荒漠灌木梭梭(Haloxylon ammodendron)周围土壤微生物的空间分布
2016-05-23曹艳峰李晨华吕光辉
曹艳峰,李 彦, 李晨华,吕光辉
1 中国科学院新疆生态与地理研究所,荒漠与绿洲生态国家重点实验室, 乌鲁木齐 830011 2 新疆大学资源与环境学院, 乌鲁木齐 830046 3 山西师范大学地理科学学院, 临汾 041000 4 中国科学院大学, 北京 100049
荒漠灌木梭梭(Haloxylon ammodendron)周围土壤微生物的空间分布
曹艳峰1,2,3,4,李彦1,*, 李晨华1,吕光辉2
1 中国科学院新疆生态与地理研究所,荒漠与绿洲生态国家重点实验室, 乌鲁木齐8300112 新疆大学资源与环境学院, 乌鲁木齐8300463 山西师范大学地理科学学院, 临汾0410004 中国科学院大学, 北京100049
摘要:以古尔班通古特沙漠荒漠生态系统中主要优势种——梭梭(Haloxylon ammodendron)冠幅内外的土壤为研究对象,通过分析从树干基部向外水分和养分变化下土壤微生物生物量、多样性和群落组成的变化规律,试图揭示荒漠生态系统中土壤微生物空间分布的基本特征。微生物生物量和多样性采用磷脂脂肪酸进行测定。结果表明:从树干基部向外,土壤微生物总生物量、多样性以及不同微生物类群的生物量均随土壤水分和养分含量降低而逐渐降低,指示了土壤微生物从裸地向内集聚分布的特征。另外,在微生物群落组成中,不同类群微生物的生物量从树干基部向外的减少程度有所不同,例如,从树干基部附近5 cm处到冠外裸地200 cm处,真菌、细菌、革兰氏阳性菌、革兰氏阴性菌的生物量分别减少了50.25%、84.06%、82.70%、92.57%,指示了细菌从裸地向内集聚分布的程度强于真菌,革兰氏阴性菌从裸地向内集聚分布的程度也较强于革兰氏阳性菌。研究表明,在资源稀缺的荒漠生态系统中,土壤微生物倾向于分布在冠内资源丰富的区域。土壤微生物的空间分布不仅与资源的丰富度有关,而且与微生物自身对环境变化的忍耐性或敏感性有关。
关键词:古尔班通古特沙漠;梭梭;微生物生物量;空间分布
在资源限制的荒漠环境中,荒漠植物和土壤资源形成了斑块状的分布格局,即“资源岛”或“肥岛”[1-3],从冠内向冠外水分和养分含量呈降低趋势[4]。土壤微生物是环境敏感的生物学指标,其生物量、多样性和群落组成与水分、养分密切相关。普遍的观点认为,与冠外土壤相比,冠内土壤拥有更高的碳、氮含量,从而支持了更高的微生物生物量和多样性[5-7]。但也有研究认为,冠内土壤中微生物的生物量和多样性高于冠外土壤,与水分和养分无关,而与微气候因子如温度和辐射有关[8-9]。因此,土壤水分和养分是否是微生物生物量和多样性从冠内向冠外变化的决定性因子仍不确定,这需要进一步研究。
在微生物群落组成中,不同的微生物类群对水分和养分变化的忍耐性或敏感性与它们自身的生理特性有关。通常认为,在水分短缺、养分贫瘠的环境中,细菌比真菌具有更强的忍耐力[10]。真菌具有更加灵活的适应策略,它们有庞大的菌丝网络,这些菌丝有利于水分和养分进行长距离运输,并可深入植物根系不可到达的领域探索新的底物[11],使真菌能够在一个地方获得碳而在另一个地方获得氮,就像植物从空气中获得碳而从土壤获得水和营养一样[12]。相比之下,细菌具有机会主义的适应策略:当底物丰富时,细菌迅速地吸收可溶性底物,且快速生长和分裂;当面临环境胁迫如干旱时,它们不得不躲避在土壤的小孔隙中,或通过平衡土壤溶质渗透势的增加,甚至休眠[13]。因此,与细菌相比,真菌对水分和养分变化的敏感性较弱。另外,在细菌群落中,革兰氏革兰氏阳性菌比革兰氏革兰氏阴性菌对水分和养分的胁迫具有更强的抵抗能力[13],被称为“耐旱的广布属”[14]。总之,不同的微生物类群对水分和养分变化的忍耐性或敏感性明显不同。那么,它们在荒漠灌木周围如何分布?为此,有必要进行综合评价。
本研究以荒漠生态系统优势种——梭梭冠幅内外的土壤为研究对象,探讨从冠内向冠外水分和养分变化下土壤微生物生物量、多样性和群落组成的变化特征,为加深认识荒漠生态系统中土壤微生物的空间分布格局,以及植物-土壤-微生物之间的相互关系提供理论基础。
1材料与方法
1.1研究地点
本实验开展于中国科学院新疆生态与地理研究所阜康荒漠生态试验站(44°17′N, 87°56′E, 475 m a.s.l)附近的梭梭(Haloxylonammodendron)原生栖息地,即古尔班通古特沙漠南缘。该区的气候属于大陆性干旱气候,冬季严寒而漫长,夏季炎热而干燥,春秋温凉而短暂,年平均降水量约为160 mm,年平均气温约为6.6 ℃。梭梭为荒漠生态系统中主要的优势种和初级生产者,在维持生态系统结构和功能上具有极其重要的作用[15-16]。地带性土壤为风沙土,其质地为壤质细砂,砂粒、粉粒和粘粒的比例分别为81.68%、16.84%、1.47%。

图1 土样采集示意图Fig.1 The sketch of soil sampling at individual scale 图中心的黑色圆点表示树干基部所在的位置;与树干基部的距离为5、40、100、200 cm的黑色圆点表示采样点;黑色箭头表示采样方向,相邻方向之间的夹角为90°
1.2野外采集
于2013年4月底,在古尔班通古特沙漠南缘北沙窝(距阜康荒漠生态系统试验站约20 km)的沙丘间平地,选择基茎(9—10 cm)、冠幅(约200 cm × 200 cm)和株高(200—210 cm)相近的梭梭3株,采用土钻法,采集0—20 cm深度的土样。以树干基部为中心,选择相邻之间夹角为90°的4个方向,沿这4个方向向外逐步画圆采集土样,将同一距离不同方向采点的土样混合成一个土样,采样点与树干基部的距离分别为5、40、100、200 cm,依次位于树干基部附近、冠幅中央、冠幅边缘、冠幅外裸地(图1)。
将采集的土样装入无菌袋,并置于保鲜箱中,运回实验室,立即过孔径为2 mm的筛子,去除植物根系、石块等杂物后,分成两份,一份用于土壤理化因子的测定,另一份保存在4 ℃的恒温冰箱中,用于土壤微生物生物量和群落组成的分析。
1.3室内测定
土壤水分含量(SWC)采用烘干法,在105 ℃下持续烘干24 h;土壤有机碳(SOC)测定采用重铬酸钾外加热法[17];土壤有效氮(AN)采用碱解扩散法[17];土壤有效磷(AP)采用分光光度计分析法[17];土壤pH值和电导率(EC)采用水土比1∶5待测液测定[17];土壤质地采用激光粒度仪分析(Sympatec GmbH, System-Partikel-Technik, and Clausthal-Zellerfeld, Germany)。
磷脂脂肪酸(PLFA)生物标记法是一种定性和定量分析土壤微生物多样性和群落组成的方法[18],故本研究采用该方法对土壤中微生物生物量和群落组成进行评价,基本过程为:运用甲醇∶氯仿∶柠檬酸缓冲液=2∶1∶0.8的提取液将土样中的总脂类提取出来,经SPE硅胶柱分离纯化得到磷脂脂肪酸(PLFAs),磷脂脂肪酸甲基化后转化为脂肪酸甲酯,采用Agilent 6890N气相色谱仪分析PLFAs的成分,内标为正十九烷酸甲酯(19∶0)[19-20]。PLFAs的鉴定采用美国MIDI公司开发的Sherlock MIS 4.5系统(Sherlock Microbial Identification System, Version 4.5, Inc., Newark, DE)。所有检测到的PLFAs用来计算其总浓度(total PLFAs)和总数量(PLFA number)。以i12:0、i13:0、a13:0、i14:0、a14:0、i15:0、a15:0、i16:0、a16:0、i17:0、a17:0标记革兰氏革兰氏阳性菌[21-22],以16:1w5t、18:1w7c、17:1w8c、cy17:0、16:1 2OH标记革兰氏革兰氏阴性菌[22-23],12:0、14:0、15:0、17:0标记普通细菌[24,25]。革兰氏阳性菌、革兰氏阴性菌和普通细菌综合代表细菌。16:1w5t和18:1w9c标记真菌[26-27]。
1.4统计分析
采用最小显著差异性(LSD)比较土壤性质和微生物指标在不同距离点之间的差异性,由SPSS 16.0软件完成。采用冗余分析法(redundancy analysis, RDA)分析土壤性质与微生物生物量及相关指标之间的相关性,由CANOCO 4.5(Microcomputer Power, NY)软件完成。在RDA分析过程中,采用前向选择(Forward selection)对土壤因子进行逐个筛选,每一步均采用蒙特卡罗(Monte-Carlo)排列检验,排列重复次数为999次,显著水平P<0.05。
2结果与分析
2.1土壤性质
从树干基部向外,土壤各理化因子随距离增加的变化趋势有所不同(图2),具体为:土壤水分、有机碳、有效磷、有效氮的含量随距离增加而逐渐降低,并且在各距离之6间具有显著的差异性(P<0.05);pH值随距离增加而不断增加,在各距离之间的差异性也显著(P<0.05);EC值随距离增加却呈波动变化,靠近树干基部5 cm处的EC值低于冠下中央40 cm的EC值(可能与树干茎流的长期冲蚀有关),而从40 cm起到冠外200 cm处,EC值不断下降,在不同距离之间没有显著的差异性(P>0.05)。
