HER-2阳性晚期乳腺癌治疗策略
2016-06-06孙婧张频
孙婧 张频
HER-2阳性晚期乳腺癌治疗策略
孙婧张频
摘要HER-2阳性晚期乳腺癌患者预后差,以抗HER-2靶向治疗为基础的综合治疗显著延长了患者的生存期、改善了预后。目前多种抗HER-2靶向药物已应用于临床,抑制HER-2通路是HER-2阳性晚期乳腺癌患者一线治疗及一线治疗进展后的基础治疗。本文简要介绍HER-2阳性晚期乳腺癌治疗相关的关键临床研究、指南推荐及今后的研究方向,以指导临床实践。
关键词HER-2阳性晚期乳腺癌治疗策略临床研究靶向治疗
作者单位:中国医学科学院肿瘤医院肿瘤内科(北京市100021)
人表皮生长因子受体-2(human epidermal growth factor receptor -2,HER-2)过表达导致细胞过度增殖、分化、凋亡减少、侵袭转移增加,HER-2阳性晚期乳腺癌患者的5年生存率较HER-2阴性者降低46%[1]。近十年来,由于多个抗HER-2药物成功研发上市,HER-2阳性晚期乳腺癌成为证据级别最高、治疗方案最成熟的晚期乳腺癌生物学亚型。本文结合国内外研究进展及治疗指南/共识,对HER-2阳性晚期乳腺癌治疗策略进行综述。
1 抗HER-2靶向治疗药物
HER-2是人表皮生长因子受体家族成员之一,其在信号通路传导中起核心作用。HER-2受体由细胞外生长因子结合区、亲脂的跨膜区、胞内区(HER-2功能区具有酪氨酸激酶的活性和ATP的结合位点)组成。HER家族受体只有形成二聚体后才能引起自身磷酸化,从而激活下游信号传导通路,导致肿瘤细胞增殖和存活[2]。
目前已上市的抗HER-2靶向药物主要为:1)针对受体细胞膜外部分的单克隆抗体:曲妥珠单抗、帕妥珠单抗、抗体-药物偶联物(antibody-drug conju⁃gate,ADC);2)针对受体细胞膜内部分的酪氨酸激酶抑制剂:拉帕替尼。曲妥珠单抗与HER-2受体细胞膜外Ⅳ区结合,阻断肿瘤细胞信号传导。帕妥珠单抗结合于HER-2受体胞外Ⅱ区,抑制HER-2同源或异源二聚体形成,继而阻止下游信号传导。两药可以互补增强对HER-2通路的抑制[3]。T-DM1(trastu⁃zumab emtansine)是新型的ADC,由曲妥珠单抗和细胞毒药物DM1(derivative of maytansine 1)连接而成,与细胞表面HER-2受体结合后内吞入细胞,释放DM1抑制微管聚集,发挥细胞毒作用,同时曲妥珠单抗发挥抗肿瘤作用。拉帕替尼是HER-1和HER-2受体酪氨酸激酶抑制剂,可同时抑制HER-1、HER-2,从而阻断下游信号传导[4]。
2 HER-2阳性晚期乳腺癌一线治疗策略
2.1以曲妥珠单抗为基础的一线化疗方案
曲妥珠单抗是最早研发上市的抗HER-2药物,可以有效抑制HER-2阳性乳腺癌进展,与多种化疗药物联合具有协同增效作用。Slamon等[5]首次证实了一线曲妥珠单抗联合化疗(包括蒽环类或紫杉类药物)与单独化疗相比,能提高HER-2阳性晚期乳腺癌的ORR(50% vs.32%,P<0.001),明显延长TTP(7.4个月vs.4.6个月,P<0.001)及OS(25.1个月vs.20.3个月,P=0.046),但化疗联合曲妥珠单抗增加心脏毒性,因此目前临床治疗不推荐蒽环类药物联合曲妥珠单抗(临床研究除外),Marty等[6]进一步肯定了上述研究的结果。紫衫类多西他赛联合曲妥珠单抗优于多西他赛。在曲妥珠单抗联合紫衫类药物基础上,Robert等[7]发现曲妥珠单抗、紫杉醇联合卡铂较安慰剂提高ORR(52%vs.36%,P=0.04)和PFS(7.1个月vs.10.7个月,P=0.03);Wardley等[8]研究证明曲妥珠单抗、多西他赛联合卡培他滨较安慰剂延长PFS(17.9个月vs.12.8个月,P=0.045),提高2年生存率(75%vs.66%)。以上均为一线治疗可选择的方案。
2.2曲妥珠单抗联合帕妥珠单抗作为一线治疗
Baselga等[9]发现曲妥珠单抗联合帕妥珠单抗能增加信号阻断效应,这与两药从不同作用位点上抑制HER-2信号通路有关。研究发现,曲妥珠单抗、多西他赛联合帕妥珠单抗与非联合组相比,双靶向联合化疗延长PFS 6.1个月(P<0.001),OS延长15.7个月(P=0.000 2);亚组分析发现,辅助或新辅助化疗、激素受体状态及检测HER-2过表达的方法均不影响生存获益[10-11]。目前双靶向联合多西他赛是HER-2阳性晚期乳腺癌一线治疗的优选方案。
2.3激素受体阳性、HER-2阳性晚期乳腺癌一线内分泌治疗
约50%HER-2阳性乳腺癌患者激素受体(hor⁃mone receptor,HR)阳性,雌激素受体(estrogen recep⁃tor,ER)与HER-2信号通路存在细胞内相互交联,HER-2过表达可导致内分泌治疗耐药。绝经后患者HR阳性、HER-2阳性晚期乳腺癌一线内分泌治疗研究中,Kaufman等[12]证明曲妥珠单抗联合阿那曲唑较阿那曲唑延长PFS1倍(4.8个月vs.2.4个月,P=0.0016),提高临床获益率(CBR)(42.7%vs.27.9%,P=0.026);Johnston等[13]证明拉帕替尼联合来曲唑较来曲唑提高ORR(37.9%vs.14.8%,P=0.021),延长PFS(8.2个月vs.3.0个月,P=0.019)。两项研究说明双通路抑制能提高疗效,恢复内分泌药物敏感性,延缓疾病进展。
2.4其他靶向药物的一线治疗
Gianni等[14]表明曲妥珠单抗、多西他赛联合贝伐珠单抗一线治疗HER-2阳性晚期乳腺癌较标准治疗无明显获益,PFS为16.5个月vs.13.7个月,P=0.0775)。Gelmon等[15]研究发现一线治疗中曲妥珠单抗联合紫杉类药物优于与拉帕替尼联合紫杉类药物,PFS为11.3个月vs.9.0个月(P=0.01)。PTEN缺失,PIK/AKT/mTOR通路激活是曲妥珠单抗耐药的原因之一,mTOR抑制剂可逆转曲妥珠单抗耐药[16]。Hurvitz等[17]在曲妥珠单抗联合紫杉醇基础上,联合依维莫司或安慰剂,结果证明HER-2阳性晚期乳腺癌一线治疗中mTOR抑制剂无优势,PFS 为14.95个月vs.14.49个月(P=0.1166),同时不良反应增加。HER-2阳性晚期乳腺癌一线治疗关键临床研究见表1。
2.5国内、外指南一线治疗的推荐
NCCN(2015年)、Giordano等[18]及Cardoso等[19]推荐曲妥珠单抗、帕妥珠单抗联合紫杉类药物作为HER-2阳性晚期乳腺癌一线治疗优选方案。曲妥珠单抗联合不同化疗药物(紫杉类等)为备选方案。HER-2阳性、HR阳性的绝经后晚期乳腺癌患者可采用曲妥珠单抗联合芳香化酶抑制剂治疗。中国抗癌协会乳腺癌专业委员会推荐,在帕妥珠单抗国内尚未上市的情况下,曲妥珠单抗联合紫杉醇或多西他赛可以作为首选的一线方案,也可加用卡铂进一步提高疗效,其他可联合药物包括长春瑞滨、卡培他滨等[20-21]。
3 一线治疗后疾病进展的治疗策略
3.1继续抑制HER-2通路
抗HER-2治疗过程中出现疾病进展,后续靶向治疗仍有应用价值。von Minckwitz等[22]发现曲妥珠单抗治疗中对于疾病进展患者,后续曲妥珠单抗联合卡培他滨的疗效优于改用卡培他滨单药(TTP:5.6个月vs.8.2个月,P=0.033 8;ORR:27%vs.48.1%,P=0.011 5),说明继续使用曲妥珠单抗仍可提高疗效,可能与曲妥珠单抗增加化疗敏感性有关。Geyer等[23]发现,对于曲妥珠单抗一线治疗后疾病进展的患者改用拉帕替尼联合卡培他滨优于卡培他滨单药(TTP:8.4个月vs.4.4个月,P<0.001),但腹泻和皮疹的发生率增加。

表1 HER-2阳性晚期乳腺癌患者一线治疗关键临床研究Table 1 Pivotal clinical trials for HER-2-positive advanced breast cancer first-line treatment
拉帕替尼与曲妥珠单抗因作用于HER-2受体的不同位点,提示二者有协同作用。曲妥珠单抗治疗后疾病进展的患者中,比较拉帕替尼联合曲妥珠单抗与拉帕替尼单药的疗效,结果联合组PFS延长4周(12.0周vs.8.1周,P=0.008),中位OS延长4.5个月(14.0个月vs.9.5个月,P=0.026),CBR提高1倍(24.7%vs.12.4%,P=0.01),不良反应两组相似[24-25]。该研究为HER-2阳性晚期乳腺癌患者提供了无化疗的双靶向治疗选择。
3.2T-DM1在二线治疗中的作用
Verma等[26]评价了T-DM1在曲妥珠单抗治疗后疾病进展患者中的应用价值。该研究中曲妥珠单抗、紫杉类治疗HER-2阳性晚期乳腺癌疾病进展患者,随机行T-DM1或拉帕替尼联合卡培他滨治疗。结果表明,T-DM1组有疗效优势(ORR:43.6%vs.30.8%,P<0.001;PFS:9.6个月vs.6.4个月,P<0.001;OS:30.9个月vs.25.1个月,P<0.001)。可见T-DM1疗效明显优于拉帕替尼联合卡培他滨,且总体耐受性更好,为二线治疗的优选药物,但该药物目前尚未在国内批准上市。
3.3抑制mTOR通路
André等[27]首次在HER-2阳性晚期乳腺癌Ⅲ期临床试验中,证明mTOR通路抑制剂依维莫司在二线治疗中获益。该研究对曲妥珠单抗联合紫杉醇类药物治疗失败的晚期乳腺癌患者,进行曲妥珠单抗、长春瑞滨联合依维莫司或安慰剂的疗效比较,依维莫司组PFS延长(7.00个月vs.5.78个月,P=0.006 7),但3~4级不良反应增加。该研究虽为HER-2阳性晚期乳腺癌二线治疗提供了选择,但治疗时需权衡利弊。
3.4国内、外指南对一线治疗后疾病进展的治疗建议
NCCN(2015年)、Giordano等[18]及Cardoso等[19]推荐二线治疗优选T-DM1,其他选择包括继续使用曲妥珠单抗同时更换化疗药物、改用拉帕替尼同时更换化疗药物,曲妥珠单抗联合拉帕替尼双靶向治疗。继续抑制HER-2通路仍为治疗的基础。中国晚期乳腺癌诊治专家共识及乳腺癌诊治指南推荐,在T-DM1未上市的情况下,可选择继续使用曲妥珠单抗同时更换化疗药物、拉帕替尼联合卡培他滨、曲妥珠单抗联合卡培他滨、曲妥珠单抗联合拉帕替尼。另外,mTOR抑制剂依维莫司也可作为二线治疗后的一种选择[20-21]。HER-2阳性晚期乳腺癌患者一线治疗后疾病进展的主要临床研究见表2。
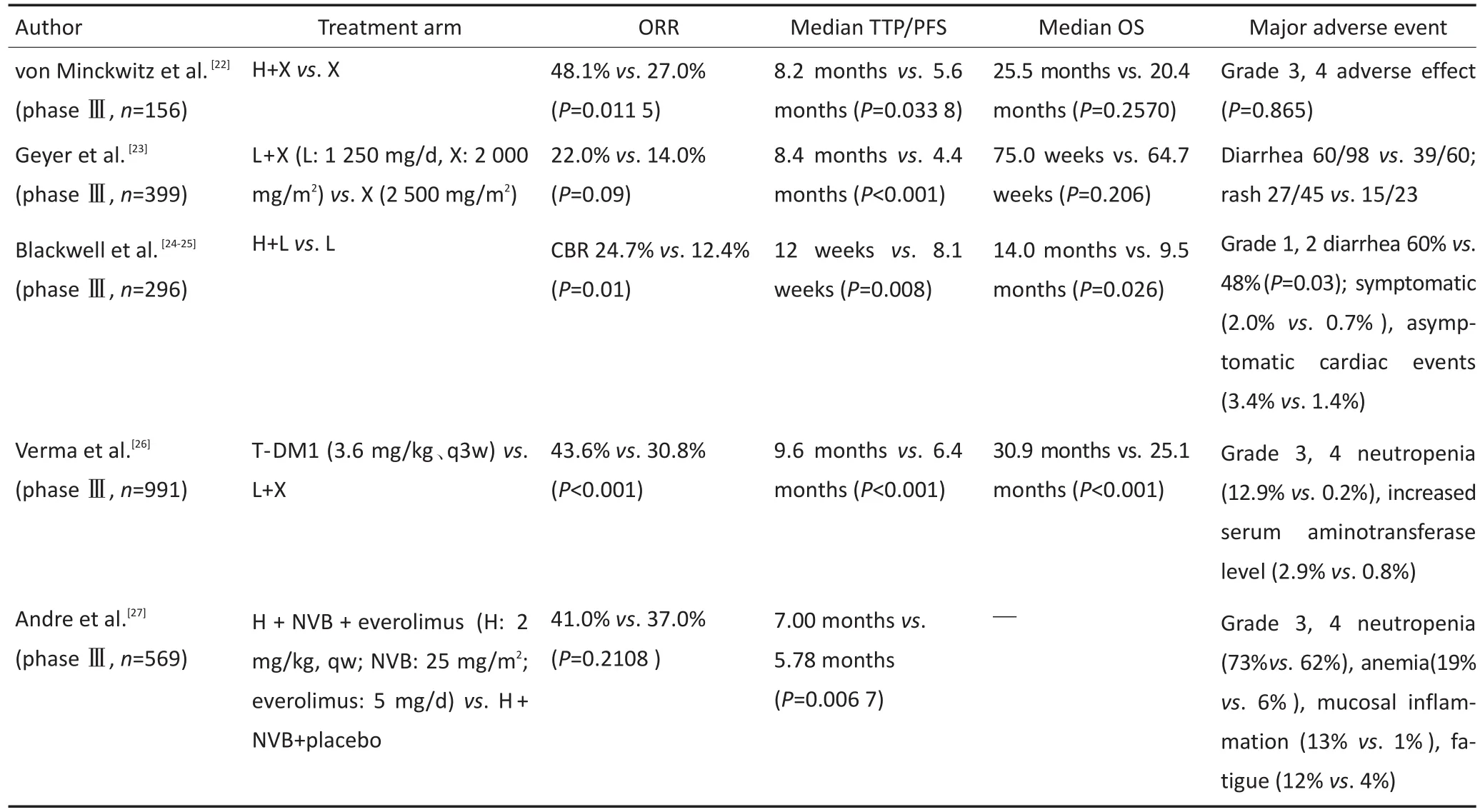
表2 HER-2阳性晚期乳腺癌患者一线治疗后疾病进展的主要临床研究Table 2 Pivotal clinical trials for disease progression of HER-2-positive advanced breast cancer beyond first-line treatment
4 结语
目前,HER-2阳性晚期乳腺癌相关的基础、转化研究正在不断深入,有望更多治疗中获益和发现潜在的预后靶点。曲妥珠单抗、帕妥珠单抗对PIK3CA野生型和突变型患者均有效,但野生型获益更多,TDM1对突变型患者获益更多。Avan等[28]研究发现,HER-2 mRNA高表达是HER-2阳性晚期乳腺癌预后良好的独立因素,HER-2下游的PIK3CA突变则提示预后较差但可在治疗中获益。多种肿瘤细胞持续表达或可诱导产生PD-1配体,在逃避活化T细胞的免疫监视中起关键作用,PD-1高表达与不良预后和低生存率相关,是一个引人注目的治疗干预靶点。目前正在招募中的PANACEA试验,旨在研究HER-2阳性晚期乳腺癌中曲妥珠单抗耐药应用抗PD-1单克隆抗体的价值。MK-3475是一种高效的、高选择性的直接阻断PD-1的人源性单克隆抗体,明显增加T细胞免疫应答,同时调节IL-2、TNF-α、IFN-γ及其他细胞因子。此外该研究将评估MK-3475是否可以逆转曲妥珠单抗耐药,提高HER-2阳性乳腺癌临床预后。肿瘤微环境作为预后和预测性指标也正在被认识,基于肿瘤的异质性,对患者进行的个体化治疗将进一步提高HER-2阳性晚期乳腺癌的治疗效果。由此可见,HER-2晚期乳腺癌未来治疗将更加精准化、个体化。
参考文献
[1]Dawood S,Broglio K,Buzdar AU,et al.Prognosis of women with metastatic breast cancer by HER-2 status and trastuzumab treatment:an institutional-based review[J].J Clin Oncol,2010,28(1):92-98.
