CO2浓度升高对三角褐指藻和旋链角毛藻种群生长的影响*
2016-04-20毛雪微刘光兴王为民陈洪举
毛雪微, 刘光兴, 2, 王为民, 陈洪举, 2**
(中国海洋大学 1. 环境科学与工程学院; 2. 海洋环境与生态教育部重点实验室, 山东 青岛 266100)
CO2浓度升高对三角褐指藻和旋链角毛藻种群生长的影响*
毛雪微1, 刘光兴1, 2, 王为民1, 陈洪举1, 2**
(中国海洋大学 1. 环境科学与工程学院; 2. 海洋环境与生态教育部重点实验室, 山东 青岛 266100)
摘要:采用室内模拟CO2加富培养的方式研究了2种pCO2(395和1000μatm)对三角褐指藻(Phaeodactylum tricornutum)和旋链角毛藻(Chaetoceros curvisetus)这2种硅藻的种群生长和溶解性无机碳的影响。研究表明:CO2浓度升高显著促进了三角褐指藻和旋链角毛藻种群的生长。三角褐指藻实验组的平均比生长率比对照组高出33.1%,旋链角毛藻实验组的平均比生长率比对照组高出13.4%。同时,CO2加富引起培养环境中溶解性无机碳(DIC)浓度升高,据此推测,未来海洋酸化将使藻生长的碳限制得到缓解。海洋酸化会促进旋链角毛藻种群密度增加,这预示着在未来酸化的环境下暴发赤潮的概率将增加,这将对海洋生态系统的稳定性和生物多样性构成威胁。
关键词:海洋酸化; 浮游植物; 硅藻; 种群生长; 三角褐指藻; 旋链角毛藻
MAO Xue-Wei, LIU Guang-Xing, WANG Wei-Min, et al. Effects of elevated CO2on the population growth ofPhaeodactylumtricornutumandChaetoceroscurvisetus[J].Periodical of Ocean University of China, 2016, 46(3): 60-66.
近年来,海洋酸化成为各沿海国家及国际研究组织日益关注的问题,并成为海洋科学领域的研究热点之一。冰芯记录表明,工业革命以前的80万年中大气CO2平均浓度基本上没有高过280ppm[1-2],表层海洋的pH平均为8.2[3]。工业革命以来,大气CO2平均浓度升高了40%,这期间海洋吸收了人为排放CO2的30%,致使海洋表层pH值平均下降了0.1,引起海洋酸化[4-6]。2013年全球大气CO2的平均浓度约为395.31ppm[7],到21世纪末大气CO2浓度可能达到1000ppm(基于典型浓度路径RCP8.5下的CMIP5 ESMs模型预估)[5],逐增的CO2排放可能会导致表层海水[H+]增加100%~150%、海洋pH降低到3亿年以来从未经历过(极端灾害性气候事件除外)的酸度值[4]。
浮游植物贡献了几乎整个海洋生态系统和全球约50%的初级生产力,是自然界物质循环和能量流动的基础,在全球气候调节、碳封存和产氧方面起着重要的作用[8-9]。有学者指出大气CO2升高导致的海洋酸化会提高海洋浮游植物的净初级生产量(Net primary production, NPP)[10]。海洋酸化对浮游植物各类群影响的研究近年来逐步开展。早期海洋酸化的实验对象大部分集中在钙化藻类[11],例如:赫氏圆石藻(Emilianiahuxleyi)和大洋桥石藻(Gephyrocapsaoceanica)的钙化量随CO2浓度增加而降低[12],但颗石藻Coccolithuspelagicus和Calcidiscusleptoporus的种群生长速率在培养环境pH为7.80~8.70不随环境pH降低而发生明显变化[13]。另外,Dason等研究发现甲藻Amphidiniumcarterae和Heterocapsaoceanica在高CO2浓度和低pH条件下生长受到抑制[14]。随着海洋酸化研究的开展,硅藻作为浮游植物的重要类群,也逐渐受到关注[15-17];pCO2水平增加至700μatm会促进牟氏角毛藻(Chaetocerosmulleri)生长并使其单位细胞光合速率增加[18],而对中肋骨条藻(Skeletonemacostatum)的研究则发现海洋酸化对该藻生长和光合固碳量的影响,取决于阳光UV辐射和海洋酸化正、负效应的综合作用[19];近岸种假微型海链藻(Thalassiosirapseudonana)受CO2浓度增加的影响同样显著[20];但有研究称CO2加富使得直舟形藻(Naviculadirecta)的比生长率降低[21]。显然,不同种类浮游植物对海洋酸化的响应存在差异。浮游植物对海洋酸化响应的差异性和研究种类的局限性,使得目前海洋酸化对浮游植物影响的认识还存在争议,也表明加大海洋酸化研究的范围和深度是十分必要的。
