碳酸氧铋光催化剂的研究进展
2016-04-25徐丽亚敖燕辉王沛芳
徐丽亚,敖燕辉,王沛芳,王 超
(浅水湖泊综合治理与资源开发教育部重点实验室, 河海大学, 江苏 南京 210098)
综述与展望
碳酸氧铋光催化剂的研究进展
徐丽亚,敖燕辉*,王沛芳,王超
(浅水湖泊综合治理与资源开发教育部重点实验室, 河海大学, 江苏 南京 210098)
摘要:光催化技术是一种将太阳能转化为化学能的绿色技术,在污染治理和能源生产方面有广阔的应用前景。近几年,碳酸氧铋光催化剂因其优异的光催化性能受到广泛关注,但因其只对紫外光具有响应和回收利用困难等问题限制了在实际生产中的应用。对碳酸氧铋进行形貌控制和改性能有效改善其光催化性能,介绍零维、一维、二维和三维结构的碳酸氧铋光催化剂的研究现状,指出三维结构的碳酸氧铋展现更佳的光催化性能。综述了采用掺杂、金属沉积和构建异质结方式对碳酸氧铋进行改性所取得的进展,提出碳酸氧铋光催化剂在降解机理、降解对象和降解环境等方面需不断努力深入研究。
关键词:催化化学;碳酸氧铋光催化剂;形貌控制;改性
CLC number:O643.36;TQ034Document code: AArticle ID: 1008-1143(2016)01-0001-06
光催化技术是一种将太阳能转化为化学能的绿色技术,在污染治理和能源生产方面应用前景广阔。光催化剂种类繁多,TiO2是最常见的光催化剂,但只对紫外光有响应,而紫外光占太阳光的5%,因此,可见光光催化剂成为研究热点。
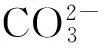
不同形貌展现出的光催化剂性能差异较大。研究者报道过多种Bi2O2CO3光催化剂的形貌,如纳米片[17]、纳米管[18]、纳米棒[19]以及花状[20]等。光催化性能的提高不仅可通过形貌结构的控制实现,更多是对光催化剂进行改性[21-24]。本文对近年来Bi2O2CO3在形貌控制和改性两大方面的研究进展进行综述,提出Bi2O2CO3光催化剂的进一步研究发展方向。
1Bi2O2CO3光催化剂的形貌控制
1.1零维及一维结构
Bi2O2CO3光催化剂的低维结构(零维及一维结构)主要有纳米粒、纳米管和纳米棒。
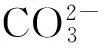
1.2二维结构
Bi2O2CO3的二维结构对纳米片的研究最多。Liu Y等[17]以尿素为碳源,用简单的水热法合成Bi2O2CO3纳米片,在降解RhB和异丙醇方面表现出较好的光催化活性。Liang H Y等[30]以十二烷基硫酸钠表面活性剂作为软模板剂合成Bi2O2CO3纳米片,并与不加十二烷基硫酸钠水热法制备的Bi2O2CO3相比,发现其在太阳光下展现出更好的光催化性能。Hu S等[31]改用十二烷基苯磺酸作为软模剂板,用水热法合成单分散性的Bi2O2CO3纳米片,指出十二烷基苯磺酸含量对Bi2O2CO3纳米片的合成具有重要影响,在形成Bi2O2CO3纳米片过程中,十二烷基苯磺酸会被吸附于Bi2O2CO3纳米粒表面,从而改变晶面的生长速率和裁缝晶体的形状,最终形成Bi2O2CO3纳米片。
1.3三维结构
三维结构具有较大的比表面积,有利于催化剂和反应物更加充分地接触,增加表面活性位,同时某些三维结构对光具有更好的吸收,展现出良好的光催化性能。Bi2O2CO3的三维结构包括花状、球状和鸡蛋状等结构。
Zhao T等[32]以柠檬酸钠作为协调剂和碳源制备出多级Bi2O2CO3微球,表现出较好的可见光光催化活性。Chen L等[15]以十六烷基三甲基溴化铵为模板剂,利用液相法合成花状Bi2O2CO3,在治理含染料污水方面效果较好。Cheng H等[33]在无模板剂和低温条件下,通过成核、生长以及自由组装三步法合成了多级花状Bi2O2CO3。
