外源氮对琼北不同类型土壤甲烷氧化能力的影响
2016-05-30齐润杰陈金霞但建国
齐润杰 陈金霞 但建国

摘 要 氮输入是影响土壤氧化吸收大气甲烷(CH4)的重要因子之一。通过对琼北5种旱地土壤进行室内培养实验,评价不同类型土壤对低浓度CH4的吸收能力及其对硝态氮和不同浓度铵态氮的响应。结果表明:火山灰土、富铁土、雏形土、铁铝土和淋溶土的CH4氧化速率分别为67.01,50.05,47.00,72.82和57.10 ng/(kg·h)。按100(mg/kg)N添加的硝态氮仅对铁铝土CH4氧化有显著的抑制效果,而按此浓度添加的铵态氮能显著降低5种土壤的CH4氧化速率。铵态氮对5种土壤CH4氧化的抑制率与铵态氮添加量均呈极显著的线性正相关。每单位(mg/kg)铵态氮添加量对铁铝土CH4氧化的抑制率最低,仅为其他4种土壤的53%~60%,这很可能跟铁铝土的高C/N和低N/P有关。因此,对这些土壤进行科学管理时,应根据土壤类型选用适宜的N肥类型,同时,还应提高土壤有机质含量,增施P肥,充分发挥土壤对大气CH4的氧化潜力。
关键词 甲烷氧化;土壤类型;硝态氮;铵态氮
中图分类号 Q938.1+3 文献标识码 A
Abstract Nitrogen inputs is one of the key factors affecting atmospheric methane consumption by soils. The soil samples collected from five litchi orchards in northern Hainan Island were incubated at 26 ℃ with gravimetric water content of 20% and an initial CH4 concentration of approximately 10 μL/L,in order to determine the effects of exogenous nitrogen on the methane oxidation in soils of different types. Average rate of CH4 oxidation without N inputs by Andosols,Ferrosols,Cambosols,Ferralsols and Luvisols was 67.01,50.05,47.00,72.82 and 57.10 ng/(kg·h),respectively. Addition of KNO3 at 100 mg N kg-1 d.w.s did not result in significant inhibition of CH4 oxidation in the soils except Ferralsols due to the higher native nitrate content. In contrast,CH4 oxidation in all soils decreased significantly after adding(NH4)2SO4 at 100 mg N kg-1 d.w.s. For all studied soils,percent inhibition by ammonia increased with the rate of ammonia application,which was fit well by linear regression. The slope for Ferralsols was only 0.10 and 53%~60% of those for other soils,indicating that the extent of ammonia inhibition of CH4 oxidation in Ferralsols was the smallest among the soils of the five types. Such response of CH4 oxidation in Ferralsols to added ammonia might be attributed to the highest C:N ratio and the lowest N:P ratio. Therefore,the correct selection of N fertilizers,improvement of organic matter status of soil,and application of P fertilizer were recommended for maintaining high CH4 oxidation rates for upland soils.
