treated with anti-TB drugs showed a higher relative weight gain (4.78%)with respect to that of the control group (4.05%), but the parameter decreased when the anti-TB group in addition received 100 μg UA/ OA (4.12%); in contrast to this, a different behavior was observed on increasing the UA/OA dose to 200 μg; in this case, the triterpene mixture did not act in the same manner. In this regard, studies-inprogress are currently being conducted to clarify this response.
High levels of urea are due to protein metabolism, which takes place in the liver and normally occurs when hepatotoxicity is induced[49,50]. A slight increase in urea levels was determ ined in the anti-TB group, and adm inistration of UA/OA (100 and 200 μg)returned this parameter to values close to those of the control group,with the 100 μg concentration being the more effective of the two. According to Awofeso[51] hepatotoxicity is a serious adverse complication of anti-TB therapy, which ranges from asymptomatic elevation of serum transam inases to acute liver failure. First-line anti-TB drugs raise the levels of hepatic enzymes AST and ALT and liver biopsies reveal lobular hepatitis[4,19]. RIF is a powerful enzyme inducer that enhances the hepatotoxicity of INH and PZA. Hepatotoxicity was detected in 1%-2% of patients treated with this drug, and high values of liver enzymes transam inases AST/ALT and ALP in plasma were reported[19,52]. An augmented level of these hepatic markers in serum may indicate cellular leakage and loss of functional integrity of the cell membrane in liver as a result of the oxygen free radicals produced by anti-TB drugs[7,53,54].
Our results after 11 treatment weeks assume the initiation of a hepatotoxic process in the group of m ice treated with anti-TB,because serum transam inases AST and ALT were significantly increased. Adm inistration of 100 μg UA/OA as a unique agent produced an important decrease of AST concentration; an effect of the same magnitude was detected in mice receiving anti-TB drugs in addition to the triterpene m ixture. The effect of UA/OA on ALT concentrations was also appreciated, although with less intensity.
Mice treated with anti-TB drugs initiated a steatosis process. Histological liver analysis showed, in samples obtained from animals administered the UA/OA mixture (100 or 200 μg) and those treated with UA/OA plus the anti-TB challenge, a sim ilar architecture to that of the controls group. In the case of the anti-TB-treated group,histological observations support the biochem ical findings, in that liver slides clearly depicted morphological alteration related with fatty accumulation. From this analysis, it is possible to support that treatment with the UA/OA mixture was able to avoid hepatic lesions induced by RIF/INH/PZA adm inistration and nearly completely prevented the development of liver steatosis. Evaluation of the UA/ OA hepatoprotector effect in the experimental model described showed that 100 μg offers better protection against damage caused by anti-TB drugs.
While it is not possible to conclude, in this prelim inary trial,the underlying mechanism of the UA/OA hepatoprotector effect against standard anti-TB drugs, we may assume the suppression of Nuclear factor-kappa beta (NF-κB) activation, inhibition of Cytochrome450 2E1 (P4502E1) expression and activity, as reported for the hepatoprotector effect of OA against CCl4[39], enhanced hepatic-glutathione regeneration capacity[41] or the upregulation of metallothionein expression mediated by TNF-α and IL-6 shown in vitro[34,54], w ithout dismissing the background that these triterpenes possess as strong antioxidants[55]. It is important to mention that additional studies are underway to evaluate the hepatoprotector effects of the triterpene mixture in this same model (Balb/c mice). Animals were administered by oral via and with more prolonged treatment periods (4 months). Another study currently being developed is that of evaluating the hepatoprotector effect of the triterpene (UA/OA) mixture administered by oral via but with higher doses of RIF/INH/PZA (30, 30, and 90 mg/kg, respectively).
The results showed that UA/OA mixture was able to prevent the steatosis induced by anti-TB drugs when was co-administered daily during 77 d by s.c. The triterpene m ixture UA/OA favored BW gain and reduced levels of AST and ALT in animals receiving anti-TB plus triterpens. The animals groups that only received triterpene m ixture or vehicle didn’t showed steatosis, and no alteration was observed on BW gain in these groups. The values of AST, ALT and ALP were slightly higher respect to vehicle group but lower than the group treated with anti-TB drugs. We have previously shown that the m ixture of UA/OA has antitubercular activity and now we are demonstrating that this mixture protects against damage caused by the basic drugs to treat TB (RIF/INH/PZA).
Conflict of interest statement
The authors declare that they have no competing interest.
Acknow ledgments
Part of this study was supported by Grant from the Instituto Mexicano del Seguro Social, projects FIS/IMSS/PROT/G12/1126 and FIS/IMSS/PROT/G14/1341.
References
[1] Ahmad F, Tabassum N. Experimental models used for the study of antihepatotoxic agents. JAD 2012; 1(2): 85-89. DOI: 10.1016/S2221-6189(13)60021-9.