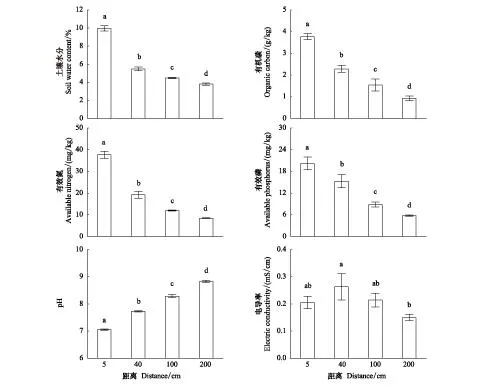
图2 土壤水分、有机碳、有效氮、有效磷、pH和电导率从树干基部向外的变化Fig.2 The changes of soil water content, organic carbon, available nitrogen, available phosphorus, pH and electric conductivity from tree base不同小写字母表示差异达显著水平(P<0.05)
2.2微生物生物量和群落结构组成
磷脂脂肪酸的浓度(nmol/g)可表示微生物的生物量[28],磷脂脂肪酸的类别数量可表示微生物的多样性[29]。从树干基部向外,微生物的总生物量和多样性以及真菌、细菌、革兰氏阳性和阴性细菌的生物量都从树干基部向外随距离增加呈减少趋势(图3,图4),表明土壤微生物从冠外裸地向内具有集聚分布的特征。

图3 PLFA总浓度和数量从树干基部向外的变化Fig.3 The changes of total PLFA concentration and number from tree base不同小写字母表示差异达显著水平(P<0.05)

图4 细菌、真菌、革兰氏革兰氏阳性菌和革兰氏革兰氏阴性菌的PLFA浓度从树干基部向外的变化Fig.4 The changes of PLFA concentrations of bacteria, fungi, Gram-positive and Gram-negative bacteria from tree base不同小写字母表示差异达显著水平(P<0.05)
在微生物群落组成中,从树干基部向外,主要类群微生物生物量的减少程度不同。例如,与树干基部5 cm处相比,冠外200 cm之间处真菌、细菌、革兰氏阳性菌、革兰氏阴性菌的生物量分别减少了50.25%、84.06%、82.70%、92.57%(图4)。另外,不同类群微生物的生物量在各距离之间的差异性也有所不同,细菌和革兰氏阴性菌的生物量在各距离之间均有显著的差异性(P<0.05),真菌生物量在40 cm和100 cm 之间(P>0.05)、100 cm和200 cm之间(P>0.05)均无显著的差异性,革兰氏阳性菌生物量在5 cm和40 cm之间(P>0.05)也没有显著的差异性(图4)。可见,从树干基部向外随距离增加,细菌生物量快速减少,而真菌生物量缓慢减少,表明从冠外裸地向内细菌集聚分布的程度强于真菌;同样,从树干基部向外,革兰氏阴性细菌生物量呈显著减少,且减少速率较高,相比之下,革兰氏阳性细菌生物量的减少程度不够显著,且减少速率较低,表明从冠外裸地向内革兰氏阴性菌的集聚分布的程度也强于革兰氏阳性菌。
2.3相关性分析
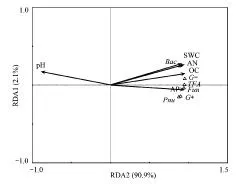
图5 微生物PLFA浓度与土壤性质之间的冗余分析(RDA) Fig.5 The redundancy analysis (RDA) of microbial PLFA concentrations and soil propertiesTPA, PAn, Bac, Fun, G+, G-分别表示总PLFA总浓度,PLFA数量,细菌,真菌,革兰氏阳性菌,革兰氏阴性菌;AP, AN, OC, SWC, pH分别表示有效磷,有效氮,有机碳,土壤水分含量,pH值
以磷脂脂肪酸分析获得的微生物总生物量、多样性和各类群微生物生物量组成微生物参数矩阵,以土壤性质作为环境变量矩阵,来分析微生物与土壤性质之间的相关性(图5)。第一轴(RDA 1)解释了总变异的90.9%,第二轴(RDA 2)解释了总变异的2.1%。土壤微生物生物量和多样性与水分(SWC)(P=0.002)、有机碳(OC)(P=0.002)、有效氮(AN)(P=0.002)、有效磷(AP)(P=0.002)显著正相关,与pH值显著负相关(P=0.002),而与电导率(EC)无显著的相关性(P=0.512)。在微生物群落组成中,不同的微生物类群与土壤水分和养分的相关性有所不同。与真菌相比,细菌和革兰氏阴性菌与土壤水分、有效氮和有机碳的相关性较强。与革兰氏阳性菌相比,革兰氏阴性菌与土壤水分、有效氮和有机碳的相关性较强。
4讨论