相较于欧美发达国家,我国无偿献血的参与程度仍有待提升,且未来无偿献血的工作重点应着眼于“固定献血人群”的建立[10]。无偿献血和保障医疗机构临床用血是一项社会系统工程。无偿献血事业的可持续发展是临床安全、足量用血的根本保证[2]。对于团体组织,政府相关部门可适当干预,如将无偿献血率作为文明单位建设的重要考核指标、作为评选先进单位的辅助条件等,使更多人群转化为固定无偿献血者,这样就可通过预约采血,缓解因高校学生寒暑假出现的临床用血措手不及的窘况,还可纠正血型偏型,提高应对突发公共事件紧急血液需求时的调配能力。
所用溶剂不同,合成的Bi2O2CO3形貌也不同。Yang L等[34]在氨水存在条件下,采用柠檬酸铋作为单一前驱体,分别选用乙二醇和水作为溶剂,以乙二醇作为溶剂合成的Bi2O2CO3为海绵状,以水作为溶剂合成的Bi2O2CO3为鸡蛋状,鸡蛋状Bi2O2CO3较海绵状Bi2O2CO3在RhB降解上表现出更佳的光催化活性。Zheng Y等[35]通过改变pH和温度,制备出不同形貌的Bi2O2CO3,并发现(001)晶面暴露的花状多级Bi2O2CO3在降解有机污染物方面优于其他形貌。
2Bi2O2CO3光催化剂的改性
对Bi2O2CO3在光催化领域的研究除了通过形貌结构的可控提高光催化性能外,主要研究焦点还有以Bi2O2CO3基制备高光催化活性和具有选择性的改性Bi2O2CO3光催化剂。对于这一目的的各种改性技术,包括金属和非金属掺杂、金属沉积及构建异质结。
2.1掺杂
掺杂改性光催化剂是通过在晶格中引入缺陷或改变结晶度的方式实现促进光生电子和空穴的有效分离,进而提高光催化活性。常用于掺杂的有金属离子(Fe3+、Co2+、Cu2+)以及非金属(N、Si)。
Li Q等[36]以双氰胺作为前驱体合成N掺杂的蜂窝状Bi2O2CO3,N掺杂Bi2O2CO3具有高效的可见光去除NO催化效果,其主要是由于N掺杂能有效缩短禁带宽度和加强可见光的吸收。Dong F等[37]以相同时间NO的可见光降解程度作比较判断N掺杂前后Bi2O2CO3光催化效果的变化,结果表明,N掺杂后,光催化效果得到明显提高。Zhao H等[38]采用水热法合成Sb掺杂的Bi2O2CO3纳米片,并通过BET和电化学阻抗谱研究指出,Sb掺杂的Bi2O2CO3纳米片光催化性能的改善是由于增大的比表面积有利于对染料的吸附以及载流子在界面的快速转移而成。Sun P等[39]制备了硅掺杂的Bi2O2CO3纳米棒,研究发现,Si—O—Bi结构使该催化剂对不同类型的染料均表现出优异的催化活性。
2.2金属沉积
金属沉积是一种提高半导体光催化剂光催化性能的有效方法,通过改变Bi2O2CO3的电子性能和光谱响应实现提高Bi2O2CO3的光催化性能。沉积在Bi2O2CO3表面的金属离子通常为光诱导电荷沉载体,有利于界面电荷转移。金属材料的性能,如金属类型、大小、浓度与制备方法对光催化的性能有巨大影响,已用在Bi2O2CO3沉积的材料有Ag、Au贵金属以及Bi金属。
Dong F等[40]通过水热法将贵金属Ag沉积到Bi2O2CO3微球上,制得的新型光催化剂对NO降解表现出比纯Bi2O2CO3微球更优异的光催化效果,指出沉积Ag后的Ag/Bi2O2CO3能吸收更多可见光,同时由于SPR效应,Ag纳米粒子可被激发,从而提高表面电子激发和界面间电子的转移。由于金属Ag的费米能级(0.4 eV)低于Bi2O2CO3的导带(0.20 eV),光生电子可能会转移到沉积的Ag颗粒上,从而创建界面处的肖特基势垒,减少电子空穴对的复合,延长光生载流子寿命。因此,在SPR效应光生电子和空穴的有效分离以及延长光生载流子寿命等协同作用下,复合光催化剂表现出更加优异的光催化效果。Peng S等[41]在PVP辅助下合成Bi2O2CO3微球,并将单分散的纳米Ag单质负载在Bi2O2CO3微球上,合成的Ag/Bi2O2CO3具有增强的光催化活性和超电容性能。Li Q等[42]将贵金属Au负载在Bi2O2CO3上制备出三维Au-Bi2O2CO3,发现其对空气中的NO具有很好的去除效果。Sun Y等[43]将Bi沉积在Bi2O2CO3微球上,制得的光催化剂在可见光下去除NO的效果与改性的对比剂Bi2O2CO3(C/TiO2、BiOI与C3N4等修饰)相比有明显优势。