本研究所选择的三角褐指藻(Phaeodactylumtricornutum)是实验室硅藻研究中的典型模式种[22],旋链角毛藻(Chaetoceroscurvisetus)广泛分布于中国近海,是比较常见的赤潮藻种[23],研究海洋酸化条件下这2种硅藻种群生长的响应及对无机碳利用的机制,将为进一步揭示海洋酸化对海洋生态系统的影响提供科学依据。
1材料与方法
1.1 实验设计
藻种来源实验所用的三角褐指藻取自中国海洋大学水产学院藻种室,旋链角毛藻取自中国海洋大学环境科学与工程学院赤潮分子生物学实验室。
培养条件取培养至对数生长期藻液接入盛“f/2”培养基[24]的1000mL锥形瓶中进行培养。三角褐指藻初始密度设为4.6×105cells/mL,旋链角毛藻初始密度设为5.0×105cells/mL。培养温度为(20±1)℃,盐度30,光照条件(139±20)μmol·m-2·s-1,光暗比例为12L∶12D。实验用海水为青岛石老人海水浴场砂滤海水,经0.45μm微孔滤膜过滤,高温高压(121℃, 0.12MPa, 20min)灭菌后,冷却至室温备用。
实验分组每种藻各设置实验组和对照组,每组各设3个平行。对照组通入过滤自然空气(395μatmCO2,2013年全球大气CO2浓度平均水平,来自NOAA/ESRL数据)。实验组通入含1000μatm CO2(2100年大气平均CO2浓度预测水平)[5,7]的过滤混合空气。通气流量均控制在0.1L/min,通气至体系底部。实验过程中的CO2浓度和通气流量由CO2加富器(CE100-6型, 武汉瑞华仪器设备有限责任公司)控制。
1.2 参数测算
培养环境pH的测定接种后通气,对藻种进行适应性培养24h。从接种次日起(第1天),每隔1d同时间取样,取已摇匀藻液10mL,立即用pH计(PB-10型, Sartorius, 德国)测定pH。
细胞计数pH测定完毕后,用中性鲁哥氏液固定藻类样品,用血球计数板、光学显微镜(Nikon YS100)进行藻密度计数。
比生长率μ的计算根据以下公式计算比生长率μ[25]:
μ=(lnN2-lnN1)/(t2-t1)。
式中:N1表示t1天藻密度;N2表示t2天藻密度。
培养环境溶解性无机碳(DIC)的测定从接种次日起(第1天),每隔1d同时间取样,取各组已摇匀藻液10mL,置于台式高速冷冻离心机(5804R, eppendorf, 德国)中离心2min (20℃,8000r/min),取上清液,用总有机碳分析仪(TOC-VCPN,岛津SHIMADZU,日本)测定其溶解性无机碳(以下简称DIC)含量。
1.3 数据处理
用SPSS 19.0软件进行数据处理,单因素方差分析(One-way ANOVA)进行显著性分析。用Origin 8.5软件绘图。
2结果
2.1 培养环境pH的变化
在整个实验过程中,CO2浓度升高引起三角褐指藻培养环境pH显著降低(p<0.05) (见图1a)。从第1天起至第7天,实验组环境pH极显著低于对照组(p<0.01),实验组培养环境平均pH降至7.94,比对照组pH (平均值8.37)低0.43。
CO2浓度升高同样引起旋链角毛藻培养环境pH极显著降低(p<0.01) (见图1b),实验组培养环境平均pH为8.01,与对照组(平均值8.47)相比降低了0.46。
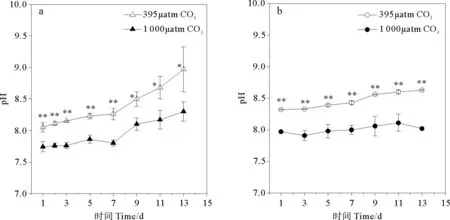
(a.代表三角褐指藻组培养环境pH,b.代表旋链角毛藻组培养环境pH。*:p<0.05,**:p<0.01,误差棒:标准偏差。a.pH inP.tricornutumculture medium ,b.pH inC.curvisetus. culture medium.*:p<0.05,**:p<0.01,Error bar:SD.)
图1不同pCO2培养条件下pH随时间的变化
Fig.1The time course of changes for pH values under differentpCO2
2.2 培养环境溶解无机碳(DIC)的变化
CO2浓度升高导致2种硅藻培养环境中DIC浓度升高(见图2),其中三角褐指藻实验组升高更明显,平均DIC浓度比对照组高出19.9% (p<0.05)。在三角褐指藻整个生长阶段中,随着时间推进,对照组DIC下降趋势较实验组明显,至第12天下降了18.0%,同时对照组pH上升趋势较实验组明显,但实验组DIC基本保持平稳。相比之下,旋链角毛藻培养环境整体DIC浓度低于三角褐指藻培养环境,且随着时间推移,其变化较平稳,处于20~25mg/L之间,实验组与对照组间差异不明显,仅在第13天有显著差别。
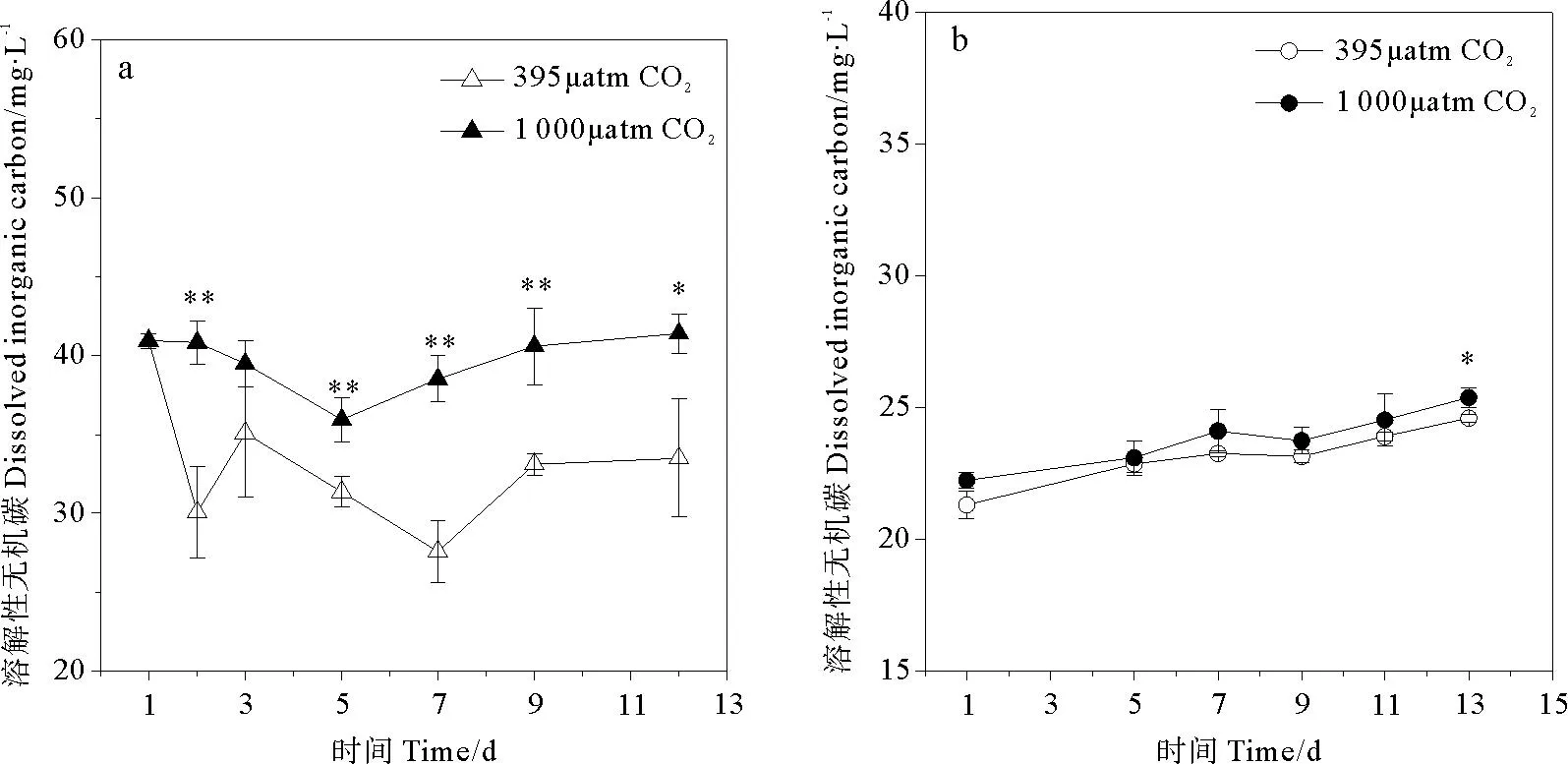
(a.代表三角褐指藻组培养环境DIC浓度,b.代表旋链角毛藻组培养环境DIC浓度。* :p<0.05,**:p<0.01,误差棒:标准偏差。a. DIC concentration inP.tricornutumculture medium , b. DIC concentration inC.curvisetus. culture medium. *:p<0.05, **:p<0.01, Error bar: SD.)
图2不同pCO2培养条件下溶解性无机碳(DIC)随时间的变化
Fig.2The time course of danges for dissolved inorganic carbon(DIC) under differentpCO2
2.3 种群生长
CO2浓度升高引起的酸化环境显著促进三角褐指藻种群的生长,不仅提高藻的生长速率,也使其生长的最大藻密度增大(见图3 a)。从第2天开始,实验组藻密度显著高于对照组(p<0.05)。第3天开始,实验组和对照组都已进入对数生长期,但是实验组藻密度明显高于对照组的藻密度(p<0.05),第5天和第7天出现极显著性差异(p<0.01)。随着时间的推进,实验组藻密度的增加更为明显,至第13天,实验组藻密度达到5.15×106cells/mL与对照组藻密度3.52×106cells/mL相比高出46.3%。
CO2浓度升高引起的酸化环境同样明显促进旋链角毛藻种群的生长(见图3 b),培养至第13天,实验组最大藻密度(3.60×106cells/mL)极显著高于对照组最大藻密度(2.81×106cells/mL) (p<0.01)。与三角褐指藻的实验结果不同的是,旋链角毛藻培养初期,实验组和对照组的藻密度只有微弱的差异,培养到第11天时,才显示出显著的差异性(p<0.05),实验组藻密度高出对照组45.3%。至实验第13天,实验组藻密度比对照组高出28.1%。
2.4 比生长率μ
结果显示,在培养初期(第1~7天),CO2浓度升高能提升三角褐指藻的比生长率(见图3a)。但在培养后期促进效果不明显。整个培养期间,三角褐指藻实验组平均比生长率为0.205d-1,高出对照组 (0.154d-1) 33.1%。相比之下,在培养初期(第1~5天)和第11天,CO2浓度升高对旋链角毛藻生长有促进作用(见图3b)。在整个培养期间,旋链角毛藻实验组平均比生长率为0.152d-1,对照组为0.134d-1。CO2加富引起旋链角毛藻平均比生长率上升13.4%。
3讨论
本研究的结果表明,在适宜温度、光照条件下,CO2浓度升高对三角褐指藻和旋链角毛藻种群生长有显著的促进作用,三角褐指藻实验组的平均比生长率比对照组高出33.1%,旋链角毛藻实验组的平均比生长率比对照组高出13.4% (见图3)。不少研究同样显示CO2浓度升高促进硅藻生长:中肋骨条藻在高浓度CO2(20.6 μmol·L-1)培养条件下的生长速率与低浓度CO2(4.5 μmol·L-1)相比提升了2‰~3‰[26];对赤道太平洋和南大洋的浮游植物群落进行CO2加富实验,发现CO2浓度增加会显著提高浮游植物的生产力并促进大型成链硅藻的生长[27-28]。Wu等研究表明,在高CO2分压((101.3±3.0) Pa, pH =7.80)条件下,三角褐指藻的生长率比对照组(39.3±1.1) Pa, pH =8.15)高出5.2%[15]。相比较而言,本研究CO2加富引起三角褐指藻平均比生长率上升33.1%(见图3),其种群促进作用比Wu等研究更显著。与硅藻的积极响应不同的是,海洋酸化能够导致海水CaCO3饱和度下降,降低多数钙化藻类的生长和钙化量[29-30],影响钙化生物的碳酸钙外壳或骨架的生成,对钙化生物产生负面影响,使得大部分的钙化生物和部分甲藻因海洋酸化而遭到抑制和威胁[14, 31-34]。据此推测,未来海洋酸化环境下,硅藻种群生长的促进将进一步影响到海洋生物群落的结构和演替,对海洋生态系统的群落稳定性造成一定影响。

(a.代表三角褐指藻组生长曲线及比生长率,b.代表旋链角毛藻组生长曲线及比生长率。* :p<0.05,**:p<0.01,误差棒:标准偏差。a. Growth curves and the specific growth rate inP.tricornutumculture medium , b. Growth curves and the specific growth rate inC.curvisetus. culture medium. *:p<0.05, **:p<0.01, Error bar: SD.)
