Comparative QTL analysis of maize seed artificial aging between an immortalized F2population and its corresponding RILs
2016-04-05BinWngZhnhuiZhngZhiyunFuZonghuLiuYnminHuJihuTng
Bin Wng, Zhnhui Zhng, Zhiyun Fu, Zonghu Liu, Ynmin Hu, Jihu Tng,*
aState Key Laboratory of Wheat and Maize Crop Science / Collaborative Innovation Center of Henan Grain Crops, Henan Agricultural University, Zhengzhou 450002, ChinabCollege of Agronomy, Henan University of Science and Technology, Luoyang 471023, China
Comparative QTL analysis of maize seed artificial aging between an immortalized F2population and its corresponding RILs
Bin Wanga,b,1, Zhanhui Zhanga,1, Zhiyuan Fua, Zonghua Liua, Yanmin Hua, Jihua Tanga,*
aState Key Laboratory of Wheat and Maize Crop Science / Collaborative Innovation Center of Henan Grain Crops, Henan Agricultural University, Zhengzhou 450002, China
bCollege of Agronomy, Henan University of Science and Technology, Luoyang 471023, China
A R T I C L E I N F O
Article history:
Received 18 May 2015
Received in revised form 22 July 2015
Accepted 6 August 2015
Available online 15 August 2015
Keywords:
Maize (Zea mays L.)
Seed vigor
Artificial aging
QTL mapping
Seed storage
A B S T R A C T
Seed aging decreases the quality and vigor of crop seeds, thereby causing substantial agricultural and economic losses in crops. To identify genetic differences in seed aging between homozygotes and heterozygotes in maize, the seeds of a set of recombinant inbred lines (RILs) and an immortalized F2(IF2) population were subjected to artificial aging treatments for 0, 2, 3, and 4 days under 45ºC and 85% relative humidity and seed vigor was then evaluated in a field experiment. Seed vigor of all entries tested decreased sharply with longer aging treatment and seed vigor decreased more slowly in heterozygotes than in homozygotes. Forty-nine QTL were detected for four measured seed vigor traits in the RIL (28 QTL) and IF2(21 QTL) populations. Only one QTL, qGP5, was detected in both populations, indicating that the genes involved in anti-aging mechanisms differed between inbred lines and hybrids. Several QTL were identified to be responsible for multiple seed vigor traits simultaneouslyinthe RILand IF2populationsunderartificialagingconditions.These QTLmay include major genes for seed vigor or seed aging. QTL qVI4b and qGE3a detected in the RIL population coincided with genes ZmLOX1 and ZmPLD1 in the same respective chromosomal regions. These QTL would be useful for screening for anti-aging genes in maize breeding.
©2015 Crop Science Society of China and Institute of Crop Science, CAAS. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
* Corresponding author. Tel.: +86 371 63558377; fax: +86 371 63558122.
E-mail address: tangjihua1@163.com (J. Tang).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science, CAAS.
1Bin Wang and Zhanhui Zhang contributed equally to this work.
http://dx.doi.org/10.1016/j.cj.2015.07.004
2214-5141/©2015 Crop Science Society of China and Institute of Crop Science, CAAS. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Crop seeds, for example, maize (Zea mays L.) kernels, are consumed directly as human food and animal feed, providing more than 70% of caloric intake worldwide. Seeds are also a fundamental component of the plant life cycle because they store the genetic information necessary for the next generation [1]. In seed production, seed quality is defined as theability to maintain high vigor and stable content during storage. Seed with high vigor usually shows advantages in growth and production potential, positively affecting germination rate, resistance to environmental stresses, and crop yield [2,3]. Seeds gradually lose their ability to germinate during long periods of storage [4]. During storage, seed vigor depends mainly on the ability to withstand prolonged storage and the deleterious effects of aging [5]. The ability of seeds to withstand storage is influenced by many factors, including seed mass, oil content, carbohydrate composition, taxonomy, seed maturity, and climate factors [6–11]. Investigating the effects of seed aging on seed vigor will facilitate understanding the mechanisms underlying seed tolerance of prolonged storage.