[2] Kumar N, Kedarisety CK, Kumar S, Khillan V, Sarin SK. Antitubercular therapy in patients with cirrhosis: challenges and options. World J Gastroenterol 2014; 20(19): 5760-5772. DOI: 10.3748/w jg.v20.i19.5760.
[3] Adhvaryu MR, Reddy NM, Vakharia BC. Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach. World J Gastroenterol 2008; 14(30): 4753-4762. DOI: 10.3748/w jg.14.4753.
[4] Ramappa V, Aithal GP. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol 2013; 3(1): 37-49. DOI: 10.1016/j.jceh.2012.12.001.
[5] Jeong I, Park JS, Cho YJ, Yoon HI, Song J, Lee CT, et al. Drug-induced hepatotoxicity of anti-tuberculosis drugs and their serum levels. J Korean Med Sci 2015; 30(2): 167-172. DOI:10.3346/jkms.2015.30.2.167.
[6] N joku DB. Drug-induced hepatotoxicity: m etabo lic, genetic and immunological basis. Int J Mol Sci 2014; 15(4): 6990-7003. DOI: 10.3390/ijms15046990.
[7] Rana SV, A ttri S, Vaiphei K, Pal R, A ttri A, Singh K, et al. Role of N-acetylcysteine in Rifampicin-induced hepatic injury of young rats. World J Gastroenterol 2006; 12(2): 287-291.
[8] Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR,et al. Protective effect of N-acetylcysteine on antituberculosis druginduced hepatotoxicity. Eur J Gastroenterol Hepatol 2010; 22(10): 1235-1238. DOI: 10.1097/MEG.0b013e32833aa11b.
[9] Sukhanov DS, Pavlova MV, Iablonskiĭ PK, Vinogradova T. Comparative efficacy of clinical use of reamberine, remaxol, and ademethionine in patients with tuberculosis of the respiratory organs and drug-induced liver injury. Antibiot Khimioter 2013; 58(1-2): 13-18.
[10] Tasduq SA, Peerzada K, Koul S, Bhat R, Johri RK. Biochem ical manifestations of anti-tuberculosis drugs induced hepatotoxicity and the effect of silymarin. Hepatol Res 2005; 31(3):132-135.
[11] Eminzade S, Uraz F, Izzettin FV. Silymarin protects liver against toxic effects of anti-tuberculosis drugs in experimental animals. Nutr Metab 2008; 5(18): 2-8. DOI: 10.1186/1743-7075-5-18.
[12] Singh M, Sasi P, Gupta VH, Rai G, Amarapurkar DN, Wangikar PP. Protective effect of curcum in, silymarin and N-acetylcysteine on antitubercular drug-induced hepatotoxicity assessed in an in vitro m odel. Hum Exp Toxicol 2012; 31(8): 788-797. DOI: 10.1177/0960327111433901.
[13] Nicoletti NF, Rodrigues-Junior V, Santos AA Jr, Leite CE, Dias AC,Batista EL Jr, et al. Protective effects of resveratrol on hepatotoxicity induced by Isoniazid and Rifampicin via SIRT1 modulation. J Nat Prod 2014; 77(10): 2190-2195. DOI: 10.1021/np5003143.
[14] Aguirre L, Portillo MP, Hijona E, Bujanda L. Effects of resveratrol and other polyphenols in hepatic steatosis. World J Gastroenterol 2014;20(23): 7366-80. DOI: 10.3748/w jg.v20.i23.7366.
[15] Upadhyay G, Kumar A, Singh MP. Effect of silymarin on Pyrogalloland Rifampicin-induced hepatotoxicity in mouse. Eur J Pharmacol 2007;565(1-3): 190-201.
[16] Pal R, Vaiphei K, Sikander A, Singh K, Rana SV. Effect of garlic on Isoniazid and Rifampicin-induced hepatic injury in rats. World J Gastroenterol 2006; 12(4): 636-639.
[17] Yogeeta S, Ragavender HRB, Devaki T. Antihepatotoxic effect of Punica granatum acetone extract against Isoniazid-and Rifampicininduced hepatotoxicity. Pharm Biol 2007; 45(8): 631-637. DOI: 10.1080/13880200701538963.
[18] Jaydeokar AV, Bandawane DD, Bibave KH, Patil TV. Hepatoprotective potential of Cassia auriculata roots on ethanol and antitubercular druginduced hepatotoxicity in experimental models. Pharm Biol 2014; 52(3): 344-355. DOI: 10.3109/13880209.2013.837075.
[19] Pillai KK, Chidam baranathan N, Mohamed HM, Jayaprakash S,Narayanan N. Hepatoprotective activity of Cnidoscolus chayamansa against Rifampicin and Isoniazid induced toxicity in Wistar rats. RJPBCS 2012; 3(2): 577-585.