土壤养分如有机质、氮是土壤中微生物种群数量的空间分布具有主要的控制作用[30]。在本研究中,微生物生物量和多样性从树干基部向外逐渐减少,其变化模式与水分和养分的变化模式类似,而且RDA结果也表明土壤水分和养分对微生物生物量和多样性变化具有显著影响,这与微生物生物量和多样性主要受土壤碳、氮影响的普遍观点基本一致[5-7]。从树干基部向外,水分含量的降低减少了含水孔隙的比例,降低了养分在土壤的迁移效率,减弱了土壤微生物与底物之间的联系[31],导致土壤微生物的生物量和活性降低。相比之下,提高水分含量可以激发微生物的活性,有利于微生物生存和发育。此外,在土壤养分中,有机碳是微生物重要的食物资源[32]。当距树干基部越近时,有机碳的含量越高,提高了微生物的生物量和多样性[7];相反,当离树干基部越远时,微生物可能会面临食物资源的短缺,使微生物的生物量和多样性降低。总之,当前结果表明,微生物的生物量和多样性随水分和养分含量的减少而降低,这与地中海区和半干旱草原区的研究结果一致[33-35]。然而,别的研究表明,微生物生物量和多样性从冠内向冠外的降低是温度和辐射的作用结果,与水分和养分无关[9]。事实上,土壤因子如有机碳、水分对生活于土壤中的微生物的影响更为直接,而温度和辐射是通过改变土壤水分和养分的含量和有效性,进而对微生物产生影响[25]。
在微生物类群中,从冠外裸地向内,真菌的集聚程度弱于细菌,RDA结果也显示水分和养分与细菌的相关性较强于真菌,表明真菌对水分和养分变化的敏感性弱于细菌。Schimel 等[13]和Strickland 等[36]研究表明,与细菌相比,真菌具有低水分和养分需求的特征,在资源稀缺的环境中仍能很好地生存和发育,在资源丰富的环境中也不会快速地生长,这支持了上述结果。由于庞大的菌丝网络,真菌能够从更大的土壤空间中吸取水分和养分,从而减弱了真菌对土壤资源的依赖性,使得它们比细菌拥有更为灵活的适应策略。相比之下,细菌属于机会主义适应策略的类群,对水分和养分的变化有较强的敏感性和依赖性[12]。另外,真菌和细菌可能具有不同的生长型。当土壤环境中有大量的营养被供给时,r-策略者将最大程度地汲取营养,并快速生长和发育;当营养变得稀缺时,r-策略者将生长速率降低,而此时k-策略者对营养的吸取才获得竞争优势[37]。基于此,细菌应归于r-策略生长型,而真菌应属于k-策略生长型。
在细菌群落中,从冠外裸地向内,与革兰氏阳性菌相比,革兰氏阴性菌集聚程度分布的程度较强,且RDA结果也显示水分、养分与革兰氏阴性菌的相关性强于革兰氏阳性菌。这表明,在冠外土壤资源较为稀缺的微生境中,革兰氏阳性菌比阴性菌具有更为较强的适应优势。Schimel等[13]和Lennon等[14]研究表明,革兰氏阳性菌是适应干旱的广布属,在资源稀缺甚至胁迫的环境中,它们具有较强的忍耐性和抵抗能力,相比之下,革兰氏阴性菌对资源胁迫的抗性较弱,更倾向于在资源丰富的环境中生存和发育,这支持了当前的研究结果。总之,革兰氏阳性菌对环境变化的忍耐性较强,而革兰氏阴性菌的忍耐性较弱。
5结论
(1)在资源稀缺的荒漠生态系统中,微生物的空间分布对土壤资源如水分和养分具有很强的依赖性,为了满足基本的生存需求,其倾向于集聚在冠内资源丰富的区域。
(2)微生物的空间分布不仅取决于资源的丰富程度,而且也取决于微生物自身对资源变化的忍耐性或敏感性。从冠外裸地向内,敏感型微生物类群(如革兰氏阴性菌)集聚分布的程度强于忍耐型微生物类群(如真菌、革兰氏阳性菌)。
参考文献(References):
[1]Maestre F T, Bowker M A, Puche M D, Hinojosa M B, Martínez I, García-Palacios P, Castillo A P, Soliveres S, Luzuriaga A L, Sánchez A M, Carreira J A, Gallardo A, Escudero A. Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecology Letters, 2009, 12(9): 930- 941.
[2]李君, 赵成义, 朱宏, 王锋. 柽柳(Tamarixspp.)和梭梭(Haloxylonammodendron)的“肥岛”效应. 生态学报, 2007, 27(12): 5138- 5147.
[3]Aguiar M R, Sala O E. Patch structure, dynamics and implications for the functioning of arid ecosystems. Trends in Ecology & Evolution, 1999, 14(7): 273- 277.
[4]Moro M J, Pugnaire F I, Haase P, Puigdefábregas J. Mechanisms of interaction between a leguminous shrub and its understorey in a semi-arid environment. Ecography, 1997, 20(2): 175- 184.
[5]Schaeffer S M, Billings S A, Evans R D. Responses of soil nitrogen dynamics in a Mojave desert ecosystem to manipulations in soil carbon and nitrogen availability. Oecologia, 2003, 134(4): 547- 553.
[6]Schaeffer S M, Evans R D. Pulse additions of soil carbon and nitrogen affect soil nitrogen dynamics in an arid Colorado Plateau shrubland. Oecologia, 2005, 145(3): 425- 433.
[7]Ewing S A, Southard R J, Macalady J L, Hartshorn A S, Johnson M J. Soil microbial fingerprints, carbon, and nitrogen in a Mojave Desert creosote-bush ecosystem. Soil Science Society of America Journal, 2007, 71(2): 469- 475.
[8]Clark J S, Campbell J H, Grizzle H, Acosta-Martìnez V, Zak J C. Soil microbial community response to drought and precipitation variability in the Chihuahuan Desert. Microbial Ecology, 2009, 57(2): 248- 260.
[9]Bachar A, Soares M I M, Gillor O. The effect of resource islands on abundance and diversity of bacteria in arid soils. Microbial Ecology, 2012, 63(3): 694- 700.
[10]Manzoni S, Schimel J P, Porporato A. Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology, 2012, 93(4): 930- 938.
[11]Joergensen R G, Wichern F. Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biology and Biochemistry, 2008 40(12): 2977- 2991.
[12]Chapin F S, Matson P A, Mooney H A. Principles of Terrestrial Ecosystem Ecology. New York: Springer-Verlag, 2002.
[13]Schimel J, Balser T C, Wallenstein M. Microbial stress-response physiology and its implications for ecosystem function. Ecology, 2007, 88(6): 1386- 1394.
[14]Lennon J T, Aanderud Z T, Lehmkuhl B K, Schoolmaster D R. Mapping the niche space of soil microorganisms using taxonomy and traits. Ecology, 2012, 93(8): 1867- 1879.
[15]Xu H, Li Y. Water-use strategy of three central Asian desert shrubs and their responses to rain pulse events. Plant and Soil, 2006, 285(1- 2): 5- 17.
[16]Xu H, Li Y, Xu G Q, Zou T. Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant, Cell & Environment, 2007, 30(4): 399- 409.
[17]鲍士旦. 土壤农化分析(第三版). 北京: 中国农业出版社, 2000.
[18]张秋芳, 刘波, 林营志, 史怀, 杨述省, 周先冶. 土壤微生物群落磷脂脂肪酸PLFA生物标记多样性. 生态学报, 2009, 29(8): 4127- 4137.
[19]Frostegård A, Tunlid A, Bååth E. Microbial biomass measured as total lipid phosphate in soils of different organic content. Journal of Microbiological Methods, 1991, 14(3): 151- 163.
[20]Wu Y P, Ding N, Wang G, Xu J M, Wu J J, Brookes P C. Effects of different soil weights, storage times and extraction methods on soil phospholipid fatty acid analyses. Geoderma, 2009, 150(1- 2), 171- 178.
[21]Zogg G P, Zak D R, Ringleberg D B, MacDonald N W, Pregitzer K S, White D C. Compositional and functional shifts in microbial communities due to soil warming. Soil Science Society of America Journal, 1997, 61(2): 475- 481.
[22]Kourtev P S, Ehrenfeld J G, Hägblom M. Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biology and Biochemistry, 2003, 35(7): 895- 905.
[23]Zelles L, Bai Q Y, Ma R X, Rackwitz R, Winter K, Beese F. Microbial biomass, metabolic activity and nutritional status determined from fatty acid patterns and poly-hydroxybutyrate in agriculturally-managed soils. Soil Biology and Biochemistry, 1994, 26(4): 439- 446.
[24]Zak D R, Ringelberg D B, Pregitzer K S, Randlett D L, White D C, Curtis P S. Soil microbial communities beneath Populus grandidentata grown under elevated atmospheric CO2. Ecological Applications, 1996, 6(1): 257- 262.
[25]Fuchslueger L, Bahn M, Fritz K, Hasibeder R, Richter A. Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytologist, 2014, 201(3): 916- 927.
[26]Frostegård A, Bååth E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biology and Fertility of Soils, 1996, 22(1- 2): 59- 65.
[27]Tornberg K, Bååth E, Olsson S. Fungal growth and effects of different wood decomposing fungi on the indigenous bacterial community of polluted and unpolluted soils. Biology and Fertility of Soils, 2003, 37(3): 190- 197.
[28]Thoms C, Gleixner G. Seasonal differences in tree species′ influence on soil microbial communities. Soil Biology and Biochemistry, 2013, 66: 239- 248.
[29]Cordova-Kreylos A L, Cao Y P, Green P G, Hwang H M, Kuivila K M, LaMontagne M G, Van De Werfhorst L C, Holden P A, Scow K M. Diversity, composition, and geographical distribution of microbial communities in California salt marsh sediments. Applied and Environmental Microbiology, 2006, 72(5): 3357- 3366.
[30]Herman R P, Provencio K R, Herrera-Matos J, Torrez R J. Resource islands predict the distribution of heterotrophic bacteria in Chihuahuan desert soils. Applied and Environmental Microbiology, 1995, 61(5): 1816- 1821.
[31]Schjønning P, Thomsen I K, Moldrup P, Christensen B T. Linking soil microbial activity to water- and air-phase contents and diffusivities. Soil Science Society of America Journal, 2003, 67(1): 156- 165.
[32]Fierer N, Schimel J P, Holden, P A. Variations in microbial community composition through two soil depth profiles. Soil Biology and Biochemistry, 2003, 35(1): 167- 176.
[33]Yuste J, Penelas J, Estiarte M, Garcia-Mas J, Mattana S, Ogaya R, Pujol M, Sardans J. Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Global Change Biology, 2011, 17(3): 1475- 1486.
[34]Sheik C S, Beasley W H, Elshahed M S, Zhou X, Luo Y, Krumholz L R. Effect of warming and drought on grassland microbial communities. The ISME Journal, 2011, 5(10): 1692- 1700.
[35]Alster C J, German D P, Lu Y, Allison S D. Microbial enzymatic responses to drought and to nitrogen addition in a southern California grassland. Soil Biology and Biochemistry, 2013, 64: 68- 79.
[36]Strickland M S, Rousk J. Considering fungal: bacterial dominance in soils-methods, controls, and ecosystem implications. Soil Biology and Biochemistry, 2010, 42(9): 1385- 1395.