With extended storage, seeds of most crops age and deteriorate. Aging and deterioration may lead to seedling abnormalities, delay field establishment, or even result in emergence failure [12]. Aging rate has been reported to be strongly influenced by seed moisture content, storage temperature, seed quality, and genetic factors [10]. At the molecular level, seed vigor and longevity are controlled mainly by protein damage and repair [13]. During the seed aging process, free radical-mediated lipid peroxidation, enzyme inactivation or protein degradation, disruption of cellular membranes, and damage to genetic material (nucleic acids) are the major factors determining loss of seed vigor [4,8,14–16]. In general, seeds are treated at high temperature and high relative humidity to accelerate seed aging for analysis of the seed aging process [12,17]. In maize, the floury parts of the seed endosperm become more corneous with aging under high temperature and high relative humidity. This physiological change is strongly associated with starch and protein changes [18]. Aged maize seeds also showed lower plasma membrane H+-ATPase activity, inhibiting germination and post-germination root growth [19].
In seed plants, a few studies have focused on the genetic mechanisms of seed aging or seed vigor [5, 11, 20–29]. In Arabidopsis, AtOGG1, PLDα1 (phospholipase D alpha), and DOG1 (delay of germination 1) have been identified as being involved in regulation of seed vigor and longevity [5,21,27,29]. Interestingly, the homolog of PLDα1 in soybean (Glycine max L.) also proved to be associated with seed longevity [28]. Quantitative trait loci (QTL) analyses seed aging and vigor have been performed in rice (Oryza sativa L.) [20,24] and wheat (Triticum aestivum L.) [23]. In maize, Li et al. reported that the mutant ZmLOX-1,2 (low lipoxygenase-1,2) showed decreased germination, suggesting that LOX-1, 2 was a factor influencing seed vigor [22]. Two proteomic analyses for maize seed germination showed that seed aging responses are regulated mainly by signal transduction, metabolism, energy, and stress-response proteins [25,26]. Some QTL have been identified to be responsible for seed vigor in maize. Presterl et al. [30] identified nine and 10 QTL for fresh seedling weight in a set of doubled haploid (DH) populations and their corresponding testcrosses, respectively, and Liu et al. [11] detected 16 seed vigor-related QTL for the seeds harvested at three developmental stages (32, 40, and 45 days after pollination). Although these genetic studies made considerable advances in dissecting the genetic mechanism of seed vigor, longevity, and aging responses, there is still much to be learned about the genetic mechanisms underlying these important traits in plants. Particularly in maize, the mechanism of seed aging is largely unknown. QTL analyses of maize seed aging will help identify the genes of the associated regulatory mechanisms.
Maize is one of the most important cereal crops in the world [31]. In 2012, the total world production of maize was 854 million tons on a total planting area of 154 million hectares [32]. The annual demand for hybrid seed for the maize production of the world is estimated to be 2.5 million tons [32]. Maize hybrids are widely used in production. Hybrid maize offers genetic mechanisms distinct from those of inbred lines for controlling various traits and metabolisms. For this reason, comparing the genetic basis of seed vigor or aging between homozygous and heterozygous materials will have important economic and agricultural significance. Previous genetic studies on maize seed vigor and aging have been developed using mainly RIL and DH populations, as well as inbred lines and hybrids [11,22,25,26,30]. However, few genetic analyses have been performed in heterozygous maize populations. In this study, we performed a comparative QTL analysis between a RILs and an IF2population to identify genetic effects of artificial seed aging on homozygous and heterozygous maize varieties.