[20] Tandon VR, Khajuria V, Kapoor B, Kour D, Gupta S. Hepatoprotective activity of Vitex negundo leaf extract against anti-tubercular drugs induced hepatotoxicity. Fitoterapia 2008; 79(7-8): 533-538. DOI:10.1016/ j.fitote.2008.05.005.
[21] Samuel AJ, Mohan S, Chellappan DK, Kalusalingam A, Ariamuthu S. Hibiscus vitifolius (Linn.) root extracts show s potent protective action against anti-tubercular drug induced hepatotoxicity. J Ethnopharmacol 2012; 141(1): 396-402. DOI: 10.1016/j.jep.2012.02.051.
[22] Anbarasu C, Rajkapoor B, Kalpana J. Protective effect of Pisonia aculeata on Rifampicin and Isoniazid induced hepatotoxicity in rats. Int J Phytomed 2011; 3: 75-83.
[23] Maity T, Ahmad A. Protective effect of Mikania scandens (l.) W illd. against Isoniazid induced hepatotoxicity in rats. Int J Pharm Pharm Sci 2012; 4(3): 466-469.
[24] Pari L, Kumar NA. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. J Med Food 2002; 5(3): 171-177.
[25] Lina SMM, Ashab I, A hm ed MI, A l-Am in M, Shah riar M. Hepatoprotective activity of Asteracantha longifolia (Nees.) extract against anti-tuberculosis drug induced hepatic damage in Sprague-Daw ley rats. PhOL 2012; 3(13-19): 13-19.
[26] Akindele AJ, Ezenw anebe KO, Anunobi CC, Adeyem i OO. Hepatoprotective and in vivo antioxidant effects of Byrsocarpus coccineus Schum and Thonn (Connaraceae). J Ethnopharmacol 2010; 129(1): 46-52. DOI:10.1016/j.jep.2010.02.024.
[27] Jiménez-Arellanes A, Luna-Herrera J, Cornejo-Garrido J, López-García S, Castro-Mussot ME, Meckes-Fischer M, et al. Ursolic and oleanolic acids as antim icrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement Altern Med 2013; 13: 258. DOI: 10.1186/1472-6882-13-258.
[28] Jiménez-Arellanes A, Meckes-Fischer M, Torres-López J, Luna-Herrera J, Hernández-Pando R, Inventors. Instituto Mexicano del Seguro Social (IMSS), CMNSXXI. Composición farmacéutica que comprende ácido ursólico y ácido oleanólico útil para el tratamiento de la tuberculosis. MX Patent 273483. 2010, Jan 18.
[29] Cornejo-Garrido J, Chamorro GA, Garduño L, Hernández R, Jiménez MA. Acute and subacute toxicity (28 days) of a m ixture of ursolic acid and oleanolic acid obtained from Bouvardia ternifolia in m ice. Bol Latinoam Caribe Plant Med 2012; 11(1): 91-102.
[30] Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 1995; 49(2): 57-68.
[31] Ikeda Y, Murakam i A, Ohigashi H. Ursolic acid: an anti- and proinflammatory triterpenoid. Mol Nutr Food Res 2008; 52(1): 26-42.
[32] Li S, Liao X, Meng F, Wang Y, Sun Z, Guo F, Li X, Meng M, Li Y, Sun C, et al. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS One 2014; 9(1): e86724. DOI: 10.1371/ journal.pone.0086724.
[33] Jeong HG, Kim HG, Hwang YP. Involvement of cytokines in the hepatic expression of metallothionein by ursolic acid. Toxicol Lett 2005; 155(3): 369-376.
[34] Vasconcelos MA, Royo VA, Ferreira DS, Crotti AE, Andrade e Silva ML, Carvalho JC, Bastos JK, Cunha WR. In vivo analgesic and anti-Inflammatory activities of ursolic acid and oleanolic acid from Miconia albicans (Melastomataceae). Z Naturforsch C 2006; 61(7-8): 477-482.
[35] Lee W, Yang EJ, Ku SK, Song KS, Bae JS. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 2013; 36(1): 94-102. DOI: 10.1007/s10753-012-9523-9.
[36] Mueller D, Triebel S, Rudako vs ki O, Richling E. Influence of triterpenoids present in apple peel on inflammatory gene expression associated with inflammatory bowel disease (IBD). Food Chem 2013;139(1-4): 339-346. DOI: 10.1016/j.foodchem.2013.01.101.
[37] Pollier J, Goossens A. Oleanolic acid. Phytochemistry 2012; 77: 10-15. DOI: 10.1016/j.phytochem.2011.12.022.
[38] Castellano JM, Guinda A, Delgado T, Rada M, Cayuela JA. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 2013; 62(6): 1791-1799. DOI: 10.2337/db12-1215.
