虾青素琥珀酸二酯的制备工艺及其结构表征*
2016-05-12周庆新李学敏薛长湖
张 婷, 徐 杰, 周庆新, 李学敏, 杨 澍, 薛长湖
(中国海洋大学食品科学与工程学院,山东 青岛 266003)
虾青素琥珀酸二酯的制备工艺及其结构表征*
张婷, 徐杰, 周庆新, 李学敏, 杨澍, 薛长湖**
(中国海洋大学食品科学与工程学院,山东 青岛 266003)
摘要:虾青素具有极强的抗氧化、抗癌变和增强免疫力等功能,但由于虾青素水溶解性(分散性)的限制致使其生物利用度和应用范围有很大的局限性。本文以丁二酸酐和合成虾青素为原料(摩尔比为10∶1),以三乙胺为催化剂(与合成虾青素的摩尔比为3∶1),合成虾青素琥珀酸二酯;经硅胶柱层析,用氯仿-甲醇(90∶10,v/v)混合液进行洗脱纯化;用高效液相色谱对合成的虾青素琥珀酸二酯进行分析,测得其纯度达95%;经紫外光谱扫描,测得其最大吸收波长为487 nm,与虾青素的最大吸收波长接近。薄层色谱结果显示,虾青素琥珀酸二酯的极性增大,LC-(APCI)MS/MS分析得到其分子量为796.5,从其裂解碎片中可得到虾青素的碎片离子m/z 561.5和m/z 579.5。通过1H NMR和DEPTQ谱进行验证,合成产物为虾青素琥珀酸二酯。
关键词:虾青素琥珀酸二酯;制备工艺;结构;液相色谱-串联质谱;核磁共振波谱
引用格式:张婷,徐杰,周庆新,等. 虾青素琥珀酸二酯的制备工艺及其结构表征[J].中国海洋大学学报(自然科学版), 2016,46(4):50-55.
ZHANG Ting, XU Jie, ZHOU Qin-Xin, et al. Preparation technology of astaxanthin succinate diester and its structure characterization[J].Periodical of Ocean University of China, 2016, 46(4): 50-55.