图3不同pCO2培养条件下的生长曲线及比生长率μ
Fig.3Diatom growth curves and the specific growth rate under differentpCO2
研究显示,CO2浓度升高引起的海水碳源增加是导致三角褐指藻和旋链角毛藻种群生长被促进的主要原因。有研究指出,海洋藻类的生长和初级生产力会受大气CO2浓度水平的限制[35-37]。CO2浓度升高可以缓解光能自养型非钙化浮游植物的碳限制,从而促进其种群的生长。Riebesell等对围隔生态系统的CO2加富实验结果表明,在CO2浓度为1 050 ppm时,12天内浮游植物群落消耗的无机碳比CO2浓度为350 ppm时增加39%,浮游植物消耗的碳氮比从6升高到8,CO2加富使浮游植物耗碳量增加从而促进初级生产力[38]。本研究数据显示,随着培养时间的推进,三角褐指藻对照组培养环境中的溶解性无机碳量有下降趋势,环境中所提供的碳量已不足以满足植物种群的生长需求;而实验组DIC处于相对饱和的稳定趋势,其环境中所提供的溶解性无机碳量足以供给植物种群生长。对照组第12天的培养环境DIC浓度比第1天下降18% (见图2 a),培养环境pH有上升趋势(见图1 a),藻密度显著低于实验组(见图3 a),说明海水中无机碳浓度限制了三角褐指藻的生长;相比之下,实验组平均DIC浓度比对照组高出19.9%,平均比生长率比对照组高出33.1%,海水DIC浓度升高缓解了三角褐指藻生长所受到的的碳限制,从而促进其种群的生长。从第2天开始,三角褐指藻实验组藻密度即显著高于对照组(p<0.05) (见图3 a),三角褐指藻对溶解性无机碳升高表现出较高的敏感度。相比较而言,旋链角毛藻对CO2加富的敏感度较低,海洋酸化对三角褐指藻生长速率的促进作用更加显著(见图3):CO2加富最终引起三角褐指藻平均比生长率上升33.1%,高于旋链角毛藻的平均比生长率(13.4%)。
本文模拟的CO2浓度升高显著促进了三角褐指藻和旋链角毛藻种群的生长,碳源增加是促进二者种群生长的主要原因。海洋酸化可能通过缓解藻类生长的碳限制促进硅藻生长,并会使得某些获益物种(例如旋链角毛藻等赤潮生物)生物量增加,提升了赤潮暴发的风险,对海洋生态系统的稳定性构成威胁。
参考文献:
[1]Feely R A, Sabine C L, Lee K, et al. Impact of anthropogenic CO2on the CaCO3system in the oceans [J]. Science, 2004, 305(5682): 362-366.
[2]Lüthi D, Le Floch M, Bereiter B, et al. High-resolution carbon dioxide concentration record 650,000-800,000 years before present [J]. Nature, 2008, 453(7193): 379-382.
[3]Feely R A, Doney S C, Cooley S R. Ocean acidification: Present conditions and future changes in a high-CO2world [J]. Oceanography, 2009, 22(4): 36-47.
[4]Caldeira K, Wickett M E. Oceanography: anthropogenic carbon and ocean pH [J]. Nature, 2003, 425(6956): 365.
[5]Stocker T F, Qin D, Plattner G K, et al. IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [M]. Cambridge, U. K. and New York: Cambridge University Press. 2013: 525-527.
[6]Van Vuuren D P, Edmonds J, Kainuma M, et al. The representative concentration pathways: An overview [J]. Climatic Change, 2011, 109: 5-31.
[7]Dlugokencky E, Tans P.Trends in atmospheric carbondioxide[EB/OL].[2015-03-11].http://www.esrl.noaa.gov/gmd/ccgg/trends/.
[8]Falkowski P G, Barber R T, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production [J]. Science, 1998, 281(5374): 200-206.
[9]McQuatters-Gollop A, Reid P C, Edwards M, et al. Is there a decline in marine phytoplankton [J]. Nature, 2011, 472(7342): 6-7.
[10]Tagliabue A, Bopp L, Gehlen M. The response of marine carbon and nutrient cycles to ocean acidification: Large uncertainties related to phytoplankton physiological assumptions [J]. Global Biogeochemical Cycles, 2011, 25(3): 3017.
[11]Ruttimann J. Oceanography: sick seas [J]. Nature, 2006, 442(7106): 978-980.
[12]Riebesell U, Zondervan I, Rost B, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2[J]. Nature, 2000, 407(6802): 364-367.
[13]Langer G, Geisen M, Baumann K H, et al. Species-specific responses of calcifying algae to changing seawater carbonate chemistry [J]. Geochemistry Geophysics Geosystems, 2006, 7(9): 9006.
[14]Dason J S, Colman B. Inhibition of growth in two dinoflagellates by rapid changes in external pH [J]. Canadian Journal of Botany, 2004, 82(4): 515-520.
[15]Wu Y, Gao K S, Riebesell U. CO2-induced seawater acidification affects physiological performance of the marine diatomPhaeodactylumtricornutum[J]. Biogeosciences Discussions, 2010, 7(3): 3855-3878.
[16]Hoppe C J M, Hassler C S, Payne C D, et al. Iron limitation modulates ocean acidification effects on Southern Ocean phytoplankton communities [J]. PloS One, 2013, 8(11): 79890.
[17]Tew K S, Kao Y C, Ko F C, et al. Effects of elevated CO2and temperature on the growth, elemental composition, and cell size of two marine diatoms: potential implications of global climate change[J]. Hydrobiologia, 2014, 741: 79-87.
[18]胡晗华,高坤山.CO2浓度倍增对牟氏角毛藻生长和光合作用的影响 [J]. 水生生物学报, 2001, 25(6): 636-639.
Hu H H, Gao K S. Effects of doubled atmospheric CO2on the growth and photosynthesis ofChaetocerosmuelleri[J]. Acta Hydrobiologica Sinica, 2001, 25(6): 636-639.
[19]Chen X, Gao K S. Effect of CO2concentrations on the activity of photosynthetic CO2fixation and extracelluar carbonic anhydrase in the marine diatomSkeletonemacostatum[J]. Chinese Science Bulletin, 2003, 48(23): 2616-2620.
[20]Li G, Campbell D A. Rising CO2interacts with growth light and growth rate to alter photosystem II photoinactivation of the coastal diatomThalassiosirapseudonana[J]. PloS One, 2013, 8(1): 55562.
[21]Torstensson A, Chierici M, Wulff A. The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatomNaviculadirecta[J]. Polar Biology, 2012, 35(2): 205-214.
[22]Bowler C, Allen A E, Badger J H, et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes [J]. Nature, 2008, 456(7219): 239-244.
[23]Wang J, Wu J. Occurrence and potential risks of harmful algal blooms in the East China Sea [J]. Science of the Total Environment, 2009, 407(13): 4012-4021.
[24]Guillard R R L, Ryther J H. Studies of marine planktonic diatoms. I.CyclotellananaHustedt andDetonulaconfervaceaCleve [J]. Canadian Journal of Microbiology, 1962, 8(2): 229-239.
[25]Guillard R R L. Handbook of Phycological Methods [M]. Cambridge: Cambridge University Press, 1973, 1: 289-312.
[26]Gervais F, Riebesell U. Effect of phosphorus limitation on elemental composition and stable carbon isotope fractionation in a marine diatom growing under different CO2concentrations [J]. Limnology and Oceanography, 2001, 46: 497-504.
[27]Tortell P D, Morel F M M. Sources of inorganic carbon for phytoplankton in the eastern Subtropical and Equatorial Pacific Ocean [J]. Limnology and Oceanography, 2002, 47(4): 1012-1022.
[28]Tortell P D, Payne C D, Li Y, et al. CO2sensitivity of Southern Ocean phytoplankton [J]. Geophysical Research Letters, 2008, 35(4): 4605.
[29]Müller M, Schulz K, Riebesell U. Effects of long-term high CO2exposure on two species of coccolithophores [J]. Biogeosciences, 2010, 7(3): 1109-1116.
[30]Beaufort L, Probert I, de Garidel-Thoron T, et al. Sensitivity of coccolithophores to carbonate chemistry and ocean acidification [J]. Nature, 2011, 476(7358): 80-83.
[31]Kleypas J A, Feely R A, Fabry V J, et al. Impacts of ocean acidification on coral reefs and other marine calcifiers: a guide for future research [R]//Report of a workshop held st. Petersburg F L: NSF, NOAA, the U. S. Geological Survey, 2005: 19-20.
[32]Langdon C, Atkinson M J. Effect of elevated pCO2on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment [J]. Journal of Geophysical Research: Oceans (1978-2012), 2005, 110(C9): 169-192.
[33]Iglesias Rodriguez M D, Halloran P R, Rickaby R E M, et al. Phytoplankton calcification in a high-CO2world [J]. Science, 2008, 320(5874): 336-340.
[34]Burns W C G. Anthropogenic carbon dioxide emissions and ocean acidification: The potential impacts on ocean biodiversity [M]// Saving Biological Diversity. Connecticut: Springer U S, 2008: 187-202.
[35]Riebesell U, Wolf-Gladrow D A, Smetacek V. Carbon dioxide limitation of marine phytoplankton growth rates [J]. Nature, 1993: 249-251.
[36]Rost B, Riebesell U, Burkhardt S, et al. Carbon acquisition of bloom-forming marine phytoplankton [J]. Limnology and Oceanography, 2003, 48: 55-67.
[37]高坤山. 海洋酸化正负效应: 藻类的生理学响应 [J]. 厦门大学学报 (自然科学版), 2011, 50(2): 411-417.
Gao K S. Positive and negative effects of ocean acidification: Physiological responses of algae [J]. Journal of Xiamen University (Natural Science), 2011, 50(2): 411-417.
[38]Riebesell U, Schulz K G, Bellerby R G J, et al. Enhanced biological carbon consumption in a high CO2ocean [J]. Nature, 2007, 450(7169): 545-548.
[39]Silverman D N. Carbonic anhydrase: Oxygen-18 exchange catalyzed by an enzyme with rate-contributing Proton-transfer steps [J]. Methods in Enzymology, 1982, 87: 732-752.
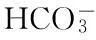
[41]Eberlein T, Van de Waal D B, Rost B. Differential effects of ocean acidification on carbon acquisition in two bloom-forming dinoflagellate species [J]. Physiologia Plantarum, 2014, 151(4): 468-479.
[42]McGinn P J, Morel F M M. Expression and regulation of carbonic anhydrases in the marine diatomThalassiosirapseudonanaand in natural phytoplankton assemblages from Great Bay, New Jersey [J]. Physiologia Plantarum, 2008, 133(1): 78-91.
[43]Aizawa K, Miyachi S. Carbonic anhydrase and CO2concentrating mechanisms in microalgae and cyanobacteria [J]. FEMS Microbiology Letters, 1986, 39(3): 215-233.
[44]Giordano M, Beardall J, Raven J A. CO2concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution [J]. Annual Review of Plant Biology, 2005, 56: 99-131.
[45]Newbold L K, Oliver A E, Booth T, et al. The response of marine picoplankton to ocean acidification [J]. Environmental Microbiology, 2012, 14(9): 2293-2307.
责任编辑朱宝象
Effect of Elevated CO2on the Population Growth ofPhaeodactylumtricornutum
andChaetoceroscurvisetus
MAO Xue-Wei1, LIU Guang-Xing1,2, WANG Wei-Min1, CHEN Hong-Ju1,2
(Ocean University of China, 1. College of Environmental Science and Engineering; 2. Key Laboratory of Marine Environment and Ecology, Ministry of Education, Qingdao 266100, China)
Abstract:Ocean acidification is increasingly become one of research hotspots of marine science. Many scholars have investigated the effect of ocean acidification on phytoplankton populations. However, there still exist academic controversy on the influence of ocean acidification on the growth characteristics and response of phytoplankton populations because of the limitation and uncertainty of ocean acidification studies. We studied two of the typical diatom species, Phaeodactylum tricornutum and Chaetoceros curvisetus (harmful algae) to assess the effect of future CO2-driven ocean acidification (the future level of the year 2100) on the growth of phytoplankton populations, aiming to predict the response of marine diatoms to future global climate change and provide data base to the research of elevated CO2 impact on marine organisms and biodiversity. CO2 enrichment experiments were carried out under specific CO2 concentrations. The control groups were bubbled with CO2 of 395μatm (ambient atmosphere level) and the experimental groups were 1,000 μatm (future scenario of 2100 year). Results showed that the environmental pH in experimental groups had reduced since the effect of CO2-driven ocean acidification and the average pH level reduced approximately 0.3~0.4. The growth of P. tricornutum and C. curvisetus was promoted significantly by elevated CO(2 )concentration. The average specific growth rate (μ) of P. tricornutum groups and C. curvisetus groups had increased by 33.1% and 13.4%, respectively, compared to their control groups. CO2-driven ocean acidification can lead to the increase of dissolved inorganic carbon (DIC) concentration in the culture environment and relieve the limitation of inorganic carbon utilization during the growing period. Then, the growth can be promoted. Since diatoms account for a large part of marine primary productivity, the positive effect of ocean acidification on their growth may have ecological consequences in marine food chain in future. Additionally, elevated CO2 may lead to the break out of harmful algal blooms (such as C. curvisetus blooms) and be a threat to the stability of marine ecosystem and biodiversity.
Key words:ocean acidification; phytoplankton; diatom; population growth; Phaeodactylum tricornutum; Chaetoceros curvisetus
DOI:10.16441/j.cnki.hdxb.20150052
中图法分类号:Q948.112
文献标志码:A
文章编号:1672-5174(2016)03-060-07
作者简介:毛雪微(1990-),女,硕士生,从事海洋浮游生物生态学研究。E-mail: maoxuewei_ouc@163.com**通讯作者: E-mail: hongjuc@ouc.edu.cn
收稿日期:2015-03-11;
修订日期:2015-06-04
*基金项目:国家自然科学基金青年基金项目(31101875) ;高等学校博士学科点专项科研基金项目(20110132120027)资助
引用格式:毛雪微, 刘光兴, 王为民, 等. CO2浓度升高对三角褐指藻和旋链角毛藻种群生长的影响[J]. 中国海洋大学学报(自然科学版), 2016, 46(3): 60-66.
Supported by Youth Fund Project of Natural Science Foundation of China, Grant (31101875); Specialized Research Fund for the Doctoral Program of Higher Education of China, Grant (20110132120027)
