Genotypic variation for seed protein and mineral content among post-rainy season-grown sorghum genotypes
2016-04-05AshokBdignnvrGirishRmchndrnGnpthi
Ashok Bdignnvr*, G. Girish, V. Rmchndrn T.R. Gnpthi
aNuclear Agriculture and Biotechnology Division, Bhabha Atomic Research Centre, Mumbai 400085, IndiabAgricultural Research Station, University of Agriculture Sciences, Gulbarga 585101, Karnataka, India
Genotypic variation for seed protein and mineral content among post-rainy season-grown sorghum genotypes
Ashok Badigannavara,*, G. Girishb, V. Ramachandrana, T.R. Ganapathia
aNuclear Agriculture and Biotechnology Division, Bhabha Atomic Research Centre, Mumbai 400085, India
bAgricultural Research Station, University of Agriculture Sciences, Gulbarga 585101, Karnataka, India
A R T I C L E I N F O
Article history:
Received 26 May 2015
Received in revised form 14 July 2015
Accepted 6 August 2015
Available online 16 August 2015
Keywords:
Mineral content
Post-rainy sorghum
Genetic correlation
Cluster analysis
A B S T R A C T
Sorghum is an important staple food crop of Asian and African countries. As a“poor man's crop”, it provides dietary starch, protein, and some vitamins and minerals. Minerals are important for various physiological functions in the human body. As a major staple crop of central and southern Indian provinces, sorghum landraces are a source of supplementary micronutrients. Concentrations of micronutrients and protein and yield parameters were studied using 112 local landraces and varieties. Univariate analysis revealed wide variation for iron (1.10–9.54 mg 100 g−1), zinc (1.12–7.58 mg 100 g−1), protein (3.50–12.60%), and grain yield (2.50–76.50 g) among the landraces. High estimates of genetic/phenotypic coefficient of variation, and genetic advances over the mean were identified for landraces and varieties. High heritabilities were also identified for yield and mineral content. Correlation estimates among the genotypes indicated that grain yield was positively correlated with copper and protein with copper and zinc. Cluster analysis based on Euclidean distance resolved all of the genotypes into three major clusters. The wide range of values with high heritability estimates may favor the use of these landraces in recombination breeding to improve nutritional quality in sorghum.
©2015 Crop Science Society of China and Institute of Crop Science, CAAS. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
* Corresponding author. Tel.: +91 22 25590543; fax: +91 22 25505151.
E-mail address: ashokmb1@gmail.com (A. Badigannavar).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science, CAAS.
http://dx.doi.org/10.1016/j.cj.2015.07.002
2214-5141/©2015 Crop Science Society of China and Institute of Crop Science, CAAS. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Sorghum is an important cereal crop grown in India under rainfed conditions and in stress-prone areas. Globally, sorghum covers an area of 42.2 Mha with grain production of 62.3 Mt and productivity of 1.5 tons ha−1[1]. Improved cultivars coupled with good management practices have increased productivity levels significantly despite decreasing acreage. In India, sorghum is grown on 6.2 Mha, accounting for 14.63% of the global area, with a production estimate of 5.3 Mt [1,2].The major factors responsible for the decline in area and production are a shift towards commercial crops such as groundnut, soybean, sunflower, and maize, and the availability of other cereals at reduced prices. In India, sorghum is grown in both rainy and post-rainy seasons, on shallow, medium, and deep soils. Although rainy-season sorghum has greater grain yield than that grown in the post-rainy season, most of it is used for industrial purposes. Post-rainy season sorghum is known for its grain quality and is cultivated mostly by marginal farmers for human consumption.
Sorghum is a good source of energy, protein, carbohydrate, vitamins and minerals. The grain contains 1.30–3.30% of ash and minerals such as phosphorus, potassium and magnesium in varying amounts. It is also an important source of iron (Fe) and zinc (Zn) and better than rice and wheat with respect to mineral nutrition [3]. Protein constitutes 12% of the grain on a dry-weight basis. There is substantial variation in total protein and amino acid profiles between sorghum varieties [4]. This variation may be attributed to the diverse range of agroclimatic conditions under which the crop is cultivated [5].
The poor digestibility of sorghum proteins is a major constraint to better utilization of the crop. It is due to the presence of antinutritive factors, including phytate, polyphenols, and kafirins [6,7]. Deficiencies in iron and zinc result in poor growth of children, reduced immunity, weakness, and morbidity [8]. Non-diversification of cereal and plant-based diets poor in micronutrients may be the major reason for micronutrient deficiency in the underdeveloped countries [9]. Dietary diversification, supplementation, fortification, and biofortification of crop plants are means of combating micronutrient malnutrition. Most of these approaches suffer from problems, but biofortification has been found to be the best way of increasing micronutrient content [10]. By this method, the bioavailability of minerals is improved by changing the genetic constitutions of food crops. Studies have even improved mineral bioavailability by reducing antinutritive properties or by soaking and germination [11]
Studies have been conducted to estimate macro- and micronutrient contents in yellow, black, and red sorghum genotypes. Studies have also been performed to reduce anti-nutritional factors and compare the effects of different physical and chemical seed treatment methods on the bioavailability of mineral nutrients. In one such study, advanced breeding lines and germplasm accessions showed higher values for Fe and Zn than popular varieties [12]. The range of Fe content was 12.10–83.40 mg kg−1and that of Zn content was 6.30–51.40 mg kg−1. Preliminary study of sorghum advanced breeding lines and a few selected germplasm accessions have indicated limited variation for grain Fe and Zn contents [13].
Identifying germplasm lines with improved yield and grain quality, especially with respect to mineral content, is an important task against the backdrop of malnutrition caused by the lack of mineral nutrients in underdeveloped countries [14]. Local landraces collected from Karnataka and Maharashtra provinces have not been studied adequately to develop lines that are suitable for various food and industrial purposes. The present investigation was accordingly planned to identify the extent of genetic variation in local landraces with respect to grain yield and mineral and protein content before their use in crossbreeding programs.
2. Material and methods
2.1. Plant material
The material used in this study comprised 92 sorghum landraces and 20 varieties, including the popular check variety M-35-1 adapted to the post-rainy season in the Indian states of Karnataka, Maharashtra, and Andhra Pradesh (Table S1). These genotypes were grown in two replications in a randomized complete block design (RCBD) at the Experimental and Gamma Field Facility, Bhabha Atomic Research Centre, Trombay, Mumbai (19°03″N, 72°93″E) during the post-rainy season in the 2013 crop season. The experiment was laid out on medium to deep black soils in two rows of 5 m length with 45 cm×15 cm spacing. All agronomic practices were followed to raise a healthy crop. The effect of soil heterogeneity (nutrient and fertility levels) was addressed by means of the replicated trial. Seed index was recorded as the weight of 100 kernels from bulk seeds from each head of the genotypes grown. Selfed seeds were harvested from each genotype and replicated grain samples (20 g) were used for mineral and protein assays.
2.2. Micronutrient estimation
Mineral (micronutrient) content of the sorghum genotypes, including copper (Cu), zinc (Zn), iron (Fe), manganese (Mn), calcium (Ca) and magnesium (Mg), were estimated by atomic absorption spectrometry. A dried seed sample of each genotype was ground to fine powder and 1 g powder was digested on a hotplate using a 5:1 mixture of Nitric acid and Perchloric acid. The digested samples were subjected to mineral analysis with a GBC 932B+ atomic absorption spectrophotometer (GBC Scientific Equipment, Melbourne, Australia) with an air–acetylene flame. The estimated concentrations of minerals were expressed as mg per 100 g of the sample.
2.3. Determination of total protein content
The nitrogen content of the sorghum genotypes was determined by the Kjeldahl method using a KEL PLUS distillation unit (Pelican Equipment, Chennai, India) [15]. A sorghum flour sample (200 mg) was digested with concentrated H2SO4in the presence of a catalyst and was heated in a chamber to 350°C. The clear solution was cooled and distilled to trap ammonia. Dissolved ammonia was estimated by titration and the nitrogen level was estimated by formula (AOAC Official Method 950.48). The crude protein content of the sample was calculated as 6.25 times its nitrogen content and expressed as a percentage.
2.4. Data analysis
Data were recorded for five plants in each accession and replication. The data were subjected to analysis of variance for each environment and for the combined data using PROC GLM of SAS 9.1 [16]. Genetic parameters were estimated to identify variability among accessions and determine genetic and environmental effects on different traits. Genotypic (σ2G), phenotypic (σ2P), and error (σ2E) variances were calculated foreach trait from the pooled ANOVA table. Phenotypic coefficient of variation (PCV) and genotypic coefficient of variation (GCV) were calculated by a standard formula [17]. The extent of variation among the genotypes was estimated as broad-sense heritability and defined as the ratio of the genetic variance (σ2G) between genotypes to the total phenotypic variance (σ2P=σ2G+σ2E) [18]. Genetic advance was calculated as GA (%) = K×σP×hbs×100, where K (selection differential at 5%) = 2.06,σPdenotes the phenotypic standard deviation, and hbsdenotes broad-sense heritability. GAM (genetic advance over mean as %) was calculated as percent of the genetic advance over the mean. To determine the genetic relationships among the different traits, Pearson correlation coefficients were calculated for every pair of traits using PROC CORR of SAS [16]. A cluster analysis of the morphological data was performed using Euclidean distances and a dendrogram was constructed [19].
3. Results
3.1. Analysis of variance (ANOVA)
The analysis of variance showed significant differences due to genotypes in grain mineral content and protein and yield traits, as indicated by their mean square values (Table 1). The coefficients of variation (CV%) ranged from 3.09% to 12.94%. A wide range of values was observed for yield (2.50–78.00 g plant−1), seed weight (2.06–4.10 g), Zn (0.76–7.58 mg 100 g−1), Fe (0.15–9.54 mg 100 g−1) and protein (3.25–14.88%). The mean values for these traits were 31.41 g plant−1, 3.13 g, 2.09 mg 100 g−1, 3.82 mg 100 g−1and 8.42%, respectively. Among the landraces studied, Rawar-1 showed the highest iron content (9.54 mg 100 g−1), whereas TSG-82 (Pop sorghum) showed the highest protein content (12.60%). The check variety, M-35-1, showed 4.84 mg 100 g−1and 11.38%, respectively. However, among the popular varieties studied, SPV-1829 showed the highest iron content (6.48 mg 100 g−1), and Muguthi the highest protein content (14.33%). One of the promising landraces, Tengalli-2, showed the highest grain yield of 78.00 g plant−1with bold pearl light yellow seeds (4.00 g per 100 seeds), in contrast to M-35-1 (28.82 g plant−1and 4.00 g per 100 seeds, respectively). The varieties outyielded the germplasm lines by an average of 35.78 g plant−1(Table 2). Although the germplasm lines showed a wide range of values for grain yield (3.0–78.0 g) the average yield (29.83 g plant−1) was lower than that of the varieties. With respect to mineral content, there was no significant difference among the varieties and germplasm lines, but the ranges for Zn, Fe, Mn, Ca, and Mg were wider for the germplasm lines than for the popular varieties.
3.2. Estimation of genetic variability parameters
The genetic coefficient of variation (GCV) for the nine traits ranged from 12.61 to 81.57%. Among the mineral components, calcium showed the highest GCV (81.57%) and PCV (81.63%), whereas magnesium showed the lowest GCV (16.65%) and PCV values (16.71%) (Table 3). Grain yield, seed weight, and protein content showed lower GCV and PCV values than mineral content. High heritability values were estimated for all the traits in this study. Genetic advance was highest for magnesium (218.38%), followed by grain yield (28.16%). Genetic advance over mean ranged from 6.49% (seed weight) to 47.55% (manganese). Broad-sense heritability values ranged from 0.76 to 0.99 for yield and mineral traits. Seed weight showed the lowest heritability values (0.76), with all other traits showing heritabilities above 0.97.
3.3. Correlation and cluster analyses
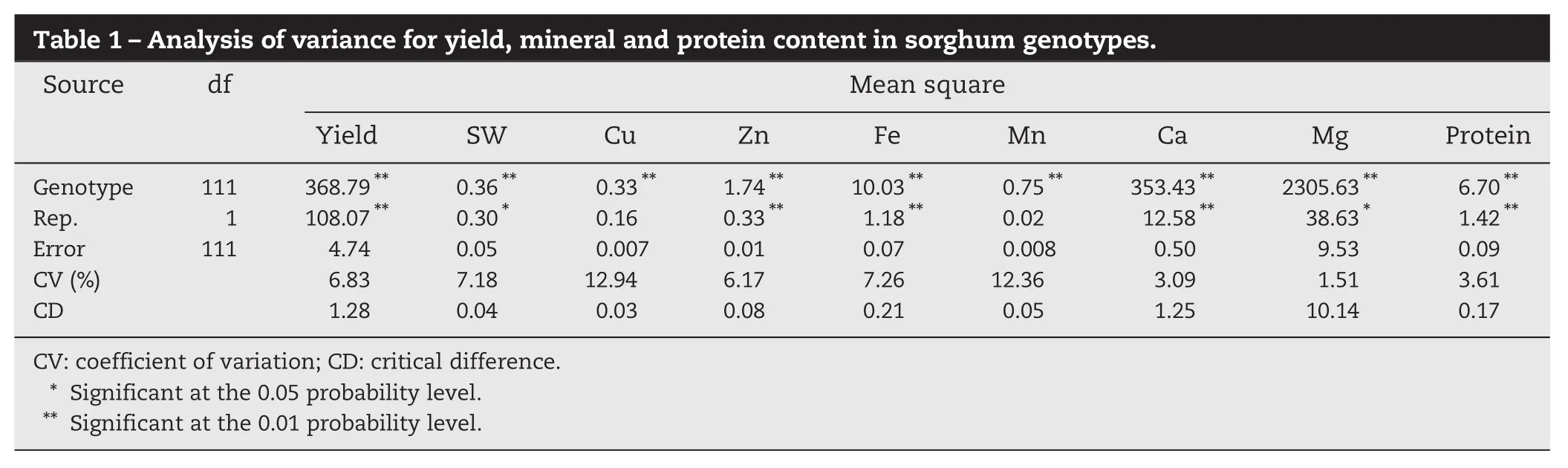
Table 1–Analysis of variance for yield, mineral and protein content in sorghum genotypes. Source df Mean square Yield SW Cu Zn Fe Mn Ca Mg Protein Genotype 111 368.79** 0.36** 0.33** 1.74** 10.03** 0.75** 353.43** 2305.63** 6.70** Rep. 1 108.07** 0.30* 0.16 0.33** 1.18** 0.02 12.58** 38.63* 1.42** Error 111 4.74 0.05 0.007 0.01 0.07 0.008 0.50 9.53 0.09 CV (%) 6.83 7.18 12.94 6.17 7.26 12.36 3.09 1.51 3.61 CD 1.28 0.04 0.03 0.08 0.21 0.05 1.25 10.14 0.17 CV: coefficient of variation; CD: critical difference. * Significant at the 0.05 probability level. ** Significant at the 0.01 probability level.
Correlation estimates between the nine traits are given in Table 4. Grain yield was significantly and positively correlated with copper content (0.28**). Protein content was positivelycorrelated with copper (0.24**) and zinc (0.27**), but negatively correlated with magnesium (−0.16*). Among the minerals, copper and zinc had significant positive correlations with manganese and calcium. Negative correlations were also observed between grain yield and magnesium (−0.32**), copper and magnesium (−0.59**), and calcium and magnesium (−0.27**).
The yield and mineral data were used to estimate Euclidean distances between the sorghum genotypes and a dendrogram wasconstructed (Fig.1).Theanalysisresolved the 112 genotypes into three clusters. Three top-yielding germplasm lines: TSG 48 (69.03 g plant−1), TSG 74 (56.00 g plant−1), and TSG 76 (78.00 g plant−1) were included in cluster III with a wide range of values for seed index (2.36–4.06 g) and protein content (5.99–13.89) (Table 5). This cluster also contained landraces showing high mineral content, especially zinc (0.81–4.68 mg g−1) and iron (1.15–9.40 mg g−1). The second cluster, comprising 43 genotypes including seven varieties, had grain yields ranging from 13.15 to 60.10 g plant−1and protein content from 5.35 to 14.56%. Mineral contents were not significantly different from thoseofgenotypesinotherclusters.Clusters Iand III,comprising 69 genotypes including eight varieties, showed moderate to high values for yield, protein, and mineral contents.

Table 3–Mean, range, and genetic variability components for yield, mineral and protein traits in sorghum genotypes. Trait Range Mean Vg Vp GCV (%) PCV (%) H2 GA GAM (%) Yield per plant 2.50–78.0 31.41 182.03 186.76 42.95 43.51 0.97 28.16 25.89 Seed weight 2.06–4.10 3.13 0.15 0.21 12.61 14.46 0.76 0.71 6.49 Cu 0.06–1.98 0.64 0.16 0.16 62.88 64.04 0.96 0.81 36.68 Zn 1.12–7.58 2.09 0.79 0.81 42.60 43.03 0.98 1.87 25.90 Fe 1.10–9.54 3.82 4.98 5.05 58.39 58.82 0.98 4.53 34.31 Mn 0.11–6.60 0.76 0.37 0.38 80.36 81.11 0.98 1.25 47.55 Ca 1.20–56.58 23.03 352.92 353.43 81.57 81.63 0.99 27.24 34.17 Mg 41.0–46.60 203.49 1148.05 1157.58 16.65 16.71 0.99 218.38 31.00 Protein 3.25–14.88 8.42 3.31 3.39 21.59 21.88 0.97 3.68 12.63 Vg and Vp: genetic and phenotypic variances; GCV and PCV: genetic and phenotypic coefficient of variation as %; H2: broad-sense heritability; GA: genetic advance; GAM: genetic advance over mean; micronutrient concentrations are in mg 100 g–1of the sample; yield and seed weight are in g.
4. Discussion
Sorghum is a rich source of mineral, vitamins, protein, and carbohydrate, which are important for human and animal consumption. Knowledge of its genetic and nutritional diversity would have a direct impact on the improvement of sorghum for quality breeding [20]. The present study was accordingly undertaken to evaluate post-rainy season sorghum genotypes for nutritional traits.
Wide variation for yield and micronutrients were noted in the germplasm lines and varieties. Wide ranges of values were observed, especially for zinc, iron and protein levels. Iron and zinc content ranged from 1.10 to 9.54 and 1.12 to 7.58 mg 100 g−1respectively in the germplasm lines. In line with the present study, earlier reports have also indicated wide ranges of values for iron (3.00–11.30 mg 100 g−1) and zinc (1.10–5.02 mg 100 g−1) in sorghum genotypes [21,22]. Grain Fe and Zn contents higher than 5 mg 100 g−1and 3.70 mg 100 g−1respectively have been recommended as potential sorghum lines for grain micronutrient enrichment [23]. The majority of Asian and African people consume sorghum as a whole grain. Although sorghum bran contains iron [24], phytate and sometimes tannins, depending on the cultivar, reduce the availability of iron [25]. However, several studies have shown that in vitro bioavailability of iron and zinc was significantly improved by soaking and germination treatments [11].
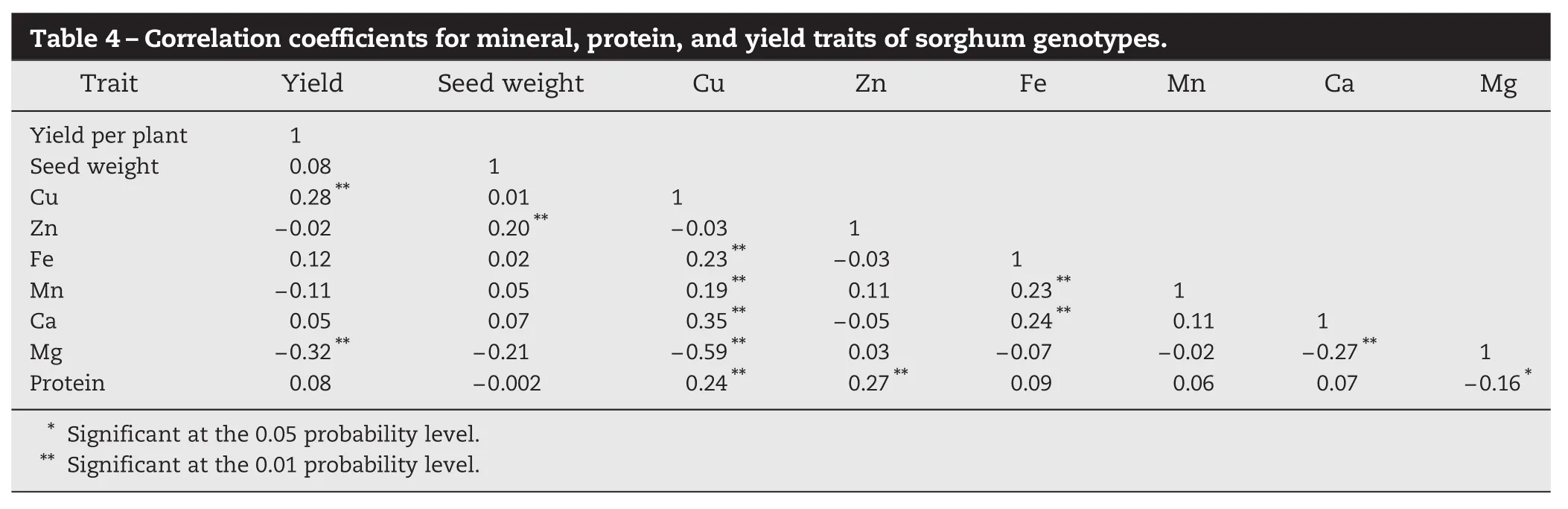
Table 4–Correlation coefficients for mineral, protein, and yield traits of sorghum genotypes. Trait Yield Seed weight Cu Zn Fe Mn Ca Mg Yield per plant 1 Seed weight 0.08 1 Cu 0.28** 0.01 1 Zn −0.02 0.20** −0.03 1 Fe 0.12 0.02 0.23** −0.03 1 Mn −0.11 0.05 0.19** 0.11 0.23** 1 Ca 0.05 0.07 0.35** −0.05 0.24** 0.11 1 Mg −0.32** −0.21 −0.59** 0.03 −0.07 −0.02 −0.27** 1 Protein 0.08 −0.002 0.24** 0.27** 0.09 0.06 0.07 −0.16* * Significant at the 0.05 probability level. ** Significant at the 0.01 probability level.
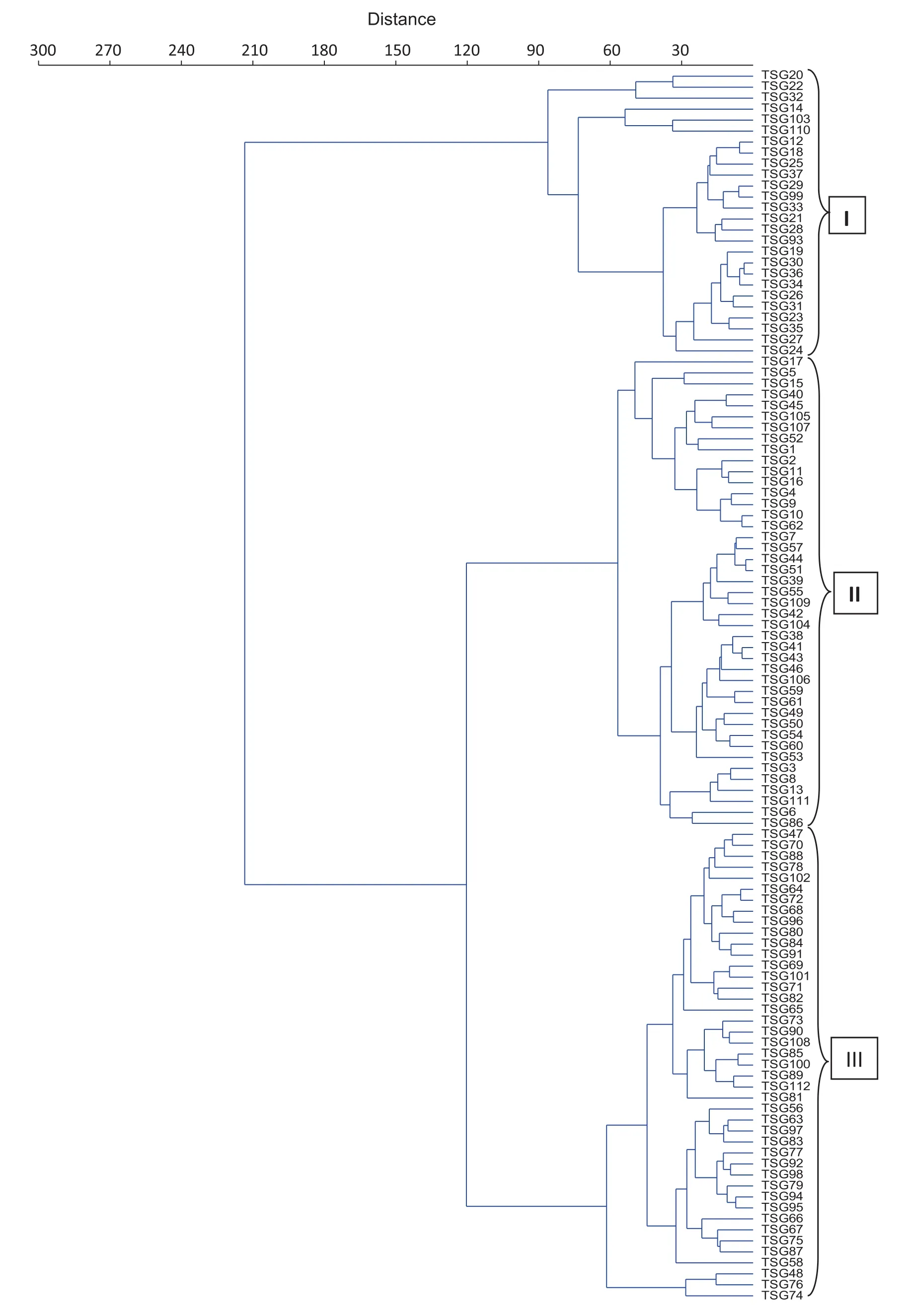
Fig. 1–Cluster analysis for yield, mineral and protein content between sorghum genotypes.
Useofsorghumasacheapsourceofproteinwouldbegreatly enhanced by effective elimination of antinutritive components and improvement of digestibility. In the present study, a wide range of values was observed for protein content (3.25–14.53%) with a mean of 8.42%. Although varieties showed a wider range of protein content (8.7%) than germplasm lines (8.46%), the difference was not significant. TSG-98 (Muguthi), a popularpost-rainy season variety known for its breadmaking quality, showed the highest protein content, 14.53%. Among the germplasm lines, TSG-82 (Pop sorghum) showed the highest protein content, 12.60%. As in this study, Jambuunathan (1984) also recorded protein contents in the range of 4.40% to 21.10% with a mean of 11.40% [4]. Among factors affecting protein content are environment, nitrogen fertilization, and genotype [26]. However seed protein in sorghum is less digestible than that of other cereals, thus reducing the bioavailability of the protein. This poor digestibility is due to extensive polymerization of kafirins upon cooking and presence of tannins in certain sorghum lines [27].
With respectto geneticparameters, calcium content showed highest GCV and PCV and magnesium the lowest. PCV values were higher than GCV for all traits studied, owing to the low influence of environment on the expression of the traits. High heritability and genetic advance for grain yield were found among the sorghum genotypes. High GCV and heritability values indicate the extent of heritable variation for the traits studied. Germplasm lines such as TSG-48 and TSG-74 not only were high-yielding but also showed significantly higher values for micronutrient levels than the control. Among the factors responsible for the wide variation in micronutrient content could be genotype, mineral concentrations and translocation rates in soil, and weather conditions. Genotypes may exhibit differing abilities to absorb nutrients from the soil [28]. Germplasm lines with high mineral content coupled with high-yielding backgrounds could be used in recombination breeding for improving the nutritional value of sorghum.
Correlations between traits are of great importance for the success of selection practiced in breeding programs. In the present study, protein content was positively correlated with copper and zinc. Copper showed significantly positive correlations with yield, protein, and all other micronutrients except zinc. Iron content was positively correlated with manganese and calcium, suggesting the possibility of combining selection for both micronutrients in a single agronomic background. However, for estimation of the effects of micronutrientimproved cultivars in human nutrition, they must be delivered in varieties that carry farmer-preferred grain traits such as earliness and seed size and color [29]. In line with the present study, cultivars with higher Zn concentration in the grain also showed high levels of protein [30]. Other studies have found negative correlations between grain yield-associated traits and Fe and zinc in maize [31] and sorghum [32].
The association between traits and their contribution to diversity can be validated by multivariate analysis. Cluster analysis based on Euclidean distance was performed to employ associations among the traits for assigning the germplasm lines and varieties to different clusters. High-yielding germplasm lines with improved micronutrient levels were grouped in cluster III. TSG-76 and TSG-48 not only were high-yielding but also showed better mineral content than the control [33]. Selection and crossing of genotypes from different clusters would help in bringing together genes favorable for yield and quality traits so as to breed tailor-made varieties [34].
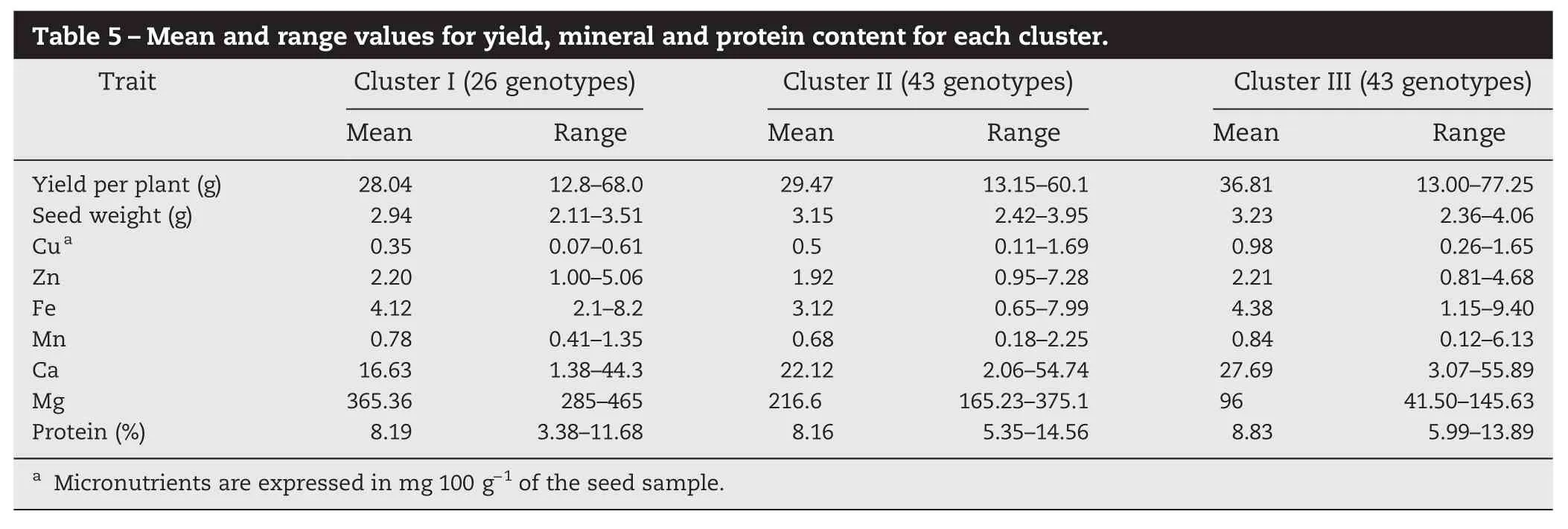
Table 5–Mean and range values for yield, mineral and protein content for each cluster. Trait Cluster I (26 genotypes) Cluster II (43 genotypes) Cluster III (43 genotypes) Mean Range Mean Range Mean Range Yield per plant (g) 28.04 12.8–68.0 29.47 13.15–60.1 36.81 13.00–77.25 Seed weight (g) 2.94 2.11–3.51 3.15 2.42–3.95 3.23 2.36–4.06 Cua 0.35 0.07–0.61 0.5 0.11–1.69 0.98 0.26–1.65 Zn 2.20 1.00–5.06 1.92 0.95–7.28 2.21 0.81–4.68 Fe 4.12 2.1–8.2 3.12 0.65–7.99 4.38 1.15–9.40 Mn 0.78 0.41–1.35 0.68 0.18–2.25 0.84 0.12–6.13 Ca 16.63 1.38–44.3 22.12 2.06–54.74 27.69 3.07–55.89 Mg 365.36 285–465 216.6 165.23–375.1 96 41.50–145.63 Protein (%) 8.19 3.38–11.68 8.16 5.35–14.56 8.83 5.99–13.89aMicronutrients are expressed in mg 100 g−1of the seed sample.
5. Conclusions
The present study was a preliminary survey of mineral contents of local landraces grown in southern and central Indian provinces. Landraces with elevated levels of mineral elements and high yielding background would lead to the addition of value to sorghum products. Owing to their high heritability, genotypes with elevated levels of mineral elements could be an effective component of functional foods and improve food nutritional quality.
Acknowledgments
The authors thank the Head, Nuclear Agriculture and Biotechnology Division, and Gamma Field Facility Section, Bhabha Atomic Research Centre, Mumbai, India for encouragement and support. The authors also thank Dr. S.N. Jamdar, NABTD, BARC, for providing the protein estimation unit.
Appendix A. Supplementary material
Supplementary material to this article can be found online at http://dx.doi.org/10.1016/j.cj.2015.07.002.
R E F E R E N C E S
[1] FAO, FAOSTAT, http://faostat3.fao.org/, 2013.
[2] D.K. Charyulu, M.C.S. Bantilan, A. Rajalaxmi, Development and diffusion of sorghum improved cultivars in India: impact on growth and variability in yield, 57th AARES Annual Conference, Sydney, New South Wales, February 5–8, 2013.
[3] S.S. Chan, E.L. Ferguson, K. Bailey, U. Fahmida, T.B. Harpe, R.S. Gibson, The concentration of iron, calcium, zinc and phytate in cereals and legumes habitually consumed by infants living in East Lombok, Indones, J. Food Compos. Anal. 20 (2007) 609–617.
[4] R. Jambunathan, U. Singh, V. Subramanian, Grain quality of sorghum, pearl millet, pigeonpea and chickpea, in: K.T. Achaya (Ed.), Proceedings of a Workshop on Interfaces Between Agriculture Nutrition And Food Science, Patancheru, India, ICRISAT, India, November 10–12, 1981.
[5] D.H. Waggle, C.W. Deyoe, F.W. Smith, Effect of nitrogen fertilization on the amino acid composition and distribution in sorghum grain, Crop Sci. 7 (1967) 367–368.
[6] B.R. Hamaker, A.A. Mohamed, J.E. Habben, C.P. Huang, B.A. Larkins, Efficient procedure for extracting maize and sorghum kernel proteins reveals higher prolamin contents than the conventional method, Cereal Chem. 72 (1995) 583–588.
[7] S. Valencia, U. Svanberg, A.S. Sanberg, J. Ruals, Processing of Quinoa (Chenopodium quinoa, Willd): effects on in vitro iron availability and phytae hydrolysis, Int. J. Food Sci. Nutr. 50 (1999) 203–208.
[8] A.J. Stein, Global impacts of human malnutrition, Plant Soil 335 (2010) 133–154.
[9] S. Gómez-Galera, E. Rojas, D. Sudhakar, C. Zhu, A.M. Pelacho, T. Capell, P. Christou, Critical evaluation of strategies for mineral fortification of staple food crops, Transgenic Res. 19 (2010) 165–180.
[10] H.E. Bouis, Special issue on improving human nutrition through agriculture, Food Nutr. Bull. 21 (2000) 351–576.
[11] E.-M.M.R. Afify, H.S. El-Beltagi, S.M. Abd El-Salam, A.A. Omran, Bioavailability of iron, zinc, phytate and phytase activity during soaking and germination of white sorghum varieties, PLoS One 6 (2011), e25512, http://dx.doi.org/10.1371/ journal.pone.0025512.
[12] K. Hariprasanna, V. Agte, J.V. Patil, Genetic control and heterosis for grain iron and zinc contents in sorghum (Sorghum bicolor), Indian J. Genet. 74 (2014) 638–643.
[13] B.V.S. Reddy, S. Ramesh, T. Longvah, Prospects of breeding for micronutrients and carotene-dense sorghums, J. SAT Agric. Res. 1 (2005) 10–14.
[14] S.B. Feil, S. Moser, S. Jampatong, P. Stamp, Mineral composition of the grains of tropical maize varieties as affected by pre-anthesis drought and rate of nitrogen fertilization, Crop Sci. 45 (2005) 516–523.
[15] B. Wathelet, Nutritional analyses of proteins and amino acids in beans (Phaseolus sp.), Biotechnol. Agron. Soc. Environ. 3 (1999) 197–200.
[16] SAS Statistical Analysis Software for Windows 9.1.3, SAS Institute Inc, Cary, NC, USA, 2010.
[17] G.W. Burton, Quantitative inheritance in Grasses, Proceedings of the Sixth International Grassland Congress 1952, pp. 227–283.
[18] R.W. Allard, Principal of Plant Breeding, John Wiley and Sons, Inc., New York, 1960 84–85.
[19]Ø. Hammer, D.A.T. Harper, P.D. Ryan, PAST: Paleontological statistics software package for education and data analysis, Palaeontol. Electron. 4 (1) (2001) 9.
[20] R.E. Dean, J.A. Dahlberg, M.S. Hopkins, S.E. Mitchell, S. Kresovich, Genetic redundancy and diversity among‘Orange' accessions in the U.S. national sorghum collection as assessed with simple sequence repeat (SSR) markers, Crop Sci. 39 (1999) 1215–1221.
[21] R. Jambunathan, Improvement of the nutritional quality of sorghum and pearl millet, Food Nutr. Bull. 2 (1980) 11–16.
[22] A.P.P. Kayode, A.R. Linnemann, J.D. Hounhouigan, M.J.R. Nout, M. Van Boekel, Genetic and environmental impact on iron, zinc, and phytate in food sorghum grown in Benin, J. Agric. Food Chem. 54 (2006) 256–262.
[23] A. Ashok Kumar, B.V.S. Reddy, B. Ramaiah, S.P. Reddy, K.L. Sahrawat, H.D. Upadhyaya, Genetic variability and plant character association of grain Fe and Zn in selected core collection of sorghum germplasm and breeding lines, J. SAT Agric. Res. 7 (2009) 1–4.
[24] S.E.O. Mahgoub, S.A. Elhag, Effect of milling, soaking, malting, heat treatment and fermentation of phytate level of four Sudanese sorghum cultivars, Food Chem. 61 (1998) 77–80.
[25] J.R. Hunt, Bioavailability of iron, zinc, and other trace minerals from vegetarian diets, Am. J. Clin. Nutr. 78 (2003) 633S–639S.
[26] Y.G. Deosthale, V. Nagarajan, K.V. Rao, Some factors influencing the nutrient composition of sorghum grain, Indian J. Agric. Sci. 42 (1972) 100–108.
[27] K.G. Duodu, J.R.N. Taylor, P.S. Belton, B.R. Hamaker, Factors affecting sorghum protein digestibility, J. Cereal Sci. 38 (2003) 117–131.
[28] A. Shergo, N.G. Shargie, A. van Biljon, M.T. Labuschagne, Diversity in starch, protein and mineral composition of sorghum landrace accessions from Ethiopia, J. Crop Sci. Biotechnol. 15 (2012) 275–280.
[29] D. Ng'uni, M. Geleta, P. Hofvander, M. Fatih, T. Bryngelsson, Comparative genetic diversity and nutritional quality variation among some important Southern African sorghum accessions [Sorghum bicolor (L.) Moench], Aust. J. Crop. Sci. 6 (2012) 56–64.
[30] A.G. Flynn, J.P. Panozzo, W.K. Gardener, The effect of copper deficiency on the baking quality and dough properties of wheat flour, J. Cereal Sci. 6 (1987) 91–98.
[31] M. Bänziger, J. Long, The potential for increasing the iron and zinc density of maize through plant breeding, Food Nutr. Bull. 20 (2000) 397–400.
[32] A. Ashok Kumar, B.V.S. Reddy, B. Ramaiah, K.L. Sahrawat, W.H. Pfeiffer, Genetic variability and character association for grain iron and zinc contents in sorghum germplasm accessions and commercial cultivars, Eur. J. Plant Sci. Biotechnol. 6 (2012) 1–5.
[33] A. Badigannavar, G. Girish, T.R. Ganapathi, Genetic variation for seed phosphorous and yield traits in Indian sorghum landraces and varieties, Crop J. 3 (2015) 358–365.
[34] A.N.G. Shergo, L.T. Labuschagne, N.G. Shargie, A. van Biljon, Multivariate analysis of nutritional diversity in sorghum landrace accessions from western Europe, J. Biol. Sci. 13 (2013) 67–74.
杂志排行
The Crop Journal的其它文章
- Single nucleotide polymorphisms linked to quantitative trait loci for grain quality traits in wheat
- Characterization of QTL for unique agronomic traits of new-plant-type rice varieties using introgression lines of IR64
- A multivariate partial least squares approach to joint association analysis for multiple correlated traits
- Comparative QTL analysis of maize seed artificial aging between an immortalized F2population and its corresponding RILs
- Analysis of simple sequence repeats in rice bean (Vigna umbellata) using an SSR-enriched library
- Intra-population genetic variance for grain iron and zinc contents and agronomic traits in pearl millet