虾青素(3,3’-二羟基-4,4’-二酮基-β,β’-胡萝卜素)是萜烯类不饱和化合物[1],是类胡萝卜素的一种,其结构如图1所示。研究发现虾青素具有很强的抗氧化活性[2-3],虾青素在清除氧自由基方面的作用是β-胡萝卜素的10多倍,VE的100多倍[4],除此之外,虾青素还具有着色[5]、光保护[6]、抗炎[7]、抗癌症[8]、增强机体免疫力[9]等多种生物学功能。虾青素易溶于氯仿、二氯甲烷、丙酮、二硫化
碳等弱极性的有机溶剂[10],不溶于水[11]。水溶性方面的限制使其在动物体内无法正常运输,降低了虾青素在动物体内的吸收和利用率,也降低了其生物学功能[12]。为提高虾青素的水溶性及其在水中的分散程度,目前研究者们也正在通过微乳化[13-14]、载体结合[15]、微囊化[16-17]等方法来实现这一目的,如HBI公司在二氯甲烷体系中,以虾青素和琥珀酸酐为原料,DMAP为催化剂经过15 h反应后合成的虾青素二琥珀酸二钠盐(Cardax)[18-19],经测定Cardax的水溶性达到了8.64mg/mL[20],研究表明,这种衍生物在心脏保护和心肌拯救方面的作用也显著优于虾青素单体[21]。
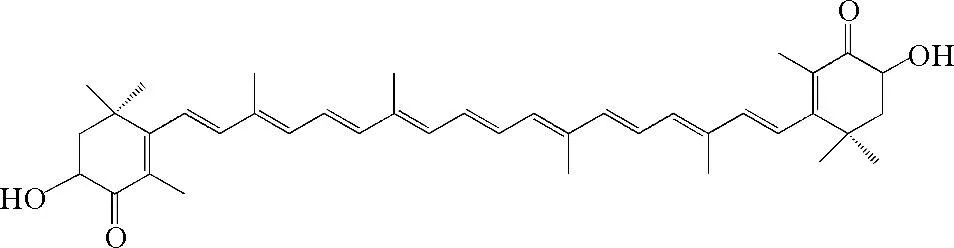
图1 虾青素结构
本文采用不同于HBI公司的一种新方法合成虾青素琥珀酸二酯,其意义在于通过暴露极性端羧基使虾青素的极性增大,这样会更加有利于虾青素与糖、氨基酸、无机盐等其他物质结合,从而增大虾青素的水溶性,提高其生物利用度。
1实验部分
1.1 实验原料及试剂
合成虾青素(含量10%)购于浙江巴士曼生物科技有限公司。
乙腈(色谱纯,德国Merck公司);甲醇、二氧六烷、三乙胺、丁二酸酐、正己烷(分析纯,天津博迪化工有限公司);丙酮、二甲基亚砜(DMSO)、三氯甲烷(分析纯,国药集团化学试剂有限公司);氘代氯仿(CDCl3,青岛腾龙微波科技有限公司);SGF254薄层色谱TLC预制硅胶板(烟台市化学工业研究所);0.22μm有机滤膜(美国Agela公司);硅胶(200~300目,青岛海洋化工厂)。
1.2 仪器和设备