[2]Yarden Y,Sliwkowski MX.Untangling the ErbB signalling network [J].Nat Rev Mol Cell Biol,2001,2(2):127-137.
[3]De Mattos-Arruda L,Cortes J.Use of pertuzumab for the treatment of HER- 2 positive metastatic breast cancer[J].Adv Ther,2013,30(7):645-658.
[4]Segovia-Mendoza M,González-González ME,Barrera D,et al.Efficacy and mechanism of action of the tyrosine kinase inhibitors gefitinib,lapatinib and neratinib in the treatment of HER-2 positive breast cancer:preclinical and clinical evidence[J].Am J Cancer Res,2015,5(9):2531-2561.
[5]Slamon DJ,Leyland-Jones B,Shak S,et al.Use of chemotherapy plus a monoclonal antibody against HER-2 for metastatic breast cancer that overexpresses HER-2[J].N Engl J Med,2001,344(11):783-792.
[6]Marty M,Cognetti F,Maraninchi D,et al.Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment:the M77001 study group[J].J Clin Oncol,2005,23(19):4265-4274.
[7]Robert N,Leyland-Jones B,Asmar L,et al.Randomized phase III study of trastuzumab,paclitaxel,and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer[J].J Clin Oncol,2006,24(18):2786-2792.
[8]Wardley AM,Pivot X,Morales-Vasquez F,et al.Randomized phase II trial of first- line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER-2-positive metastatic breast cancer[J].J Clin Oncol,2010,28(6):976-983.
[9]Baselga J,Gelmon KA,Verma S,et al.Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy[J].J Clin Oncol,2010,28(7):1138-1144.
[10]Baselga J,Cortés J,Kim SB,et al.Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer[J].N Engl J Med,2012,366(2):109-119.
[11]Swain SM,Baselga J,Kim SB,et al.Pertuzumab,trastuzumab,and docetaxel in HER-2-positive metastatic breast cancer[J].N Engl J Med,2015,372(8):724-734.
[12]Kaufman B,Mackey JR,Clemens MR,et al.Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive,hormone receptor-positive metastatic breast cancer:results from the randomized phase III TAnDEM study[J].J Clin Oncol,2009,27(33):5529-5537.
[13]Johnston S,Pippen J Jr,Pivot X,et al.Lapatinib combined with letrozole versus letrozole and placebo as first- line therapy for postmenopausal hormone receptor-positive metastatic breast cancer [J].J Clin Oncol,2009,27(33):5538-5546.
[14]Gianni L,Romieu GH,Lichinitser M,et al.AVEREL:a randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER-2-positive locally recurrent/ Metastatic breast cancer[J].J Clin Oncol,2013,31(14):1719-1725.
[15]Gelmon KA,Boyle FM,Kaufman B,et al.Lapatinib or trastuzumab plus taxane therapy for human epidermal growth factor receptor 2-positive advanced breast cancer:final results of NCIC CTG MA.31 [J].J Clin Oncol,2015,33(14):1574-1583.
[16]O'Brien NA,McDonald K,Tong L,et al.Targeting PI3K/mTOR overcomes resistance to HER2-targeted therapy independent of feedback activation of AKT[J].Clin Cancer Res,2014,20(13):3507-3520.
[17]Hurvitz SA,Andre F,Jiang Z,et al.Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER-2-positive advanced breast cancer(BOLERO-1):a phase 3,randomized,double-blind,multicentre trial[J].Lancet Oncol,2015,16 (7):816-829.
[18]Giordano SH,Temin S,Kirshner JJ,et al.Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer:American Society of Clinical Oncology clinical practice guideline[J].J Clin Oncol,2014,32(19):2078-2099.
[19]Cardoso F,Costa A,Norton L,et al.ESO-ESMO 2nd international consensus guidelines for advanced breast cancer(ABC2)[J].Breast,2014,23(5):489-502.
[20]Chinese Anti-Cancer Association Committee of Breast Cancer Society.Chinese expert consensus for diagnosis and treatment of advanced breast cancer(2015 edition)[M].Beijing:People's Medical Publishing House,2015.[中国抗癌协会乳腺癌专业委员会.中国晚期乳腺癌诊治专家共识(2015版)[M].北京:人民卫生出版社,2015.]
[21]Chinese Anti-Cancer Association Committee of Breast Cancer Society.Chinese Anti- Cancer Association Committee guidelines andstandards for breast cancer diagnosis and treatment(2015 edition)[J].Chin Oncol,2015,25(9):641-703.[中国抗癌协会乳腺癌专业委员会.中国抗癌协会乳腺癌诊治指南与规范(2015版)[J].中国癌症杂志,2015,25(9):641-703.]
[22]von Minckwitz G,du Bois A,Schmidt M,et al.Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer:a german breast group 26/breast international group 03-05 study[J].J Clin Oncol,2009,27(12):1999-2006.
[23]Geyer CE,Forster J,Lindquist D,et al.Lapatinib plus capecitabine for HER-2-positive advanced breast cancer[J].N Engl J Med,2006,355(26):2733-2743.
[24]Blackwell KL,Burstein HJ,Storniolo AM,et al.Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive,trastuzumab-refractory metastatic breast cancer[J].J Clin Oncol,2010,28(7):1124-1130.
[25]Blackwell KL,Burstein HJ,Storniolo AM,et al.Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer:final results from the EGF104900 Study[J].J Clin Oncol,2012,30(21):2585-2592.
[26]Verma S,Miles D,Gianni L,et al.Trastuzumab emtansine for HER-2-positive advanced breast cancer[J].N Engl J Med,2012,367(19):1783-1791.
[27]André F,O'Regan R,Ozguroglu M,et al.Everolimus for women with trastuzumab- resistant,HER- 2- positive,advanced breast cancer (BOLERO-3):a randomized,double-blind,placebo-controlled phase 3 trial[J].Lancet Oncol,2014,15(6):580-591.
[28]Avan A,Maftouh M,Ghayour Mobarhan M,et al.Biomarker analysis in CLEOPATRA:searching for a sensitive prognostic factor in breast cancer[J].J Clin Oncol,2015,33(15):1711-1712.
(2016-03-02收稿)
(2016-05-10修回)
(编辑:张抿校对:杨红欣)
孙婧专业方向为肿瘤内科临床与基础研究。E-mail:legendsun001@163.com

·专家论坛·

张力教授中国医学科学院,北京协和医学院,北京协和医院呼吸科主任医师、硕士生导师。主要从事呼吸系统疾病,尤其肺癌的临床、科研和教学工作。参加了30余项国内外临床新药的研发工作。任中华医学会肺癌学组成员、中国交叉科学学会委员、《计算生命科学杂志》(英文)副主编、《国际呼吸杂志》第六届编辑委员会委员和中国肺癌联盟成员,北京分联盟副主席。Journal of Thoracic Oncology中文版编委。获得2015年国家科技进步一等奖。
Therapeutic strategy for HER-2-positive advanced breast cancer
Jing SUN,Pin ZHANG
Department of Medical Oncology Cancer Institute/Hospital,Peking Union Medical College and Chinese Academy of Medical Sciences,Beijing 100021,China
Correspondence to:Pin ZHANG;E-mail:zhang_pin@sina.com
AbstractPatients with HER-2-positive advanced breast cancer were associated with poor prognosis.Meanwhile,HER-2-targeted therapy has dramatically improved survival and prognosis among breast cancer patients.Over the years,multiple HER-2-targeting drugs stepped into clinical practice,and the targeted agents are now considered as the standard of care in the first-line setting and beyond.This review basically summarizes the importance of HER-2-targeted therapy,the significance of the clinical trial results,and the clinical practice guidelines for the management of patients with HER-2-positive advanced breast cancer.
Keywords:HER-2-positive,advanced breast cancer,therapeutic strategy,clinical trials,targeted therapy
doi:10.3969/j.issn.1000-8179.2016.10.237
通信作者:张频zhang_pin@sina.com
作者简介