2.3构建异质结
与价带匹配的半导体物质构建异质结是对Bi2O2CO3改性最广泛的方法,构建异质结可实现光生电子与空穴的有效分离,减少光生电子与空穴的结合率,实现光催化活性的加强。与具有磁性的Fe2O3和Fe3O4构建异质结还可以实现对复合光催化的回收利用。
Bi2O2CO3可与Bi2O3、Bi2S3和BiOX (X=Cl,Br,I)等铋系半导体物质形成异质结。Cai G等[44]采用热处理法将对可见光有很好响应的β-Bi2O3(禁带宽度为2.47 eV)与只对紫外光有响应的Bi2O2CO3(禁带宽度约为3.5 eV)相结合制备了异质结光催化剂β-Bi2O3/Bi2O2CO3,可见光响应范围(400~500) nm,禁带宽度最低可达2.79 eV,对可见光有很好的利用。同时,光生电子和空穴能在β-Bi2O3和Bi2O2CO3界面转移,促进光生载流子的分离,从而提高光催化活性。Liang N等[45]将窄禁带宽度的Bi2S3(1.38 eV)负载在Bi2O2CO3上形成具有可见光催化活性的n-n型Bi2S3/Bi2O2CO3异质结。Cao J等[46]制备出(BiO)2CO3/BiOX(X=Cl,Br,I)p-n型异质结光催化剂,该光催化剂与纯(BiO)2CO3和BiOX相比,在可见光下对甲基橙具有很好的降解效果。将3种p-n型异质结光催化剂进行比较,其光催化活性为(BiO)2CO3/BiOCl<(BiO)2CO3/BiOBr<(BiO)2CO3/BiOI。
研究者还将Bi2O2CO3与磁性材料相结合,制备出形成利于回收的磁性Bi2O2CO3基异质结。Zhu G等[47]将Fe3O4负载在球状和花状Bi2O2CO3上,制备出两种形貌的Fe3O4/Bi2O2CO3,通过对甲基橙和亚甲基蓝的降解实验结果发现,花状的Fe3O4/Bi2O2CO3光催化活性更好,同时花状的Fe3O4/Bi2O2CO3在磁场作用下能够被快速回收利用,而且Fe3O4/Bi2O2CO3再次利用时仍保持很好的光催化活性。Hu D等[48]选用Fe2O3负载到(001)晶面暴露的Bi2O2CO3上,制备出Bi2O2CO3@Fe2O3磁性异质结光催化剂,当Fe2O3负载质量分数为5%时达到最佳比例,该最佳Bi2O2CO3@Fe2O3磁性光催化剂在可见光降解RhB和混合染料以及对其回收利用方面表现出良好的性能。
3结语与展望
形貌结构的不同,使Bi2O2CO3光催化剂的光催化效果差异明显,可以通过形貌的控制,实现对Bi2O2CO3光催化活性的调控。与零维、一维和二维结构的Bi2O2CO3相比,三维结构的Bi2O2CO3具有更好的光催化活性,这主要是由于三维的Bi2O2CO3有更大的比表面积,对被降解的物质具有更好的吸附,反应活性位也增加,从而表现出良好的光催化活性。此外,还发现(001)晶面暴露的Bi2O2CO3具有更好的光催化活性。因此,理想的Bi2O2CO3光催化剂的结构是(001)晶面暴露的具有高比表面积的三维结构。
虽然Bi2O2CO3具有优异的光催化活性、良好的光稳定性以及无毒等优点,但因只对紫外光有响应,粉末状难以再利用,限制了其实际应用。通过金属和非金属掺杂,金属沉积和构建异质结等改性技术可制备出具有高催化活性、能回收利用和可见光响应型Bi2O2CO3光催化剂,加快Bi2O2CO3光催化剂的实际使用。
对Bi2O2CO3光催化剂的研究仍需要不断努力深入:(1) 对Bi2O2CO3光催化作用的机理、影响因素没有深入研究了解,如光催化剂的形貌、晶态如何具体作用光催化性能;(2) 如今的Bi2O2CO3光催化剂主要用于对水中甲基橙、亚甲基蓝和罗丹明B等染料的降解,对水中持久性有机污染物的研究需要进一步深入;(3) 降解对象以水溶液中的污染物为主,对甲醛和苯等大气污染的研究较少;(4) Bi2O2CO3光催化剂降解以单一污染物为主,对复杂水环境的多种污染物降解研究非常少。
参考文献:
[1]Sood S,Umar A,Kumar Mehta S,et al.α-Bi2O3nanorods:an efficient sunlight active photocatalyst for degradation of Rhodamine B and 2,4,6-trichlorophenol[J].Ceramics International,2015,41(3):3355-3364.
[2]Cuéllar E L,Martínez-De La Cruz A,Rodríguez K H L,et al.Preparation of γ-Bi2MoO6thin films by thermal evaporation deposition and characterization for photocatalytic applications[J].Catalysis Today,2011,166(1):140-145.
[3]Cheng J,Wang C,Cui Y,et al.Visible light photocatalytic properties and thermochromic phenomena of nanostructured BiOCl microspheres[J].Journal of Materials Science & Technology,2014,30(11):1130-1133.
[4]Zhou Y,Shuai L,Jiang X,et al.Visible-light-driven photocatalytic properties of layered double hydroxide supported-Bi2O3modified by Pd(Ⅱ) for methylene blue[J].Advanced Powder Technology,2015,26(2):439-447.
[5]Albuquerque R,Neves M C,Mendonça M H,et al.Adsorption and catalytic properties of SiO2/Bi2S3nanocomposites on the methylene blue photodecolorization process[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2008,328(1/3):107-113.
[6]Xue Y,Wang X.The effects of Ag doping on crystalline structure and photocatalytic properties of BiVO4[J].International Journal of Hydrogen Energy,2015,40(17):5878-5888.
[7]Ying C,Chen X,Ji S,et al.Preparation of HPA/Bi2WO6and its photocatalytic properties for denitrification[J].Journal of Fuel Chemistry and Technology,2014,42(8):978-985.
[8]Li J,Liu X,Sun Z,et al.Mesoporous yolk-shell structure Bi2MoO6microspheres with enhanced visible light photocataly-tic activity[J].Ceramics International,2015,41(7):8592-8598.