Key words Methane oxidation;Soil type;Nitrate;Ammonia
doi 10.3969/j.issn.1000-2561.2016.08.015
甲烷(CH4)是地球大气中的一种温室气体,百年时间尺度上单分子CH4增温潜势是CO2的26倍[1]。CH4对全球变暖贡献约占17%,仅次于CO2[2]。过去200年来,人类活动导致大气CH4浓度增加,至2014年大气CH4浓度已达1.83 μL/L,是工业革命前的2.54倍[2]。大气CH4浓度的持续升高是CH4排放源增加或吸收汇减小的结果[3]。土壤是大气CH4主要的生物汇,土壤中的微生物每年氧化消耗15~45 Tg CH4[4-6],其氧化过程由隶属γ-变形菌纲(Gammaproteobacteria)(即Type Ⅱ型)甲烷氧化菌来完成,包括Methylocystis属、山地土壤菌群α(USCα)和γ(USCγ)等,这些甲烷氧化菌拥有高亲和力的甲烷单加氧酶(MMO)[6-9]。氮输入(施N肥或N沉降)是影响土壤氧化吸收大气CH4的重要因子之一,其效果可归为3类:抑制作用、促进作用和无影响[10-12]。CH4氧化对氮输入的响应往往与所施N肥的种类和数量以及土壤N背景值有关,施用铵态氮的抑制效果比硝态氮更强[13],铵态氮对CH4氧化的抑制作用随铵态氮添加量的加大而增大[14]。“氮饱和”(N-saturated)成熟林土中N添加使CH4氧化显著减少[15-16],而“氮限制”(N-limited)森林土壤的N水平未达抑制临界值,故施N对这些土壤CH4吸收无显著影响[15,17]。此外,土壤对大气CH4的氧化吸收能力跟土壤的类型有关,Akiyama等[18]和Morishita等[19]曾报道,火山灰土的CH4氧化速率远高于其他几种土壤。
海南岛地处热带,既具有热带海洋性气候,又受热带季风气候的影响,成为中国热带土壤最集中分布、土壤资源最丰富的地区[20]。本研究在琼北选取不同土纲的5种荔枝土壤,探讨不同氮浓度及氮肥种类对土壤CH4氧化能力的影响,有助于寻求适宜的管理措施,以增强热带亚热带林地土壤对大气CH4的氧化潜力。
1 材料与方法
1.1 研究地区概况
研究区处于低纬度热带北缘(19°11′-20°04′ N,108°56′-110°42′E),属热带海洋性季风气候。全年日照时间长,光照充足,年日照1 800~2 300 h,年平均气温22.4~24.0 ℃。降雨充沛,年平均降雨量1 434.6~2 447.1 mm[21]。地貌类型以火山岩台地为主,土壤类型以砖红壤为主,水稻土零星分布,局部有火山灰土和石质土。土地利用类型以耕地为主,其次为园地和林地[22]。选取分属于火山灰土、富铁土、雏形土、铁铝土和淋溶土等土纲的5种荔枝园土壤进行研究,它们分别是:火山灰土,位于海口市琼山区永兴镇雷虎村荔枝园,砂土,土壤呈黑色;富铁土,位于儋州市三都乡德义岭北坡荔枝园,黏土,土壤呈红棕色;雏形土,位于儋州市乌石至那大公路45 km处荔枝园,粉砂黏壤土,土壤呈黄橙色;铁铝土,位于海口市琼山区三门坡镇荔枝园,黏土,土壤呈棕色;淋溶土,位于海口市琼山区内村南100 m处荔枝园,砂壤土,土壤呈橙色[20]。
1.2 方法
1.2.1 土样采集与处理 于2014年12月下旬采用五点采样法,在除去地表凋落物和表层土壤后的样点上采集5~15 cm土壤,混合均匀后用塑料袋运回实验室,自然条件下风干,过2 mm筛,一部分土壤用于理化性质的测定(表1)[23],另一部分土壤用于室内培养实验。
1.2.2 室内培养实验 称取相当于20 g干土的供试土样于150 mL厌氧瓶中,分别加入含有0.4、1.2、2、4、6 mg N的(NH4)2SO4和2 mg N的KNO3作为各种外源氮处理,添加无菌去离子水作为不加氮处理(即对照),用无菌去离子水调节土壤水含量至20%;注入一定体积的高浓度CH4标准气体使瓶内顶空CH4浓度达10 μL/L左右[7,24],每处理设3次重复;将所有厌氧瓶放置到26 ℃的黑暗条件下培养16 d,期间每3 d用Agilent 7890a气相色谱仪测定一次瓶内CH4浓度,以已知浓度的CH4标准气体作参照进行计算。根据瓶内顶空CH4浓度随时间变化的直线斜率,计算出CH4氧化速率。外源氮对每种土壤CH4氧化的抑制率按下列公式计算:
PI=(1-Rt/Rc)×100
其中,PI为土壤CH4氧化抑制率(%);Rt为外源氮处理的CH4氧化速率;Rc为不添加氮处理的CH4氧化速率。
1.3 数据分析
采用Excel 2003、SAS 9.1统计软件进行单因素方差分析、相关性分析及Duncan多重比较和作图。
2 结果与分析
2.1 外源硝态氮和铵态氮对土壤CH4氧化速率的影响
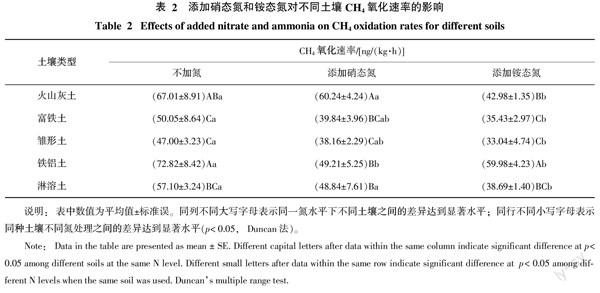
在不添加外源氮条件下,5种土壤对CH4的氧化能力有一定的差异(表2)。铁铝土的CH4氧化速率高,为72.82 ng/(kg·h),火山灰土[67.01 ng/(kg·h)]次之。富铁土、雏形土和淋溶土的CH4氧化速率介于47.00~57.10 ng/(kg·h),这3种土壤之间没有显著差异(p>0.05)。土壤的各种理化参数中(表1),仅C/N和全磷与CH4氧化速率的相关性达到了显著水平,均呈正相关关系,其相关系数分别为0.928和0.911。
按100(mg/kg)N添加硝态氮后,以火山灰土的CH4氧化速率最高,其值为60.24 ng/(kg·h),雏形土的CH4氧化速率最低,仅为38.16 ng/(kg·h)(见表2)。火山灰土CH4氧化速率与其他4种土壤CH4氧化速率之间的差异均达到显著水平(p<0.05)。
在添加铵态氮[100(mg/kg)N]条件下,铁铝土CH4氧化速率为59.98 ng/(kg·h),显著高于其他4种土壤的CH4氧化速率(p<0.05)(表2)。富铁土和雏形土的CH4氧化速率比较低,介于33.04~35.43 ng/(kg·h)。
外源硝态氮和铵态氮对CH4氧化的抑制效果随土壤种类而异。除铁铝土外,铵态氮对其余4种土壤CH4氧化的抑制作用均高于硝态氮(表2)。硝态氮仅对铁铝土的CH4氧化有显著的抑制作用(p<0.05),其抑制率高达32.42%。铵态氮对5种土壤的CH4氧化均有显著的抑制作用(p<0.05),抑制率介于17.64%~35.86%。
2.2 添加不同浓度铵态氮对土壤CH4氧化速率的影响
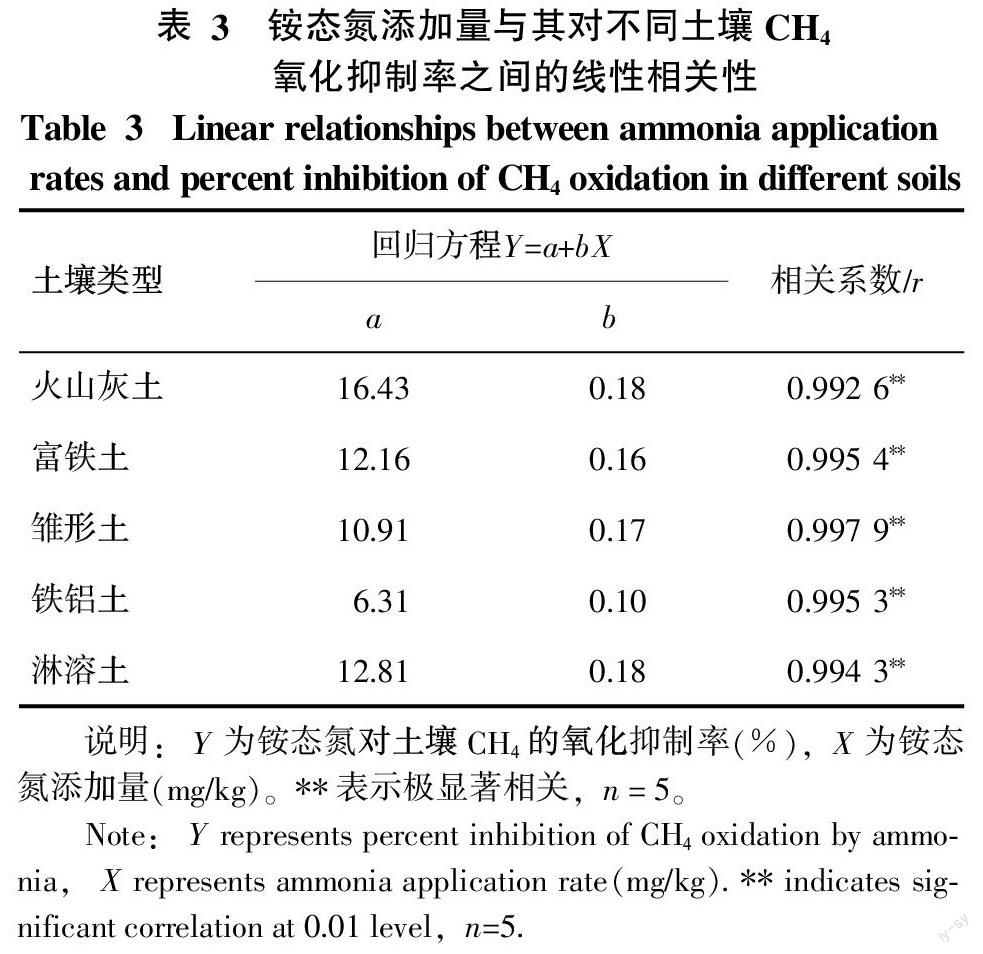
按20~300(mg/kg)N添加铵态氮后,5种土壤的CH4氧化速率均随铵态氮浓度的增加而下降。铵态氮对每种土壤CH4氧化的抑制率与铵态氮的添加量均呈极显著的正相关(表3)。表3中回归方程的参数b用来表征单位铵态氮添加量对土壤CH4氧化的抑制潜能。铁铝土的参数b值仅为0.10,而其他4种土壤的b值比较接近,介于0.16~0.18。由此可见,外源铵态氮对铁铝土CH4氧化的抑制潜能比其他4种土壤低40%~47%。
3 讨论与结论
本研究采用室内培养实验评价了5种荔枝土壤对低浓度CH4的氧化能力的影响,其氧化速率大小排序为:铁铝土>火山灰土>淋溶土>富铁土>雏形土。国外的研究曾证实,火山灰土对大气CH4的吸收能力远高于其他土壤[18-19,25]。
不同类型的土壤理化特性不同。土壤CH4氧化速率与土壤理化特征值的相关分析结果表明,土壤C/N和全磷含量愈高,CH4氧化速率愈高。铁铝土和火山灰土的C/N和全磷含量位列前2位,所以,这2种土壤有较强的CH4氧化能力。富铁土和雏形土拥有较低的C/N和全磷,其CH4氧化速率显著低于前2种土壤。Tamai等[26]对树龄为30~40年的日本扁柏林的研究表明,土壤对大气CH4的吸收与土壤C/N呈显著正相关。Veraart等[27]发现土壤P含量跟CH4氧化呈正相关。增施P肥能提高土壤中大气CH4的氧化速率[16]。土壤C/N和全磷对CH4氧化的影响应该与相关微生物群落结构的变化有关,土壤C/N和P含量是影响土壤细菌群落结构的主要因子[28-29]。长期施N可使高亲和力TypeⅡ型甲烷氧化菌种群数量减少70%以上[30]。土壤中微生物的活性因缺P而被抑制[31],施用P肥可使土壤微生物的生物量显著增加[32-33]。
土壤CH4氧化速率可能还受土壤铝含量的影响。在本研究中,5种土壤均为酸性土,雏形土的交换性铝含量最高,为3.42 cmol/kg。当pH<4.8时,土壤铝的溶解性会显著增加,难溶性铝转变成交换性铝,添加水溶性铝盐能抑制土壤对大气CH4的氧化能力[24,26,34],所以,一旦土壤pH偏低或出现酸化,铝会大量溶出,土壤对大气CH4的氧化能力就会降低。在温带森林土壤[35]和热带土壤[36]均发现了铝毒对CH4氧化的抑制作用。铁铝土的交换性铝含量也较高,仅次于雏形土,但是前者含有较多的P,而P能吸附铝[37],可减轻铝对甲烷氧化菌的毒害作用[15,38]。
土壤CH4氧化对外源氮的响应往往与外源氮的种类和数量以及土壤N背景值有关。本研究结果表明,按100(mg/kg)N添加硝态氮后,只有铁铝土的CH4氧化过程受到显著的抑制,这可能跟该土壤的硝态氮背景值较高有关,铁铝土硝态氮含量高达67.88 mg/kg,其他4种土壤仅为13.46~18.79 mg/kg。按100(mg/kg)N添加的铵态氮对5种土壤的CH4氧化均有显著的抑制作用。除铁铝土外,铵态氮对CH4氧化的抑制作用均大于硝态氮。Li等[39]在亚热带雏形土的研究中也观测到类似的现象,铵态氮对CH4氧化有更强的抑制效果。国外研究者还证实,施用尿素后黄杉林火山灰土[25]和亚热带草原富铁土[40]中大气CH4的氧化过程受到明显的抑制。在本研究中,铵态氮对5种土壤CH4氧化的抑制作用均随铵态氮添加量的加大而增大,关于铵态氮的这种浓度效应已有报道,如温带淋溶土[14]和亚热带雏形土[39]。然而,每单位(mg/kg)铵态氮添加量对CH4氧化的抑制潜能跟土壤类型有密切的关系。每单位铵态氮添加量对铁铝土CH4氧化的抑制潜能比其他4种土壤都低,这可能跟土壤的C/N和N/P有关,在5种土壤中,铁铝土拥有最高的C/N和最低的N/P。土壤C/N是指土壤有机质中的有机碳总量和氮素总量之比,其大小可反映有机质的分解状况,被认为是氮素矿化能力的标志。土壤氮的总矿化速率跟土壤C/N呈负相关[41-43],所以,高C/N意味着土壤内部铵态氮的供应量少。铁铝土铵态氮背景值的确比其他4种土壤都低(见表1)。此外,有研究表明,施P肥能有效缓解氮沉降对CH4氧化的抑制作用[16],此现象意味着N/P变小时外源氮对土壤CH4氧化的抑制潜能会下降。
综上所述,琼北5种不同类型旱地土壤对CH4的吸收能力有一定的差异。外源硝态氮和铵态氮对土壤CH4氧化的抑制效果随土壤的类型、C/N和全P而异。因此,对这些土壤进行科学管理时,应根据土壤类型选用适宜的N肥类型;同时,还应提高土壤有机质含量,增施P肥,进而促使土壤对大气CH4的氧化能力维持在较高的水平上。
参考文献
[1] IPCC. Climate Change 2013: The Physical Science Basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change[M]. Cambridge: Cambridge University Press, 2013, 1-1535.
[2] World Meteorological Organization. The state of greenhouse gases in the atmosphere based on global observations through 2014[J]. WMO greenhouse gas bulletin, 2015, 11: 1-4.
[3] Dalal R C, Allen D E, Livesley S J, et al. Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: a review[J]. Plant and Soil, 2008, 309(1-2): 43-76.
[4] Conrad R. The global methane cycle: recent advances in understanding the microbial processes involved[J]. Environmental Microbiology Reports, 2009, 1(5): 285-292.
[5] Curry C L. The consumption of atmospheric methane by soil in a simulated future climate[J]. Biogeosciences, 2009, 6(11): 2 355-2 367.
[6] Kolb S. The quest for atmospheric methane oxidizers in forest soils[J]. Environmental Microbiology Reports, 2009, 1(5): 336-346.
[7] Lima A B, Muniz A W, Dumont M G. Activity and abundance of methane-oxidizing bacteria in secondary forest and manioc plantations of Amazonian Dark Earth and their adjacent soils[J]. Frontiers in Microbiology, 2014, 5: 550.
[8] Knief C. Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker[J]. Frontiers in Microbiology, 2015, 6(487): 1-38.
[9] 张坚超, 徐镱钦, 陆雅海.陆地生态系统甲烷产生和氧化过程的微生物机理[J]. 生态学报, 2015, 35(20): 6 592-6 603.
[10] Bodelier P L E. Interactions between nitrogenous fertilizers and methane cycling in wetland and upland soils[J]. Current Opinion in Environmental Sustainability, 2011, 3(3): 379-388.
[11] 程淑兰, 方华军, 于贵瑞, 等.森林土壤甲烷吸收的主控因子及其对增氮的响应研究进展[J]. 生态学报, 2012, 32(15): 4 914-4 923.
[12] Shrestha R K, Strahm B D, Sucre E B. Greenhouse gas emissions in response to nitrogen fertilization in managed forest ecosystems[J]. New Forests, 2015, 46(2): 167-193.
[13] Wang Y, Cheng S, Fang H, et al. Simulated nitrogen deposition reduces CH4 uptake and increases N2O emission from a subtropical plantation forest soil in Southern China[J]. PLOS ONE, 2014, 9(4): e93 571.
[14] Hütsch B W. Methane oxidation in arable soil as inhibited by ammonium, nitrite and organic manure with respect to soil pH[J]. Biology and Fertility of Soils, 1998, 28(1): 27-35.
[15] Zhang W, Mo J, Zhou G, et al. Methane uptake responses to nitrogen deposition in three tropical forests in southern China[J]. Journal of Geophysical Research, 2008, 113(D11): 3 078.
[16] Zhang T, Zhu W, Mo J. Increased phosphorus availability mitigates the inhibition of nitrogen deposition on CH4 uptake in an old-growth tropical forest, southern China[J]. Biogeosciences, 2011, 8(9), 2 805-2 813.
[17] Veldkamp E, Koehler B, Corre M D. Indications of nitrogen-limited methane uptake in tropical forest soils[J]. Biogeosciences Discussions, 2013, 10(3): 6 007-6 037.
[18] Akiyama H, Morimoto S, Tago K, et al. Relationship between ammonia oxidizers and N2O and CH4 fluxes in agricultural fields with different soil types[J]. Soil Science and Plant Nutrition, 2014, 60(4): 520-529.
[19] Morishita T, Sakata T, Takahashi M, et al. Methane uptake and nitrous oxide emission in Japanese forest soils and their relationship to soil and vegetation types[J]. Soil Science and Plant Nutrition, 2007, 53(5): 678-691.
[20] 龚子同, 张甘霖, 漆智平.海南岛土系概论[M]. 北京: 科学出版社, 2004: 99-211.
[21] 海南省人民政府. 海南年鉴2013[M]. 海口: 海南年鉴社, 2013: 60-61.
[22] 海南省地质调查院. 琼北火山岩区农田土壤重金属生态地球化学评价报告[R]. 海口: 海南省地质调查院, 2010.
[23] 鲍士旦. 土壤农化分析(第三版)[M]. 北京: 中国农业出版社, 2000.
[24] Nanba K, King G M. Response of atmospheric methane consumption by Maine forest soils to exogenous aluminum salts[J]. Applied and Environmental Microbiology, 2000, 66(9): 3 674-3 679.
[25] Shrestha R K, Strahm B D, Sucre E B, et al. Fertilizer management, parent material and stand age influence forest soil greenhouse gas fluxes[J]. Soil Science Society of America Journal, 2014, 78(6): 2 041-2 053.