[37]Fontaine S, Mariotti A, Abbadie L. The priming effect of organic matter: a question of microbial competition?. Soil Biology and Biochemistry, 2003, 35(6): 837- 843.
ThespatialdistributionofsoilmicrobesaroundadesertshrubofHaloxylon ammodendron
CAOYanfeng1,2,3,4,LIYan1, *,LIChenhua1,LVGuanghui2
1 State Key Laboratory of Desert and Oasis Ecology, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumq 830011, China 2 College of Resources and Environment Science, Xinjiang University, Urmuqi 830046, China 3 College of Geographical Science, Shanxi Normal University, Linfen 041000, China 4 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract:‘Fertile islands’ or ‘resource islands’ are considered to be a featured pattern in desert ecosystems. Water and nutrient contents are found to be higher in under- than inter-shrub soils. One general idea is that soil water and nutrients have a close correlation with microbes. However, it remains controversial on whether the spatial distribution of soil microbes around shrubs is determined by soil water and nutrients of fertile islands. In addition, different microbial groups, such as fungi, bacteria, Gram-positive and Gram-negative bacteria, have distinct sensitivity or tolerance to the changes of soil water and nutrients. How these microbes distribute around shrubs and their relations with soil water and nutrients calls for further study. In the current study, under- and inter-shrub soils of Haloxylon ammodendron (height 200—210 cm, basal diameter 9—10 cm and crown width 200 × 200 cm), a dominant species in desert ecosystems of Gurbantunggut Desert, were studied. Soil samples were collected in circles with radii of 5, 40, 100 and 200 cm respectively from tree base in horizontal directions, and from depths of 0—20 cm. The sampling points of 5, 40, 100 and 200 cm represent the vicinity of tree base, the center of canopy, the edge of canopy and barren interspaces of shrubs, respectively. Soil samples from four directions, with angles between the adjacent directions at 90°, were mixed to obtain one composted sample. By analyzing changes in soil microbial biomass, diversity and community composition with changes of soil water and nutrients outward from tree base, the features of spatial distribution of soil microbes around shrubs was revealed. Microbial biomass, abundance and community composition were examined using phospholipid fatty acids. Redundancy analysis (RDA) was conducted to assess the correlations between soil properties and microbes. With the decreasing of soil water and nutrient content from the vicinity of tree base to interspaces of shrubs, total microbial biomass and diversity, as well as the biomass of different microbial groups, decreased gradually, indicating an increase of soil microbes inward from interspaces of shrubs. In addition, for the microbial composition in the soil, the biomass of different microbial groups had different reduction rate outward from tree base. For example, from the vicinity of tree base (i.e. 5 cm) to interspaces (i.e. 200 cm), the biomass of fungi, bacteria, Gram-positive and Gram-negative bacteria reduced 50.25%、84.06%、82.70%、92.57%, respectively. RDA results also showed that soil water, available nitrogen and organic carbon had a stronger correlation with bacteria comparing to fungi, and Gram-negative bacteria also correlated stronger than Gram-positive bacteria. This demonstrated that inward from interspaces, the increases of bacteria was more significant than fungi, and that of Gram-negative bacteria was more significant than Gram-positive bacteria. Our study suggested that soil microbes tended to distribute in soil resource-rich areas under the shrub canopy in resource-poor desert ecosystems. The spatial distribution of soil microbes was influenced more directly by soil properties than microclimate factors. Furthermore, the spatial distribution of soil microbes was not only determined by soil resource abundance, but also by the tolerance or sensitivity of different groups of microbes to environmental variations.
Key Words:Gurbantunggut Desert; Haloxylon ammodendron; microbial biomass; spatial distribution
DOI:10.5846/stxb201408041552
*通讯作者
Corresponding author.E-mail: liyan@ms.xjb.ac.cn
收稿日期:2014- 08- 04; 网络出版日期:2015- 04- 16
基金项目:国家自然科学基金项目(41301102, 41371079)
曹艳峰,李彦, 李晨华,吕光辉.荒漠灌木梭梭(Haloxylonammodendron)周围土壤微生物的空间分布.生态学报,2016,36(6):1628- 1635.
Cao Y F, Li Y, Li C H, Lü G H.The spatial distribution of soil microbes around a desert shrub ofHaloxylonammodendron.ActaEcologicaSinica,2016,36(6):1628- 1635.