2. Materials and methods
2.1. Development of the experimental population
Nongda 108 (Huang C×Xu 178) is an elite hybrid that was widely planted in China around 1999–2002. The parent Huang C has low seed vigor but high lysine content and stress resistance. Xu 178 is a high-nitrogen-efficiency inbred line with high seed vigor and low lysine content. A set of 166 RILs was constructed by single-seed descent from the hybrid. Using this RIL population, an IF2population was constructed using random RIL single-crossing. According to the procedure described by Hua et al. [33,34], 166 RILs were randomly divided into two groups, each consisting of 83 RILs. Then, pairwise crosses were made randomly between the lines of two groups without repeats, so that 83 different crosses were generated. The procedure was repeated three times, resulting in 249 (83×3) pairwise crosses between the two groups of RILs. Because insufficient seeds were harvested from six of these single crosses, 243 crosses (the IF2population) were used in this study.
2.2. Artificial aging treatment of seeds
The seeds of all plant materials, including RILs, the IF2population, two parents (Huang C and Xu 178), and the hybrid Nongda 108, were multiplied in winter 2010 in Hainan province. After harvest, the ears of the materials were fully dried under natural conditions, and uniform ears from each material were selected and threshed by hand. For each material, 1200 kernels were collected from the middle part of the ear and divided into four portions for artificial aging treatments with 300 kernels each. For ensuring uniform treatment conditions, the seed of each portion of the RILpopulation was placed in a mesh bag, which was then placed in an incubator (Thermoline Scientific, NSW, Australia. Plant growth cabinet 1100L) under a regime of 45±1ºC and 85% relative humidity [12,17]. Samples of seeds from the RIL population were artificially aged for 2, 3, or 4 days using the same incubator. The remaining untreated portions were used as the control. The seeds of the IF2population were artificially treated for 2, 3, and 4 days like those of the RIL population. For each IF2single cross, 1200 kernels were also divided into four portions of 300 kernels each for artificial aging treatments in the incubator.
2.3. Field evaluation and measurements of maize seed vigor parameters
The seed vigor of artificially aged seeds and the control was evaluated in a field trial. Before seed sowing, all the seeds were soaked in 0.1% (w/v) carbendazim (AccuStandard Inc.) solution for 12 h to prevent fungal infection. The treated and untreated seeds of the RILs, IF2population, the two parents, and the hybrid (Nongda 108) were planted in a randomized complete block design with three replications on the farm of Henan Agricultural University, Zhengzhou, Henan province. During the field experiment, from June 24 to July 15, 2011, the average temperature ranged from 22 to 33ºC. The soil of the experimental field, a silt loam, contained 13.8% of organic matter, 55.9 mg kg–1of available nitrogen, 47.2 mg kg–1of available phosphorus, and 139.5 mg kg–1of available potassium. Before sowing, the field was irrigated enough (the soil moisture reached 20±1%) to ensure seed germination. In each replication, 100 seeds of each entry were sown by hand uniformly in a single row 5 m long and 0.67 m wide. Four seed vigor parameters, including germination percentage (GP), germination energy (GE), germination index (GI), and vigor index (VI), were measured for each entry. The seedlings were counted and sampled 3, 5, and 7 days after the first seedlings emerged. At each sampling, 20 normal seedlings of each entry were taken, dried in an oven at 70ºC for 24 h, and weighed. GE was calculated as the number of seedlings on day 3 divided by the total number of seeds×100. GP was calculated as the number of seedlings on day 7 divided by the total number of seeds×100. GI was calculated as ∑Gt/Dt×100, where Dt is the germination time and Gt is the number of germinated seeds at that germination time. The VI wascalculatedas GI×S,where Sistheseedlingweightonday3 (ISTA 2012). Average values of the three replicates in each measured traitwere calculated and usedas raw data for further analyses. Phenotypic description was performed using IBM SPSS Statistics 19.0 software [35].
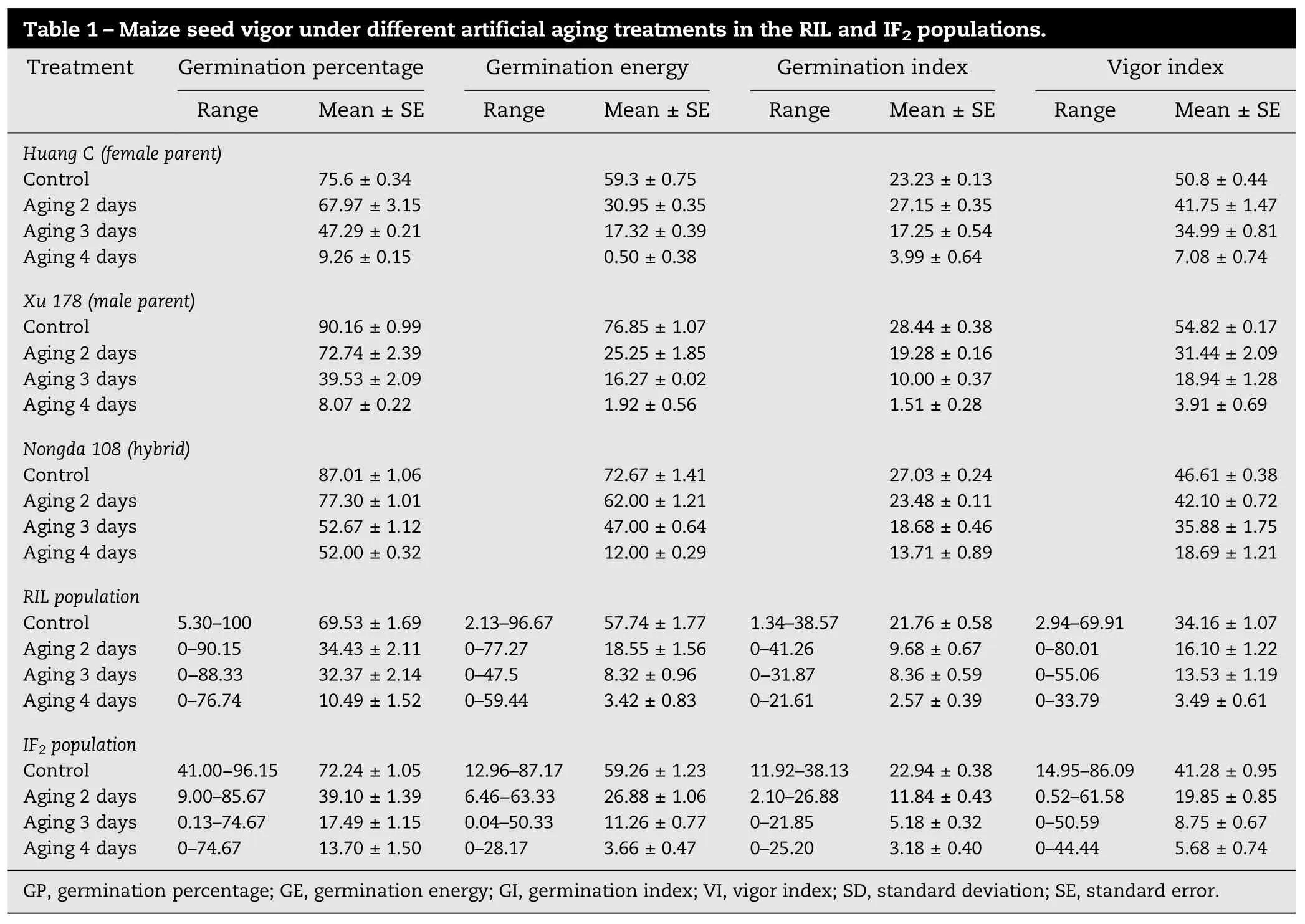
Table 1–Maize seed vigor under different artificial aging treatments in the RIL and IF2populations. Treatment Germination percentage Germination energy Germination index Vigor index Range Mean±SE Range Mean±SE Range Mean±SE Range Mean±SE Huang C (female parent) Control 75.6±0.34 59.3±0.75 23.23±0.13 50.8±0.44 Aging 2 days 67.97±3.15 30.95±0.35 27.15±0.35 41.75±1.47 Aging 3 days 47.29±0.21 17.32±0.39 17.25±0.54 34.99±0.81 Aging 4 days 9.26±0.15 0.50±0.38 3.99±0.64 7.08±0.74 Xu 178 (male parent) Control 90.16±0.99 76.85±1.07 28.44±0.38 54.82±0.17 Aging 2 days 72.74±2.39 25.25±1.85 19.28±0.16 31.44±2.09 Aging 3 days 39.53±2.09 16.27±0.02 10.00±0.37 18.94±1.28 Aging 4 days 8.07±0.22 1.92±0.56 1.51±0.28 3.91±0.69 Nongda 108 (hybrid) Control 87.01±1.06 72.67±1.41 27.03±0.24 46.61±0.38 Aging 2 days 77.30±1.01 62.00±1.21 23.48±0.11 42.10±0.72 Aging 3 days 52.67±1.12 47.00±0.64 18.68±0.46 35.88±1.75 Aging 4 days 52.00±0.32 12.00±0.29 13.71±0.89 18.69±1.21 RIL population Control 5.30–100 69.53±1.69 2.13–96.67 57.74±1.77 1.34–38.57 21.76±0.58 2.94–69.91 34.16±1.07 Aging 2 days 0–90.15 34.43±2.11 0–77.27 18.55±1.56 0–41.26 9.68±0.67 0–80.01 16.10±1.22 Aging 3 days 0−88.33 32.37±2.14 0–47.5 8.32±0.96 0−31.87 8.36±0.59 0–55.06 13.53±1.19 Aging 4 