[9]Hao H,Xu Y,Liu P,et al.BiOCl nanostructures with different morphologies:tunable synthesis and visible-light-driven photocatalytic properties[J].Chinese Chemical Letters,2015,26(1):133-136.
[10]Zhang W,Dong F,Xiong T,et al.Synthesis of BiOBr-graphene and BiOBr-graphene oxide nanocomposites with enhanced visible light photocatalytic performance[J].Ceramics International,2014,40(7):9003-9008.
[11]Xia J,Yin S,Li H,et al.Enhanced photocatalytic activity of bismuth oxyiodine (BiOI) porous microspheres synthesized via reactable ionic liquid-assisted solvothermal method[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2011,387(1/3):23-28.
[12]Gan H,Zhang G,Huang H.Enhanced visible-light-driven photocatalytic inactivation of escherichia coli by Bi2O2CO3/Bi3NbO7composites[J].Journal of Hazardous Materials,2013,250-251:131-137.
[13]Wang Q,Zheng L,Chen Y,et al.Synthesis and characterization of novel PPy/Bi2O2CO3composite with improved photocatalytic activity for degradation of Rhodamine-B[J].Journal of Alloys and Compounds,2015,637:127-132.
[14]Zhao Z,Zhou Y,Wang F,et al.Polyaniline-decorated {001} facets of Bi2O2CO3nanosheets:in situ oxygen vacancy formation and enhanced visible light photocatalytic activity[J].ACS Applied Materials & Interfaces,2015,7(1):730-737.
[15]Chen L,Huang R,Yin S,et al.Flower-like Bi2O2CO3:facile synthesis and their photocatalytic application in treatment of dye-containing wastewater[J].Chemical Engineering Journal,2012,193-194:123-130.
[16]Cao X,Zhang L, Chen X,et al.Persimmon-like (BiO)2CO3microstructures:hydrothermal preparation,photocatalytic properties and their conversion into Bi2S3[J].CrystEngComm,2011,13:1939-1945.
[17]Liu Y,Wang Z,Huang B,et al.Preparation,electronic structure,and photocatalytic properties of Bi2O2CO3nanosheet[J].Applied Surface Science,2010,257(1):172-175.
[18]Chen R,So M,Yang J,et al.Fabrication of bismuth subcarbonate nanotube arrays from bismuth citrate[J].Chemical Communications,2006,21:2265-2266.
[19]Cheng G,Yang H,Rong K,et al.Shape-controlled solvothermal synthesis of bismuth subcarbonate nanomaterials[J].Journal of Solid State Chemistry,2010,183(8):1878-1883.
[20]Ma J,Zhu S,Shan Q,et al.Facile Synthesis of flower-like (BiO)2CO3@MnO2and Bi2O3@MnO2nanocomposites for supercapacitors[J].Electrochimica Acta,2015,168:97-103.
[21]Tian N,Huang H,Guo Y,et al.A g-C3N4/Bi2O2CO3composite with high visible-light-driven photocatalytic activity for rhodamine B degradation[J].Applied Surface Science,2014,322:249-254.
[22]Wang Q,Yun G,Bai Y,et al.Photodegradation of rhodamine B with MoS2/Bi2O2CO3composites under UV light irradiation[J].Applied Surface Science,2014,313:537-544.
[23]Song P,Xu M,Zhang W.Sodium citrate-assisted anion exchange strategy for construction of Bi2O2CO3/BiOI photocatalysts[J].Materials Research Bulletin,2015,62:88-95.
[24]Hu Y,Dong C,Wu K,et al.Synthesis of Ag3PO4-Bi2O2CO3composites with high visible-light photocatalytic activity[J].Materials Letters,2015,147:69-71.
[25]Alivisatos A.Semiconductor Clusters,Nanocrystals,and quantum dots[J].Science,1996,271:933-937.