[39] Jeong HG. Inhibition of cytochrome P4502E1 expression by oleanolic acid: hepatoprotective effects against carbon tetrachloride-induced hepatic injury. Toxicol Lett 1999; 105(3): 215-22. DOI: 10.1016/S0378-4274(99)00004-1.
[40] Liu Y, Hartley DP, Liu J. Protection against carbon tetrachloride hepatotoxicity by oleanolic acid is not mediated through metallothionein. Toxicol Lett 1998; 95(2): 77-85.
[41] Yim TK, Wu WK, Pak WF, Ko KM. Hepatoprotective action of an oleanolic acid enriched extract of Ligustrum lucidum fruits is mediated through an enhancement on hepatic glutathione regeneration capacity in mice. Phytother Res 2001; 15(7): 589-592.
[42] Liu J, Liu Y, Mao Q, K laassen CD. The effects of 10 triterpenoid compounds on experimental liver injury in m ice. Fund Appl Toxicol 1994;22(1): 34-40.
[43] Liu J. Oleano lic acid and urso lic acid: research perspectives. J Ethnopharmacol 2005; 100(1-2): 92-94.
[44] Saravanan R, Viswanathan P, Pugalendi KV. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci 2006; 78(7): 713-718.
[45] Wu CR, Hseu YC, Lien JC, Lin LW, Lin YT, Ching H, et al. Triterpenoid contents and anti-inflammatory properties of the methanol extracts of Ligustrum species leaves. Molecules 2010; 16(1): 1-15. DOI: 10.3390/ molecules16010001.
[46] Jiménez-Arellanes MA, Gutiérrez-Rebolledo G, Rojas-Tomé S, Meckes-Fischer M. Medicinal plants, an important reserve of antimycobacterial and antitubercular drugs: an update. J Infect Dis Ther 2014; 2(6): 1000185. DOI: 10.4172/2332-0877.1000185.
[47] Senousy BE, Belal SI, Draganov PV. Hepatotoxic effects of therapies for tuberculosis. Nat Rev Gastroenterol Hepatol 2010; 7(10): 543-56. DOI: 10.1038/nrgastro.2010.134.
[48] Enríquez-Cortina C, Almonte-Becerril M, Clavijo-Cornejo D, Palestino-Domínguez M, Bello-Monroy O, Nuño N, et al. Hepatocyte grow th factor protects against Isoniazid/Rifampicin-induced oxidative liver damage. Toxicol Sci 2013; 135(1): 26-36. DOI:10.1093/toxsci/kft134.
[49] Saba AB, Oyagbem i AA, Azeez OI. Amelioration of carbon tetrachlorideinduced hepatotoxicity and haemotoxicity by aqueous leaf extract of Cnidoscolus aconitifolius in rats. Niger J Physiol Sci 2010; 25(2): 139-147. [50] Waring WS, Stephen AF, Robinson OD, Dow MA, Pettie JM. Serum urea concentration and the risk of hepatotoxicity after paracetamol overdose. QJM 2008; 101(5): 359-363. DOI: 10.1093/qjmed/hcn02.
[50] Awofeso N. Anti-tuberculosis medication side effects constitute major factor for poor adherence to tuberculosis treatment. Bull World Health Org 2008; 86(3): B-D. DOI: 10.2471/BLT.07.043802.
[52] Devarbhavi H. Adaptation and antituberculosis drug-induced liver injury. Am J Respir Crit Care Med 2012; 186(4): 387-388.
[53] Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008; 245(3): 194-205. DOI: 10.1016/j.tox. 2007.11.021.
[54] Kim KA, Lee JS, Park HJ, Kim JW, Kim CJ, Shim IS, et al. Inhibition of cytochrome P450activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci 2004; 74(22): 2769-2779.
[55] Somova LO, Nadar A, Rammanan P, Shode FO. Cardiovascular,antihyperlipidem ic and anti-oxidant effect of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003; 10(2-3): 115-121.
doi:Document heading 10.1016/j.apjtm.2016.05.015
*Corresponding author:Adelina Jiménez-Arellanes, Unidad de Investigación Médica en Farmacología, UMAE Hospital de Especialidades, Centro Médico Nacional-Siglo XXI, Instituto Mexicano del Seguro Social, México City, Mexico.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Predicted pattern of Zika virus infection distribution with reference to rainfall in Thailand
- Effect of partial splenic embolization on the immune function of cirrhosis patients with hypersplenism
- Perfusion of gastrodin in abdom inal aorta for alleviating spinal cord ischem ia reperfusion injury
- Study on the effect and mechanism of the dysfunction of CD4+T cells in the disease process of chronic cardiac failure
- Influence on radiosensitivity of lung glandular cancer cells when ERCC1 gene silenced by targeted siRNA
- Experimental study on the inhibition effect of m iR-106a inhibitor on tumor grow th of ovarian cancer xenografts m ice