[26] Tamai N, Takenaka C, Ishizuka S, et al. Methane flux and regulatory variables in soils of three equal-aged Japanese cypress(Chamaecyparis obtusa)forests in central Japan[J]. Soil Biology and Biochemistry, 2003, 35(35): 633-641.
[27] Veraart A J, Steenbergh A K, Ho A, et al. Beyond nitrogen: the importance of phosphorus for CH4 oxidation in soils and sediments[J]. Geoderma, 2015, (259-260): 337-346.
[28] Nielsen U N, Osler G H R, Campbell C D, et al. The influence of vegetation type, soil properties and precipitation on the composition of soil mite and microbial communities at the landscape scale[J]. Journal of Biogeography, 2010, 37(7): 1 317-1 328.
[29] Kuramae E E, Yergeau E, Wong L C, et al. Soil characteristics more strongly influence soil bacterial communities than land-use type[J]. FEMS Microbiology Ecology, 2012, 79(1): 12-24.
[30] Maxfield P J, Hornibrook E R C, Evershed R P. Acute impact of agriculture on high-affinity methanotrophic bacterial populations[J]. Environmental Microbiology, 2008, 10(7): 1 917-1 924.
[31] Cleveland C C, Townsend A R, Schmidt S K. Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies[J]. Ecosystems, 2002, 5(7): 680-691.
[32] Liu L, Gundersen P, Zhang T, et al. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China[J]. Soil Biology and Biochemistry, 2012, 44(1): 31-38.
[33] Liu L, Zhang T, Gilliam F S, et al. Interactive effects of nitrogen and phosphorus on soil microbial communities in a tropical forest[J]. PLOS ONE, 2013, 8(4): 61.
[34] Tamai N, Takenaka C, Ishizuka S. Water-soluble al inhibits methane oxidation at atmospheric concentration levels in Japanese forest soil[J]. Soil Biology and Biochemistry, 2007, 39(7): 1 730-1 736.
[35] Benstead J, King G M. The effect of soil acidification on atmospheric methane uptake by a Maine forest soil[J]. FEMS Microbiology Ecology, 2001, 34(3): 207-212.
[36] Hassler E, Corre M D, Tjoa A, et al. Soil fertility controls soil-atmosphere carbon dioxide and methane fluxes in a tropical landscape converted from lowland forest to rubber and oil palm plantations[J]. Biogeosciences, 2015, 12(12): 5 831-5 852.
[37] Frossard E, Brossard M, Hedley MJ, et al. Reactions controlling the cycling of P in soils[M]∥Tiessen H. Phosphorus in the global environment: transfers, cycles and management. New York: John Wiley & Sons,1995: 107-137.
[38] Bradford M A, Ineson P, Wookey P A, et al. The effects of acid nitrogen and acid sulphur deposition on CH4 oxidation in a forest soil: a laboratory study[J]. Soil Biology and Biochemistry, 2001, 33(1): 1 695-1 702.
[39] Li X, Cheng S, Fang H, et al. The contrasting effects of deposited NH4+ and NO3- on soil CO2, CH4 and N2O fluxes in a subtropical plantation, Southern China[J]. Ecological Engineering, 2015, 85: 317-327.
[40] Scheer C, Grace P R, Rowlings D W, et al. Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia[J]. Plant and Soil, 2010, 345(1): 47-58.
[41] Booth M S, Stark J M, Rastetter E. Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data[J]. Ecological Monographs, 2005, 75: 139-157.
[42] Hogberg M N, Chen Y, Hogberg P. Gross nitrogen mineralisation and fungi-to-bacteria ratios are negatively correlated in boreal forests[J]. Biology and Fertility of Soils, 2007, 44(2): 363-366.
[43] Christenson L M, Lovett G M, Weathers K C, et al. The influence of tree species, nitrogen fertilization, and soil C to N ratio on gross soil nitrogen transformations[J]. Soil Science Society of America Journal, 2009, 73(2): 638-646.