1100系列高效液相色谱仪,配二极管阵列检测器(DAD)(美国Agilent公司),G6410B三重四极杆质谱仪,配大气压化学电离源(APCI)(美国Agilent公司);紫外-可见分光光度计(日本岛津公司);核磁共振波谱仪(德国Bruker公司);Milli-Q Elix-5超纯水系统(美国Millipore公司);DF-101S磁力搅拌器(上海羌强设备有限公司);硅胶柱(规格φ22 mm × 1100 mm,北京慧德易科技有限责任公司)。
1.3 实验方法
1.3.1 虾青素琥珀酸二酯的合成将合成虾青素原料用二氧六烷溶解,以丁二酸酐和合成虾青素为原料(摩尔比为10∶1),以三乙胺为催化剂(与合成虾青素的摩尔比为3∶1),充入氮气密封使其隔绝氧气。在50℃下搅拌15 h,反应结束后冷却至室温,加入0.1% HCl溶液将生成的虾青素琥珀酸二酯沉淀出来,冻干后即所得产物。
1.3.2 虾青素琥珀酸二酯的纯化取60g硅胶(200~300目),105℃高温活化3h后用氯仿匀浆后湿法装柱,将填料充分沉降,用一定量洗脱液匀速淋洗直至柱平衡。再用少量的氯仿溶解3g样品后上样,用氯仿-甲醇100∶0、97∶3、90∶10、0∶100的比例依次淋洗。
1.3.3 薄层色谱分析采用氯仿:甲醇:水(80∶15∶1,v/v/v)和正己烷:丙酮(4∶1,v/v)2种展开剂,通过薄层色谱初步分析生成的虾青素衍生物。
1.3.4 紫外光谱扫描将虾青素琥珀酸二酯和合成虾青素原料各称取10~15mg,用DMSO溶解后稀释到适合浓度(吸光度值范围在0.2~0.8之间)进行光谱扫描,扫描波长范围200~800nm。
1.3.5 LC(APCI)MS/MS分析
色谱条件流动相乙腈(A):水(B),溶剂梯度[22]:0~6min:40%A,60%B;7~9min:A(40%~98%),B(2%~60%);10~30min:A(98%),B(2%);30~35min:A(98%~40%),B(2%~60%);色谱柱:Agilent Zorbax XDB C18(150mm × 4.6mm, 5μm),流速1mL/min;检测器:二极管阵列检测器(DAD),检测波长486nm;柱温35℃。样品用甲醇溶解。通过液相色谱峰面积百分比计算虾青素琥珀酸二酯的纯度。