days 0–76.74 10.49±1.52 0–59.44 3.42±0.83 0–21.61 2.57±0.39 0–33.79 3.49±0.61 IF2population Control 41.00−96.15 72.24±1.05 12.96–87.17 59.26±1.23 11.92–38.13 22.94±0.38 14.95–86.09 41.28±0.95 Aging 2 days 9.00–85.67 39.10±1.39 6.46−63.33 26.88±1.06 2.10–26.88 11.84±0.43 0.52–61.58 19.85±0.85 Aging 3 days 0.13–74.67 17.49±1.15 0.04–50.33 11.26±0.77 0–21.85 5.18±0.32 0–50.59 8.75±0.67 Aging 4 days 0–74.67 13.70±1.50 0–28.17 3.66±0.47 0–25.20 3.18±0.40 0–44.44 5.68±0.74 GP, germination percentage; GE, germination energy; GI, germination index; VI, vigor index; SD, standard deviation; SE, standard error.
2.4. QTL mapping
冷榨和热榨的汉麻籽油在5种不同的储存环境中,温度和光照均影响油脂的颜色、透明度和气味;温度变化越大,光照越强,油脂颜色褪色明显,透明度降低,易产生刺激性气味,稳定性越低。GC-MS方法分析2种不同榨油方式的汉麻籽油脂肪酸成分相同,含量有所差别。油脂在UVC (200 ~ 275 nm)和UVB(270 ~ 320 nm)处有很强的吸收峰,可以较好的吸收紫外线,适合作为防晒霜原料;储存汉麻籽油时,要尽可能避免让油脂与空气接触,应该采用低温避光或者采取真空、惰性气体保存油脂,防止油脂被氧化。
A genetic linkage map for the RIL population was constructed using 217 SSR markers with Mapmaker 3.0 software [36]. The map included 10 chromosomes spanning a total of 2438.2 cM, with an average interval of 11.2 cM [37]. The genotypes of eachIF2cross were deduced from the marker genotypes of their RIL parents, and QTL mapping in the IF2population was performed using the molecular linkage map of the RIL population [33,38].
QTL mapping for the two populations was performed using the composite interval mapping method and Model 6 of the Zmapqtl module of QTL Cartographer 2.5 [36]. The logarithm of odds (LOD) threshold was calculated using 1000 permutations at P = 0.05. A scanning interval of 2 cM between markers and putative QTL with a window size of 10 cM window was used to detect QTL. The background control of marker cofactors was set by forward–backward stepwise regression with five controlling markers. QTL effects in the IF2population were estimated according to criteria suggested by Stuber et al. (1987): d/a = dominance effects/additive effects; A, additive (d/a = 0–0.20); PD, partial dominance (d/a = 0.21–0.80); D, dominance (d/a = 0.81–1.20); and OD, overdominance (d/a>1.20) [39].
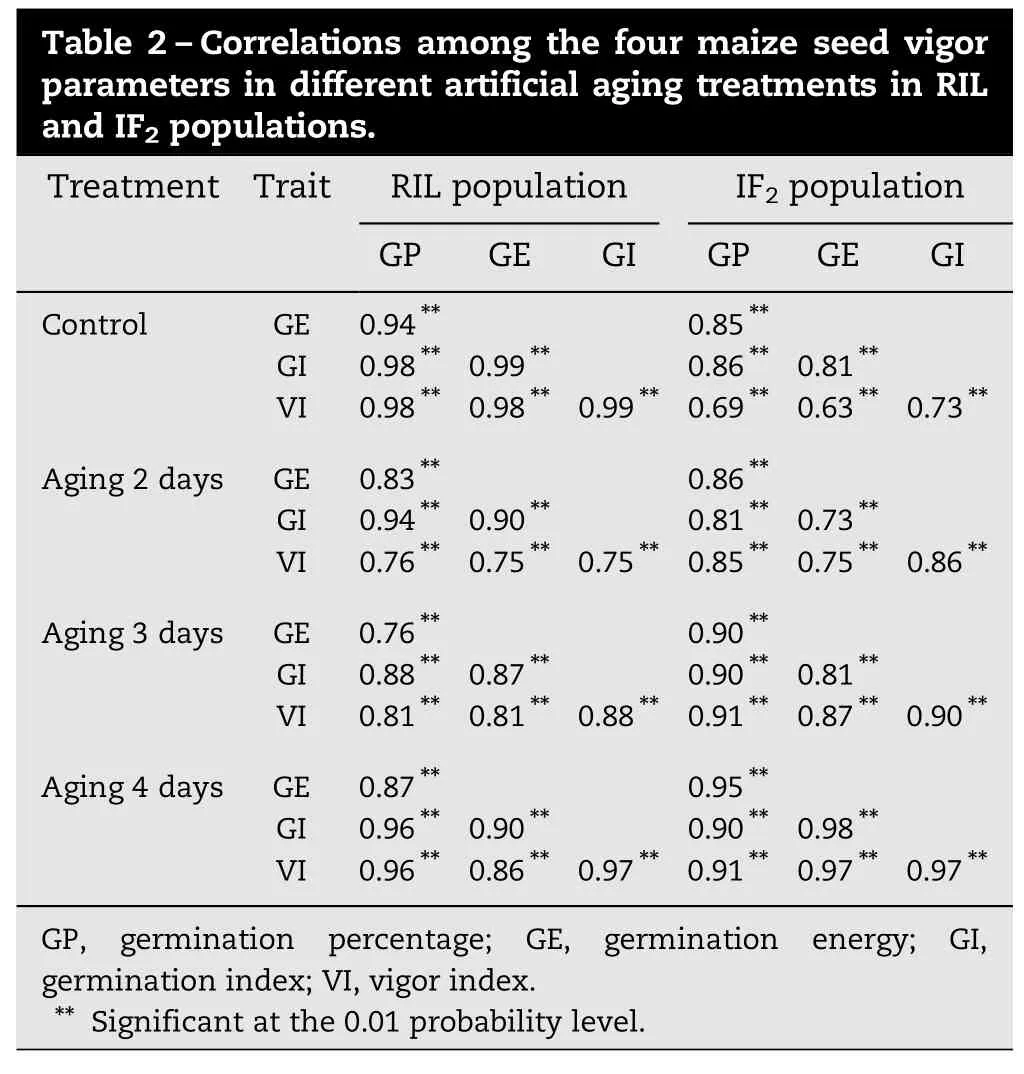
Table 2–Correlations among the four maize seed vigor parameters in different artificial aging treatments in RIL and IF2populations. Treatment Trait RIL population IF2population GP GE GI GP GE GI Control GE 0.94** 0.85** GI 0.98** 0.99** 0.86** 0.81** VI 0.98** 0.98** 0.99** 0.69** 0.63** 0.73** Aging 2 days GE 0.83** 0.86** GI 0.94** 0.90** 0.81** 0.73** VI 0.76** 0.75** 0.75** 0.85** 0.75** 0.86** Aging 3 days GE 0.76** 0.90** GI 0.88** 0.87** 0.90** 0.81** VI 0.81** 0.81** 0.88** 0.91** 0.87** 0.90** Aging 4 days GE 0.87** 0.95** GI 0.96** 0.90** 0.90** 0.98** VI 0.96** 0.86** 0.97** 0.91** 0.97** 0.97** GP, germination percentage; GE, germination energy; GI, germination index; VI, vigor index. ** Significant at the 0.01 probability level.
3. Results
3.1. Variations of seed vigor in the RIL and IF2population
In the control (no aging treatment) (Table 1), the four measured parameters of seed vigor were generally higher in both parents and the hybrid than in the RIL and the IF2populations. Under artificial aging treatment, the seed vigor of all materials decreased significantly, and the parent Xu 178 showed the most drastic decrease among the RIL, the IF2population, both parents, and the hybrid. In contrast, the seed vigor in the hybrid Nongda 108 and the IF2population showed less decrease than did the inbred lines (two parents and the RILs). Four days after treatment, the hybrid and the IF2population had higher seed vigor than the inbred lines but showed different trends of decrease. The seed vigor decrease in the IF2population and in the hybrid was continuous. In the RILs, however, the decrease showed a marked slowdown during aging for 2 to 3 days. The results confirmed that the anti-aging mechanisms involved in hybrids and inbred lines were different. The four parameters were positively correlated with one another during the artificial aging treatments in both the RIL and IF2populations (P<0.05) (Table 2). The four treatments were positively correlated with one another for each seed vigor parameter in both populations (P<0.05), with the exception of the control and 3-day aging treatments in the IF2population (Table 3).
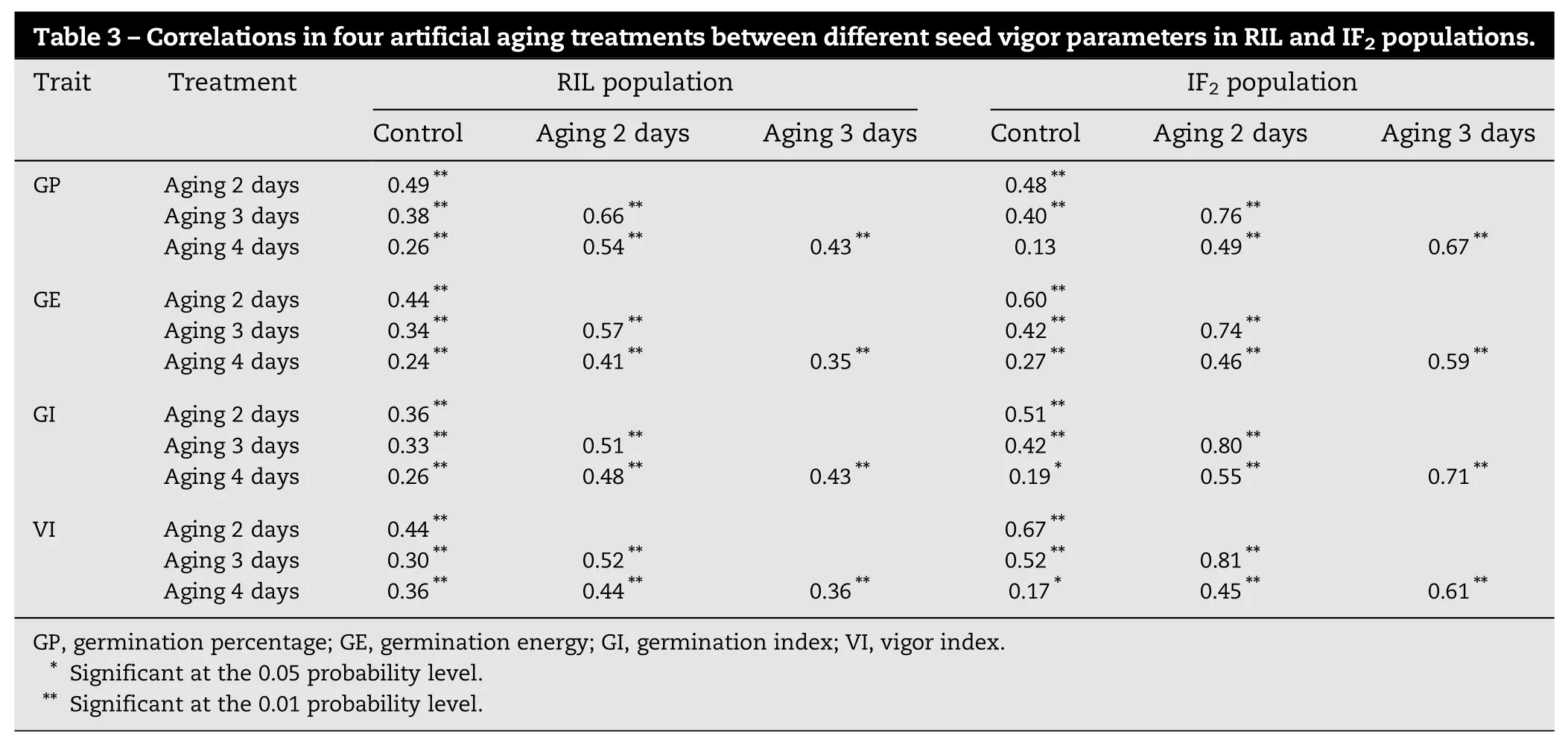
Table 3–Correlations in four artificial aging treatments between different seed vigor parameters in RIL and IF2populations. Trait Treatment RIL population IF2population Control Aging 2 days Aging 3 days Control Aging 2 days Aging 3 days GP Aging 2 days 0.49** 0.48** Aging 3 days 0.38** 0.66** 0.40** 0.76** Aging 4 days 0.26** 0.54** 0.43** 0.13 0.49** 0.67** GE Aging 2 days 0.44** 0.60** Aging 3 days 0.34** 0.57** 0.42** 0.74** Aging 4 days 0.24** 0.41** 0.35** 0.27** 0.46** 0.59** GI Aging 2 days 0.36** 0.51** Aging 3 days 0.33** 0.51** 0.42** 0.80** Aging 4 days 0.26** 0.48** 0.43** 0.19* 0.55** 0.71** VI Aging 2 days 0.44** 0.67** Aging 3 days 0.30** 0.52** 0.52** 0.81** Aging 4 days 0.36** 0.44** 0.36** 0.17* 0.45** 0.61** GP, germination percentage; GE, germination energy; GI, germination index; VI, vigor index. * Significant at the 0.05 probability level. ** Significant at the 0.01 probability level.
3.2. QTL detection for seed vigor in RIL population under aging treatments
In the RIL population, a total of 28 QTL were identified for the four seed vigor parameters under different artificial agingtreatments across the whole genome with the exception of chromosomes 8 and 9 (Table 4; Fig. 1). In the control, eight QTL were identified for the four seed vigor traits with the additive effects originating in the parent Xu 178. These QTL explained 8.12–15.70% of the total phenotypic variance. