WR-2721口服肠溶生物降解微囊的制备研究
2016-05-07阿古拉许彦来张少麟
阿古拉,许彦来,张少麟
WR-2721口服肠溶生物降解微囊的制备研究
阿古拉,许彦来,张少麟
[摘要]目的通过制备WR-2721口服肠溶生物降解微囊,从而改善其口服利用度。方法以高分子可生物降解的丙烯酸树脂Eudragit L 100-55为囊材,用喷雾干燥技术制备微囊;利用横移激光散射图案在介质中的分布测量微粒直径、形状及粒径分布;微囊体外溶出度实验及体内血液及各组织分布使用高效液相色谱法(HPLC)测定含量;小鼠口服微囊1 h后全身照射6 Gy的γ射线观察30 d存活率实验。结果3批微囊90%的直径在(2.8±0.24)μm,微囊表面光滑、圆球形,得囊率为80%;体外24 h释放率分别为(95.35±2.13)%(37℃)和(89.68±3.17)%(4℃),且37℃的释放率高于4℃;30 d存活率实验WR-2721微囊组为80%、WR-2721口服组为50%、辐射对照组为0%、空白对照组和腹腔注射组为100%。结论WR-2721口服微囊有显著的辐射防护作用。
[关键词]WR-2721;微囊;辐射防护;口服剂型;Eudragit
[作者单位]266071山东青岛,济南军区青岛第一疗养院中医科
WR-2721为1999年被美国FDA批准上市的用于对化疗药肾毒性以及减轻肿瘤患者因放射引起的损伤[1],也是一种放射效果良好的辐射防护及细胞保护剂[2]。但本品有较强的不良反应,如恶心呕吐、血压降低、口干等反应[3-4],此外排泄过快,半衰期只有0.9 min,并且静脉给药在15 min内快速滴入[5]。为了降低毒性、方便给药、提高稳定性,本研究拟将WR-2721由高分子材料包裹成囊,制成肠溶微囊,避开胃酸的水解,以降低其不良反应,延长半衰期,提高口服生物利用度。
1 仪器与试剂
1.1仪器
美国Angilent1100高效液相色谱仪,Buchi B191(瑞士)喷雾干燥器,85-2A磁力搅拌器,RCZ-6B2型药物溶出度仪,sigma3-18k高速控温离心机,Milli-Q型去离子水制备仪。甲醇、乙腈为色谱纯,其他试剂为分析纯,水为去离子水,对照品由中国药品生物制品检定所提供。
1.2色谱条件
根据样品分离要求色谱法的分析条件为:分离柱C18(Angilent 150 mm×3.9 mm,5μm);流动相为乙腈-水(0.1 mol/L氯乙酸与5 mmol/L的辛烷磺酸钠,用三乙胺调pH至3.0)(15:85),流速1.0 ml/min;检测器为电化学检测器(Hg/Au电极),氧化电位设定为+0.2 V;注射量为20μl。
1.3方法
1.3.1微囊的制备取高分子基质适量,溶于无水乙醇中备用,另精确称取WR-2721适量溶解于去离子水,加入相关的表面活性剂,在磁力搅拌器作用下加入到高分子溶液,使得溶液被乳化。用小型的带有1.0 mm喷嘴标准的喷雾干燥Buchi B191(瑞士)进行喷雾干燥。进气口的温度、液体流动速度和喷雾空气压缩速度分别设置为65℃,3.5 m l/min,600 L/h。得白色均匀粉末,冷冻保存。
1.3.2测定微粒直径分布将样品溶于水中进行分型,并且使用Frauenhofer方法计算粒径分布。粒径计算是假设存在球形颗粒,并且是以球形体积的计算量为基础的。对于每个样品,都要以流动的去离子水为背景。对于微室中的微球样品(大约2 mg)要计数120 s。去除背景后,计算粒径分布。每个试验重复3次。
1.3.3微囊药物体外溶出度溶出介质为经脱气处理的pH7.4磷酸盐缓冲液900ml、转速为100 r/min,温度设为为4℃和37℃。将适量微囊M(g),装进透析袋中,然后再放入转篮中,根据图2定时取样5 ml,用微孔滤膜过滤,并补入等量介质,取滤液用高效液相色谱法同法测定含量,并计算出浓度为C(μg/ml)。1.3.4小鼠30 d活存率实验取Balb/c小鼠,50只,8~10周,体质量18~22 g,分5组,分别为正常空白对照组、辐射对照组、WR-2721原料药物口服组和微囊组、WR-2721腹腔注射组。剂量为口服WR-2721 50 mg/kg及微囊(含WR-2721相当于50 mg/kg)腹腔注射WR-2721 5 mg/kg(照射前30 min给药),在军事医学科学院动物实验中心适应性饲养一周,照前1 h给药后Co60照射,照射剂量为6 Gy,观察各组小鼠30 d的活存情况并进行统计。
1.3.5小鼠各组织及血液分布[6]取Balb/c小鼠48只,组别分WR-2721口服50 mg/kg和微囊50 mg/kg,采血时间:照射后30、60、120、180 min,每个时间点取3只鼠,眼眶取血0.4 ml,立刻加冰蛋白沉淀液1 ml(1 mol/L高氯酸,2.7 mmol/L EDTA)沉淀蛋白,处死动物,另取心、肝、肺、脑、胃、脾、肾各加上述蛋白沉淀液1 ml,用眼科剪刀剪碎,在12 000 rpm/min(-4℃)离心10 min,取上清液,保存在-70℃冰箱待测。
2 结果
2.1测定微囊直径分布
使用Frauenhofer方法计算囊径分布,囊径计算是假设存在球形颗粒,并且是以球形体积的计算量为基础的。3批微囊的直径分布较均匀,90%微囊的直径在(2.8±0.24)μm,得囊率为80%,如图1。
2.2微囊药物体外溶出度
微囊在2种温度下24 h的释放结果如图2,在最初的2 h快速释放,并在4 h释放60%的药物,在24 h释放率分别为(95.35±2.13)%(37℃)和(89.68±3.17)%(4℃);且24 h体外释放率37℃的释放率高于4℃,达到缓慢释放药物的目的。
2.3 30 d存活率实验
30 d存活率实验显示WR-2721微囊组为80%,WR-2721口服组为50%,辐射对照组为0%,空白对照组和腹腔注射均为100%,无1例死亡。见图3。
2.4小鼠各组织及血液分布
如图4所示,微囊在120 min浓度增加,并在180 min时全面超过WR-2721口服组,显示出制备成微囊后的缓释作用。
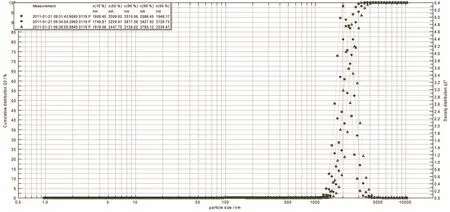
图1 3批微囊的直径分布图
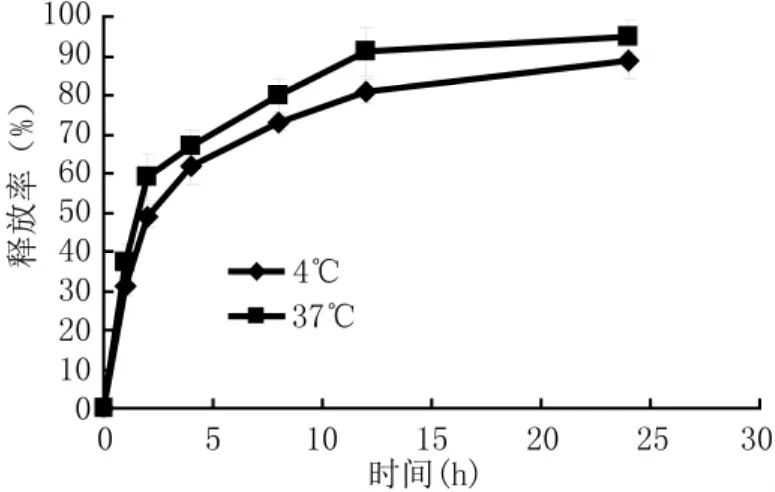
图2 不同温度下3批微囊药物体外溶出度
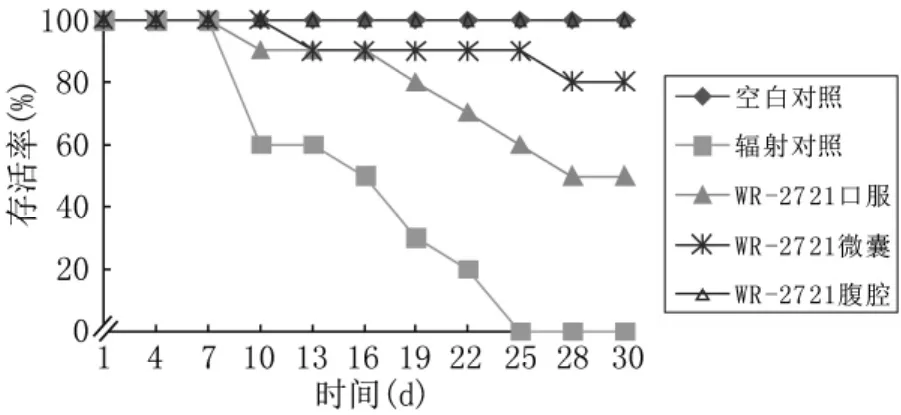
图3 各组小鼠30 d活存率比较
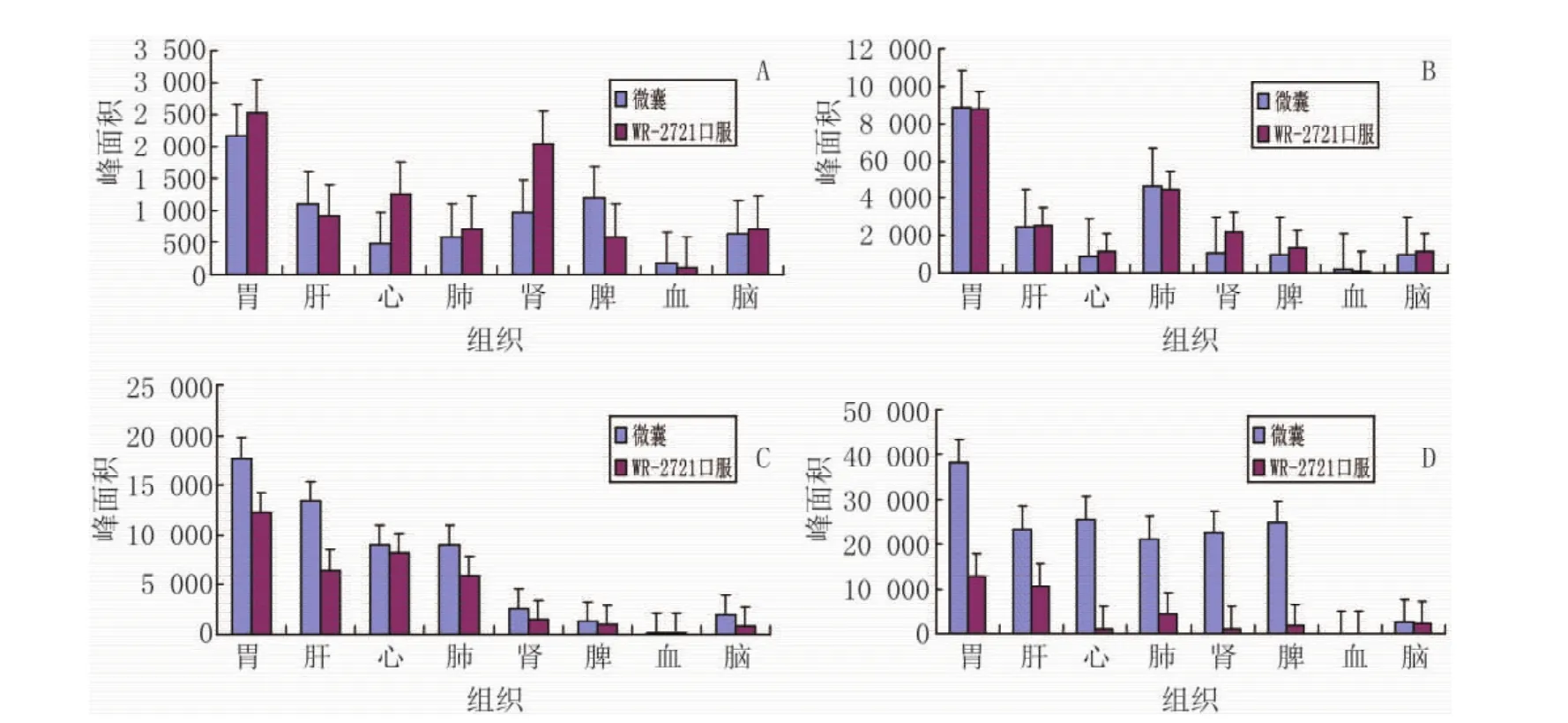
图4 不同给药方法各组织及血液中WR-2721分布比较
3 讨论
用干燥喷雾法制得的微囊有制备简单、快速、有效成分降解少且工艺易控制等优点,制备后的微囊稳定大大提高,有望开发出口服的WR-2721新剂型。
从微囊的体外溶出度及体内组织分布图来看有一定的缓释控释作用,体外释放实验表明,制成微囊后温度对药物的影响大大降低,有利于保存使用。
[参考文献]
[1]Haigentz M Jr,Kim M,Sorich J,etal.Phase Istudy of amifostine as a cytoprotector of the gemcitabine/cisplatincombination in patientswith advanced solid malignancies[J].Anticancer Drugs,2003,14(4):321-326.DOI:10.1097/01.cad.0000065049.82984.d8.
[2]Jatoi A.Aggressivemultimodality therapy for patients with locally advanced esophageal cancer:is there a role for amifostine[J].Semin Oncol,2003,30(6 Suppl18):72-75.
[3]Cassatt DR,Fazenbaker CA,Bachy CM,et al.Preclinicalmodeling of improved amifostine(Ethyol)use in radiation therapy[J].Semin Radiat Oncol,2002,12(1 Suppl 1):97-102.
[4]Schuchter LM,Hensley ML,Meropol NJ,et al.2002 update of recommendations for the use of chemotherapyand radiotherapy protectants:clinical practice guidelines of the American Society of Clinical Oncology[J].JClin Oncol,2002,20(12):2895-2903.
[5]Bonner HS,Shaw LM.New dosing regimens for amifostine:a pilot study to compare the relative bioavailability of oral and subcutaneous administration with intravenous infusion[J].J Clin Pharmacol,2002,42(2):166-174.
[6]Haznedar S,Dortun B.Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide[J].Int J Pharm,2004,269(1):131-140.
(本文编辑:林永丽)
Development of biodegradable enteric m icrocapsules of WR-2721 for oral delivery
AGula,Xu Yanlai,Zhang Shaolin
(Department of Chinese Medicine,First Qingdao Sanitarium,CPLA,Qingdao 266071,China)
[Abstract]Objective Amifostine(WR-2721)is the first FDA-approved cyto-protective agent,which is prescribed to reduce certain side-effects during chemotherapy of ovarian,non-small cell lung cancer,and in the radiation treatment for head-and-neck cancer patients.However,due to the fact that intravenous infusion of the drug was the main route ofmedication and assimilation was low if taken orally,its wide application was therefore affected clinically.For this reason,we intended to develop biodegradable entericmicrocapsules ofWR-2721 for oral delivery,so as to improve the bioavailability of the drug.MethodsThe preparation of themicrocapsule wasmade by using the high-molecular and biodegradable Eudragit L100-55 as its capsulematerial and by the spray drying technique.The diameter,shape,particle size distribution and yield rate of the capsules weremeasured by transversal distributionmeasurement of laser light scattering pattern inmedium particle diameter.High performance liquid chromatography(HPLC)was used to detect in vitro release of the drug and the drug amount remaining in themicrocapsule at a specific sampling time.One hour after medication,radioprotective efficacy of the new drug formulation was determined by 30-daymice survival ratewith whole-body exposure to6Gy.Results The diameter of90%microcapsules of the 3 batcheswas(2.8±0.24)μm.The shape of themicrocapsulewas sphericalwith a smooth surface,and the yield rate was 80%.In vitro release rate in 24 h was respectively[(95.35±2.13)%]at 37℃and[(89.68± 3.17)%]at4℃,with the release rate at37℃being higher than that at4℃.The 30-day survival rate for theWR-2721 microcapsule group was 80%,the rate for the WR-2721 oral group was 50%,the rate for the radiation group was 0%,and the rates for the blank control group and the abdominal injection group were as high as 100%.Conclusion As compared with the oral WR-2721 group,the WR-2721 microcapsule had significant radio-protective effect.
[Key words]WR-2721;Amifostine;Microcapsule;Radioprotection;Oral delivery;Eudragit
(收稿日期:2015-07-21)
[基金项目]济南军区立项课题(JN13W056)
[中图分类号]R94
[文献标识码]A[DOI]10.3969/j.issn.1009-0754.2016.01.020