质谱条件大气压化学电离源(APCI),正离子扫描模式;干燥气温度350℃,干燥气流速5L/min,蒸发室温度400℃,雾化气压力40psi,毛细管电压4500V,流速1mL/min;一级质谱采集范围m/z100~1200,子离子扫描采集范围m/z100~1000。
1.3.6 核磁共振波谱分析通过硅胶柱纯化得到的产物样品进行核磁共振分析以进一步证明其结构,溶剂采用CDCl3,温度:室温。1H NMR条件:扫描频率(SF):600 MHz,谱宽(TD):32768,扫描次数(NS):32;DEPTQ条件:扫描频率(SF):150MHz,谱宽(TD):65536,扫描次数(NS):28。
2结果与讨论
2.1 薄层色谱分析结果
表1为10%虾青素原料(A)和虾青素合成反应产物(B)分别在正己烷:丙酮展开剂和氯仿:甲醇:水展开剂中对应点的Rf值。其中在正己烷:丙酮展开剂中B的Rf值为0.02,靠近原点,极性增大的原因可能是虾青素的母体结构连接酸酐后暴露出羧基端。在氯仿-甲醇-水的展开剂中B分离出清晰的4个点。
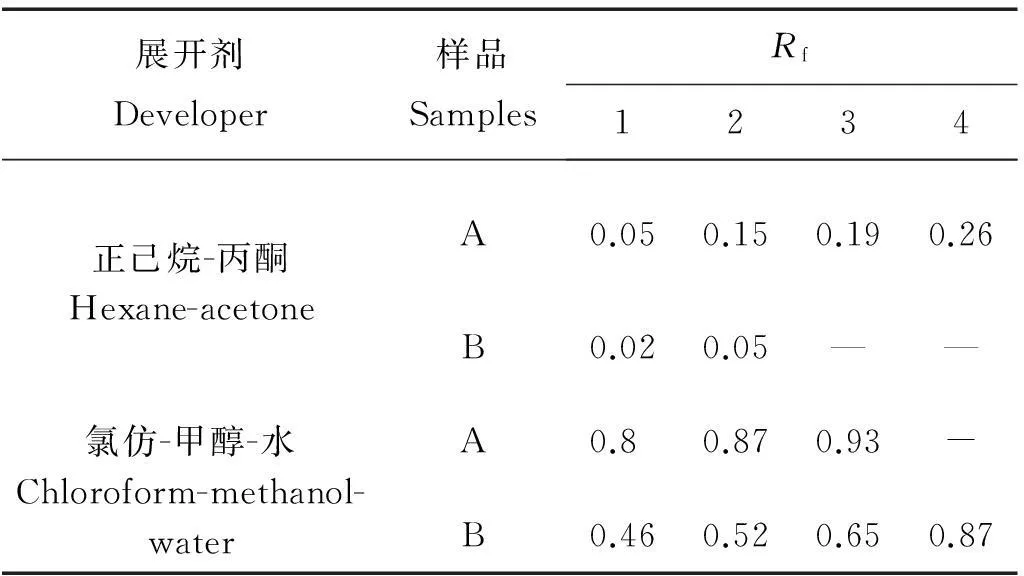
表1 薄层色谱分析结果
2.2 虾青素琥珀酸二酯的纯化结果
为验证虾青素琥珀酸二酯的结构,进一步对反应后的产物采用硅胶柱进行纯化,以除去其中未反应的虾青素和大极性的杂质,氯仿-甲醇硅胶柱的具体洗脱条件和结果如表2所示。
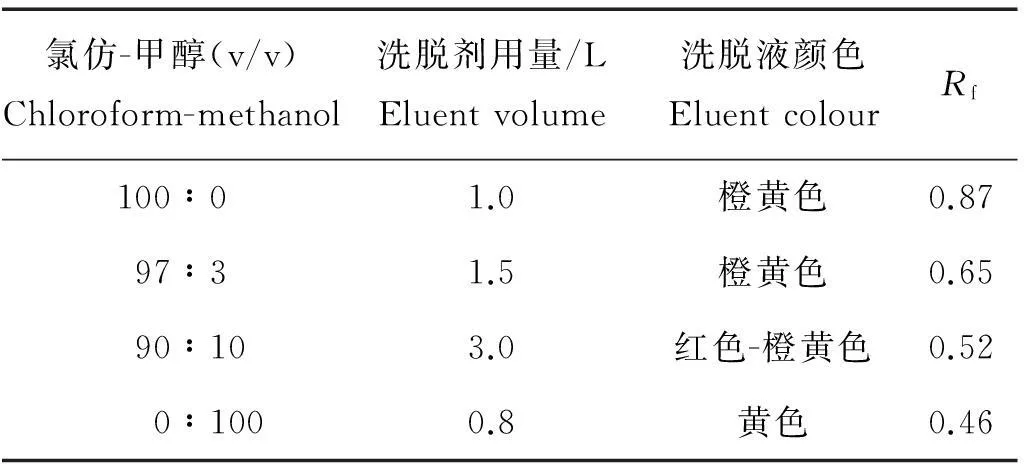
表2 洗脱条件
2.3 虾青素琥珀酸二酯的光谱扫描结果
虾青素和虾青素琥珀酸二酯的光谱扫描结果如图2所示,虾青素的最大吸收波长483 nm,虾青素琥珀酸二酯的最大吸收波长487 nm。可能由于虾青素琥珀酸二酯多了2个羧酸形成4个酯键致使其最大吸收波长发生红移[23],向长波方向移动,但是其最大吸收波长与虾青素相差并不大。
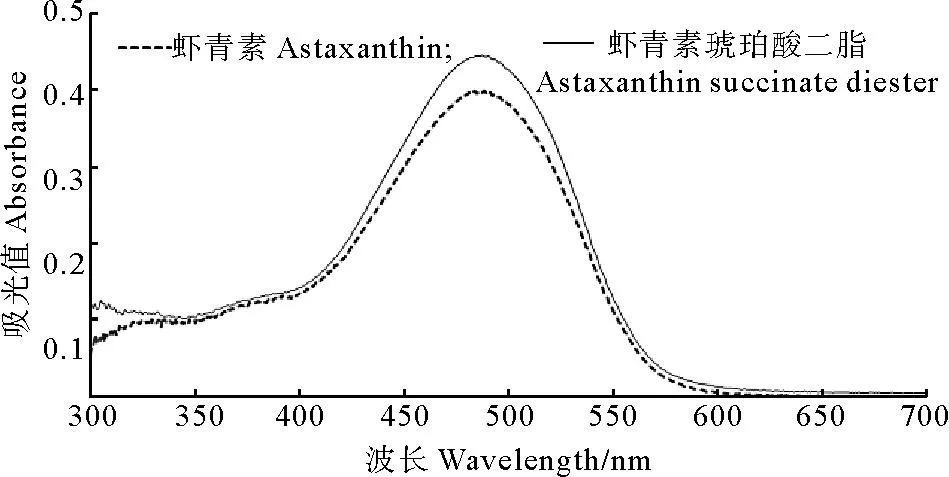
图2 虾青素和虾青素琥珀酸二酯的UV-Vis扫描图
2.4 LC(APCI)MS/MS检测虾青素琥珀酸二酯
图3为10%虾青素原料、反应后产物和纯化后的虾青素琥珀酸二酯的高效液相色谱图,合成虾青素的保留时间是15~18 min,虾青素琥珀酸酯的保留时间是11~14 min,说明反应后产物的极性增大。由峰面积所占的比例得到纯化后虾青素琥珀酸二酯的纯度达95%。
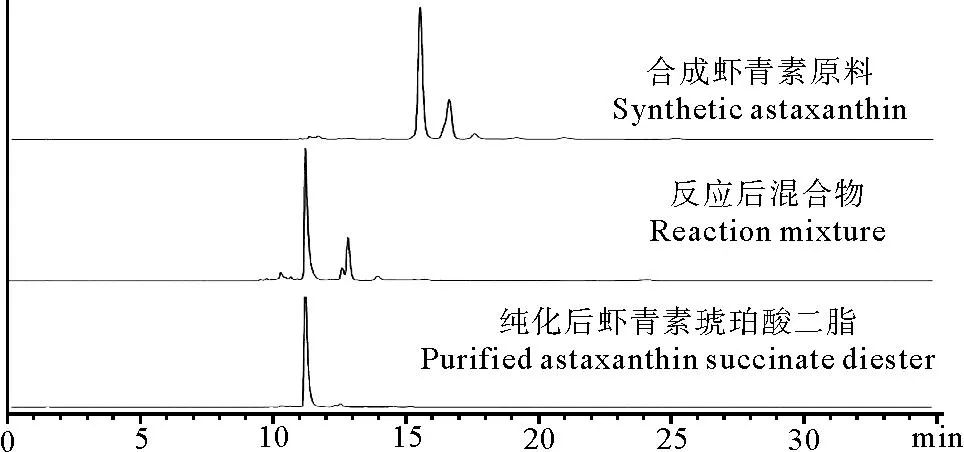
图3 虾青素和虾青素琥珀酸二酯的高效液相色谱图
为验证生成的虾青素衍生物的结构,本文通过LC-(APCI)MS的一级质谱图和二级质谱图具体解析衍生物的结构,在文献[24]的研究中曾采用LC-(ESI)MS验证虾青素琥珀酸二酯的分子量为796.5,但是ESI源由于能量原因不能裂解中等极性的虾青素,所以本实验中采用高能量的APCI源。纯化后的虾青素琥珀酸二酯一级质谱显示m/z797.6为虾青素琥珀酸二酯质子化的准分子离子峰[M+H]+;m/z779.5为[M+H-H2O]+,即虾青素琥珀酸二酯失去一分子水所产生的碎片离子峰。
图4为虾青素琥珀酸二酯的二级质谱图及裂解规律,分析得到虾青素琥珀酸二酯的裂解规律是以失去琥珀酸或是失水为主要特征,其中m/z697.5为[M+H-C4H4O3]+,是虾青素琥珀酸二酯失去一侧的琥珀酸产生的碎片离子峰;m/z579.5和m/z561.7为虾青素骨架的特征离子碎片。可以判定虾青素的主要结构没有在反应中被破环。
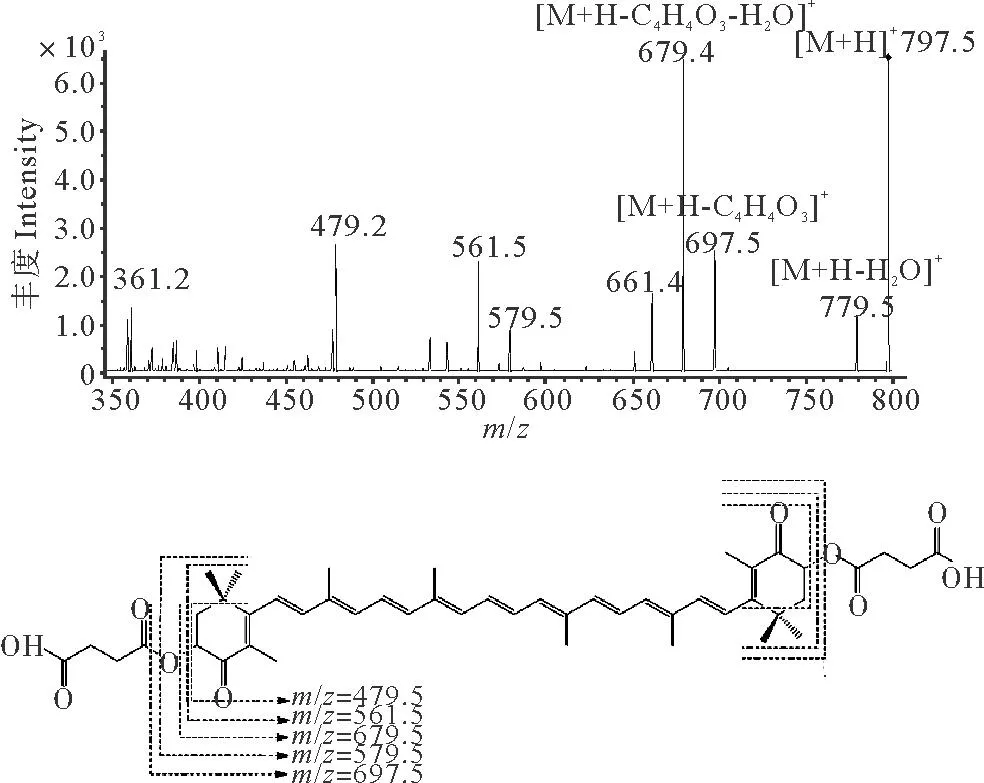
图4 虾青素琥珀酸二酯的二级质谱图及主要碎片裂解途径
2.5 核磁共振鉴定虾青素琥珀酸二酯的结构
采用1H NMR和DEPTQ谱鉴定虾青素琥珀酸二酯的结构。虾青素琥珀酸二酯的1H NMR (CDCl3) 化学位移和积分结果如表3和图5所示。再通过DEPTQ谱验证目标产物的碳架结构,如图6所示,DEPTQ(CDCl3) 化学位移(δ,ppm):194.1,176.0,171.4,161.8,142.5,139.8,136.8,135.2,134.5,133.9,130.7,128.2,124.6,123.1,71.5,59.1,50.9,42.5,39.5,37.1,30.5,29.7,29.0,28.6,26.3,22.6,20.8,14.1,13.1,12.8。经验证合成反应所生成的产物即虾青素琥珀酸二酯。

图5 虾青素琥珀酸二酯的DEPTQ图

质子Proton化学位移(δ)Chemicalshift/ppm积分结果Integralresults7/7',8/8',10/10',11/11'12/12',14/14',15/15'6.75~6.1714H3/3'5.672H22/22',23/23'2.85~2.738H19/19',20/20',2/2'2.09~1.9516H17/17'1.926H16/16'1.386H18/18'1.256H
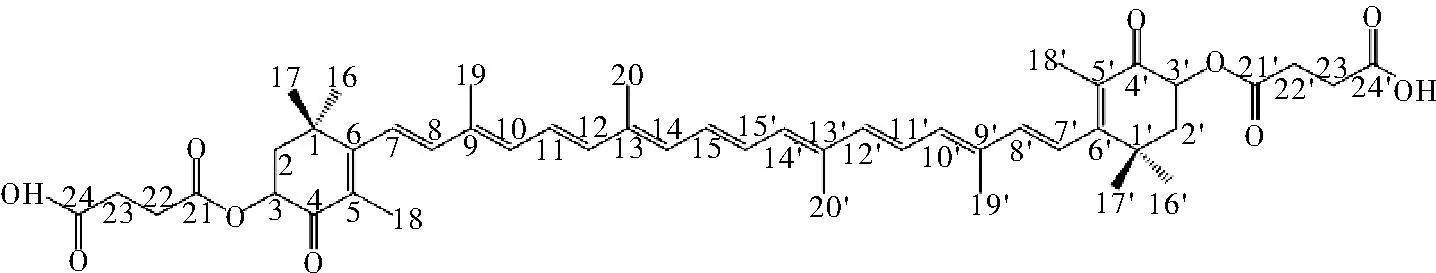
图6 虾青素琥珀酸二酯的结构
3结语
本文以合成虾青素和琥珀酸酐为原料经三乙胺催化成功合成虾青素琥珀酸二酯,纯化后纯度可达95%,通过光谱和色谱手段验证了虾青素琥珀酸二酯的结构。本实验以毒性相对于二氯甲烷更低的二氧六烷为反应溶剂体系,以及成本更低的三乙胺为催化剂合成虾青素琥珀酸二酯,此制备工艺更有利于工业化生产。虾青素琥珀酸二酯作为一种中间体,相比较于虾青素极性增大,其暴露的羧基端可使合成其他高水溶性虾青素衍生物更加容易,为扩展虾青素的应用范围、提高虾青素的生物利用度等提供基础。
参考文献:
[1]朱明军, 宗敏华. 虾青素研究进展[J]. 食品工业科技, 2000(2): 79-81.
ZHU Mingjun, ZONG Minhua. The research progress of astaxanthin[J]. Journal of Science and Technology of Food Industry, 2000(2): 79-81.
[2]何强强, 惠伯棣, 宫平, 等. 类胡萝卜素代谢物的生物学活性研究进展[J]. 食品科学, 2011, 32(15): 289-295.
HE Qiangqiang, HUI Bodi, GONG Ping, et al. Research progress in bioactivities of carotenoid metabolites[J]. Journal of Food Science, 2011, 32(15): 289-295.
[3]Naguib Y M A. Antioxidant activities of astaxanthin and related carotenoids[J]. Journal of Agricultural and Food Chemistry, 2000, 48(4): 1150-1154.
[4]Miki W. Biological functions and activities of animal carotenoids[J]. Pure and Applied Chemistry, 1991, 63(1): 141-146.
[5]Sommer T R, Potts W T, Morrissy N M. Utilization of microalgal astaxanthin by rainbow trout (Oncorhynchusmykiss)[J]. Aquaculture, 1991, 94(1): 79-88.
[6]Guerin M, Huntley M E, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition[J]. Trends in Biotechnology, 2003, 21(5): 210-216.
[7]Liu X J, Wu Y H, Zhao L C, et al. Determination of astaxanthin inHaematococcuspluvialisby first-order derivative spectrophotometry[J]. Journal of AOAC International, 2011, 94(6): 1752-1757.
[8]Jyonouchi H, Sun S, Iijima K, et al. Antitumor activity of astaxanthin and its mode of action[J]. Nutrition and Cancer, 2000, 36(1): 59-65.
[9]Park J S, Chyun J H, Kim Y K, et al. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans[J]. Nutrition and Metabolism, 2010, 7(1): 18.
[10]陶姝颖, 明建. 虾青素功能特性及其在功能食品中的应用研究进展[J]. 食品工业, 2012, 33(8): 110-112.
Tao Shuying, Ming Jian. Research advance on the functional characteristics of astaxanthin and its application in functional food[J]. Journal of Food Industry, 2012, 33(8): 110-112.
[11]Lockwood S F, O'Malley S, Mosher G L. Improved aqueous solubility of crystalline astaxanthin (3, 3′-dihydroxy-β, β-carotene-4, 4′-dione) by Captisol (sulfobutyl ether β-cyclodextrin)[J]. Journal of Pharmaceutical Sciences, 2003, 92(4): 922-926.
[12]Horn D. Preparation and characterization of microdisperse bioavailable carotenoid hydrosols[J]. Die Angewandte Makromolekulare Chemie, 1989, 166(1): 139-153.
[13]Anarjan N, Tan C P. Developing a three component stabilizer system for producing astaxanthin nanodispersions[J]. Food Hydrocolloids, 2013, 30(1): 437-447.
[14]Anarjan N, Nehdi I A, Tan C P. Protection of astaxanthin in astaxanthin nanodispersions using additional antioxidants[J]. Molecules, 2013, 18(7): 7699-7710.
[15]Naess S N, Elgsaeter A, Foss B J, et al. Hydrophilic carotenoids: Surface properties and aggregation of crocin as a biosurfactant[J]. Helvetica Chimica Acta, 2006, 89(1): 45-53.
[16]Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea F M, et al. Microencapsulation of astaxanthin in a chitosan matrix[J]. Carbohydrate Polymers, 2004, 56(1): 41-45.
[17]Bustos-Garza C, Yáez-Fernández J, Barragán-Huerta B E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin fromHaematococcuspluvialisusing several encapsulation wall materials[J]. Food Research International, 2013, 54(1): 641-649.
[18]Showalter L A, Osterlie M. Plasma appearance and tissue accumulation of non-esterified, free astaxanthin in C57BL/6 mice after oral dosing of a disodium disuccinate diester of astaxanthin (Heptax TM)[J]. Comparative Biochemistry and Physiology C-toxicology & Pharmacology, 2004, 137: 227-236.
[19]Cardounel A J, Dumitrescu C, Zweier J L, et al. Direct superoxide anion scavenging by a disodium disuccinate astaxanthin derivative: relative efficacy of individual stereoisomers versus the statistical mixture of stereoisomers by electron paramagnetic resonance imaging[J]. Biochemical and Biophysical Research Communications, 2003, 307(3): 704-712.
[20]Laura M H, Samuel F L. Up regulation of connexin 43 protein expression and increased gap junctional communication by water soluble disodium disuccinate astaxanthin derivatives[J]. Cancer Letters, 2004, 211(1): 25-37.
[21]Gross G J, Zsout B, Ferenc Z, et al. Cardioprotection and myocardial salvage by a disodium disuccinate astaxanthin derivative (Cardax TM)[J]. Life Science, 2004, 75: 215-224.
[22]Jackson H L, Cardounel A J, Zweier J L, et al. Synthesis, characterization, and direct aqueous superoxide anion scavenging of a highly water-dispersible astaxanthin-amino acid conjugate[J]. Bioorganic & Medicinal Chemistry Letters, 2004, 14(15): 3985-3991.
[23]惠伯棣. 类胡萝卜素化学及生物化学[M]. 北京:中国轻工业出版社, 2005: 148-152.
HUI Bodi. Carotenoids chemistry and biochemistry[M]. Beijing: China Light Industry Press, 2005: 148-152.
[24]Frey D A, Kataisto E W, Ekmanis J L, et al. The efficient synthesis of disodium disuccinate astaxanthin (Cardax)[J]. Organic Process Research & Development, 2004, 8(5): 796-801.
责任编辑朱宝象
Preparation Technology of Astaxanthin Succinate Diester and its Structure Characterization
ZHANG Ting, XU Jie, ZHOU Qing-Xin, LI Xue-Min, YANG Shu, XUE Chang-Hu
(College of Food Science and Engineering, Ocean University of China, Qingdao 266003, China)
Abstract:Astaxanthin has many powerful functions including antioxidation, immune system improvement, prevention of a series of chronic diseases such as cardiovascular disease, cancer, diabetes among others. However, the limitation of astaxanthin aqueous solubility reduces its bioavailability and application range. In order to improve the aqueous solubility of astaxanthin, many other researchers committed themselves to overcome these limitations. Their trials included microencapsulation and structure improvement. Furthermore, microencapsulation has some restrictions like low transparency and inferior stability. Hence, we adopted chemical synthesis to improve astaxanthin polarity and solubility. Our researches included also the structural characterization with high performance liquid chromatography (HPLC), liquid chromatography/mass spectrometer and spectrum methods like ultraviolet spectrum scanning, nuclear magnetic resonance spectrum. In this study, astaxanthin succinate diester was synthesized with succinic anhydride and synthetic astaxanthin (the molar ratio is 10∶1), and catalyzed by triethyl amine (the molar ratio to synthetic astaxanthin is 3∶1). Then with silica gel column chromatography, the astaxanthin succinic diester was purified by eluting with chloroform: methanol (90∶10, v/v). The purity of astaxanthin succinate diester was 95% as was determined with HPLC. The maximum absorption wavelength of astaxanthin succinic diester was 487 nm which was similar to that of astaxanthin. TLC result showed that the polarity of astaxanthin succinic diester increased. The spectrum of LC-(APCI) MS/MS illustrated that the molecule weight of astaxanthin succinic diester was 796.5, and the fragment ions of astaxanthin m/z 561.5 and m/z 579.5 were discovered simultaneously. Finally, the analysis of1H NMR and DEPTQ spectrum showed that the derivative was astaxanthin succinic diester.We employed a new method for synthesizing astaxanthin succinic diester with dioxane reaction system, which possesses low toxicity and can be amplified in scale. This research systematically characterized the astaxanthin succinic diester with several chromatography and spectrum methods, which provided the basic data for future studies. Astaxanthin succinic diester terminated with carboxyl at both sides, which would magnify the polarity of astaxanthin. In addition, it would be an intermediate to synthesize with other high aqueous solubility materials such as sugars, proteins, amino acids among others, which will enlarge the application and bioavailability of astaxanthin.
Key words:astaxanthin succinate diester; preparation process; structure; liquid chromatography-mass spectrometry; nuclear magnetic resonance spectrum
DOI:10.16441/j.cnki.hdxb. 20150087
中图法分类号:O656.5
文献标志码:A
文章编号:1672-5174(2016)04-050-06
作者简介:张婷(1991-),女,硕士生。E-mail:specialhuiyi@126.com**通讯作者: E-mail:xuech@ouc.edu.cn
收稿日期:2015-03-12;
修订日期:2015-05-29
*基金项目:国家自然科学基金项目(31571864);“泰山学者攀登计划”专项经费资助
Supported by Natural Science Foundation of China(31571864);The Special Funds for “Taishan Scholar Climbing Plan”