[26]Shi W,Yu J,Wang H,et al.Hydrothermal synthesis of single-crystalline antimony telluride nanobelts[J].Journal of the American Chemical Society,2006,128(51):16490-16491.
[27]Dong F,Li Q,Zhang W,et al.Synthesis of flower-like,pinon-like and faceted nanoplates (BiO)2CO3micro/nanostructures with morphology-dependent photocatalytic activity[J].Materials Chemistry and Physics,2013,142(1):381-386.
[28]Qin F,Li G,Wang R,et al.Template-free fabrication of Bi2O3and (BiO)2CO3nanotubes and their application in water treatment[J].Chemistry-A European Journal,2012,18(51):16491-16497.
[29]Xu Y,Zhang Z,Zhang W.Inlay of Bi2O2CO3nanoparticles onto Bi2WO6nanosheets to build heterostructured photocatalysts[J].Dalton Transactions,2014,43(9):3660-3668.
[30]Liang H Y,Yang Y X,Tang J C,et al.Photocatalytic properties of Bi2O2CO3nanosheets synthesized via a surfactant-assisted hydrothermal method[J].Materials Science in Semiconductor Processing,2013,16(6):1650-1654.
[31]Hu S,Xu C,Zhang B,et al.Solvothermal synthesis of Bi2O2CO3nanoplates for efficient photodegradation of RhB and phenol under simulated solar light irradiation[J].Bulletin of Korean Chemical Society,2014,35(10):2935-2940.
[32]Zhao T,Zai J,Xu M,et al.Hierarchical Bi2O2CO3microspheres with improved visible-light-driven photocatalytic activity[J].CrystEngComm,2011,13(12):4010-4017.
[33]Cheng H,Huang B,Yang K,et al.Facile template-free synthesis of Bi2O2CO3hierarchical microflowers and their associated photocatalytic activity[J].ChemPhysChem,2010,11(10):2167-2173.
[34]Yang L,Han Q,Zhu J,et al.Synthesis of egg-tart shaped Bi2O2CO3hierarchical nanostructures from single precursor and its photocatalytic performance[J].Materials Letters,2015,138:235-237.
[35]Zheng Y,Duan F,Chen M,et al.Synthetic Bi2O2CO3nanostructures:novel photocatalyst with controlled special surface exposed[J].Journal of Molecular Catalysis A:Chemical,2010,317(1/2):34-40.
[36]Li Q,Liu H,Dong F,et al.Hydrothermal formation of N-doped (BiO)2CO3honeycomb-like microspheres photocatalysts with bismuth citrate and dicyandiamide as precursors[J].Journal of Colloid and Interface Science,2013,408:33-42.
[37]Dong F,Wang R,Li X,et al.Hydrothermal fabrication of N-doped (BiO)2CO3:structural and morphological influence on the visible light photocatalytic activity[J].Applied Surface Science,2014,319:256-264.
[38]Zhao H,Tang J,Lai Q,et al.Enhanced visible light photocatalytic performance of Sb-doped (BiO)2CO3nanoplates[J].Catalysis Communications,2015,58:190-194.
[39]Sun P,Jin Y,Zhao Y,et al.Novel hierarchical nanorods of silicon-doped Bi2O2CO3and its photocatalytic activity[J].Nano,2014,9(8):68-78.
[40]Dong F,Li Q,Zhou Y,et al.In situ decoration of plasmonic Ag nanocrystals on the surface of (BiO)2CO3hierarchical microspheres for enhanced visible light photocatalysis[J].Dalton Transactions,2014,43(25):9468-9480.
[41]Peng S,Li L,Tan H,et al.Monodispersed Ag nanoparticles loaded on the PVP-assisted synthetic Bi2O2CO3microspheres with enhanced photocatalytic and supercapacitive performances[J].Journal of Materials Chemistry,2013,1(26):7630-7638.
[42]Li Q,Hao X,Guo X,et al.Controlled deposition of Au on (BiO)2CO3microspheres:the size and content of Au nanoparticles matter[J].Dalton Transactions,2015,44(19):8805-8811.
[43]Sun Y,Zhao Z,Dong F,et al.Mechanism of visible light photocatalytic NOx oxidation with plasmonic Bi cocatalyst-enhanced (BiO)2CO3hierarchical microspheres[J].Physical Chemistry Chemical Physics,2015,17(16):10383-10390.
[44]Cai G,Xu L,Wei B,et al.Facile synthesis of β-Bi2O3/Bi2O2CO3nanocomposite with high visible-light photocatalytic activity[J].Materials Letters,2014,120:1-4.
[45]Liang N,Zai J,Xu M,et al.Novel Bi2S3/Bi2O2CO3heterojunction photocatalysts with enhanced visible light responsive activity and wastewater treatment[J].Journal of Materials Chemistry A,2014,2(12):4208.
[46]Cao J,Li X,Lin H,et al.Surface acid etching of (BiO)2CO3to construct (BiO)2CO3/BiOX(X=Cl,Br,I) heterostructure for methyl orange removal under visible light[J].Applied Surface Science,2013,266:294-299.
[47]Zhu G,Hojamberdiev M,Katsumata K,et al.Heterostructured Fe3O4/Bi2O2CO3photocatalyst:synthesis,characterization and application in recyclable photodegradation of organic dyes under visible light irradiation[J].Materials Chemistry and Physics,2013,142(1):95-105.
[48]Hu D,Zhang K,Yang Q,et al.Super-high photocatalytic activity of Fe2O3nanoparticles anchored on Bi2O2CO3nanosheets with exposed {001} active facets[J].Applied Surface Science,2014,316:93-101.
Recent advances in bismuthyl carbonate photocatalysts
XuLiya,AoYanhui*,WangPeifang,WangChao
(Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes of Ministry of Education, Hohai University, Nanjing 210098, Jiangsu, China)
Abstract:Photocatalytic technology is a kind of green technology,which can convert solar energy into chemical energy.Photocatalytic technology is the most promising ways for solving pollution control and energy production.Bi2O2CO3 photocatalyst has attracted increasing attention due to its excellent photocatalytic activity.However,Bi2O2CO3 only absorbs UV light and has difficulty recycling,which restricts its practical application under solar light.The photocatalytic activity of Bi2O2CO3 can be effectively improved by morphology control and modification of Bi2O2CO3.The research status of zero-dimensional,one-dimensional,two-dimensional and three-dimensional structures of Bi2O2CO3 photocatalyst was introduced,and it was pointed out that Bi2O2CO3 with three-dimensional structure exhibited the best photocatalytic activity.The progress in the modification methods of Bi2O2CO3 by doping,metal deposition and the construction of the heterojunction was reviewed.Finally,the further development of Bi2O2CO3 photocatalyst in the degradation mechanism,degradation objects and degradation environment and so on should be made.
Key words:catalytic chemistry; Bi2O2CO3 photocatalyst; morphology control; modification
中图分类号:O643.36;TQ034
文献标识码:A
文章编号:1008-1143(2016)01-0001-06
doi:10.3969/j.issn.1008-1143.2016.01.001 10.3969/j.issn.1008-1143.2016.01.001
作者简介:徐丽亚,1991年生,女,浙江省金华市人,在读硕士研究生, 研究方向为光催化。
基金项目:国家自然科学基金委创新群体(51421006);优秀青年基金(51422902);重点基金(41430751);面上基金(51479065);江苏省自然科学基金面上项目(BK20141417);教育部创新团队(IRT13061);江苏高校优势学科建设工程资助项目
收稿日期:2015-07-18
通讯联系人:敖燕辉,1980年生,男,副教授,研究方向为基于光催化技术的水污染控制技术、环境友好材料的开发与应用、清洁能源的开发与利用以及水资源保护与水环境修复相关技术。
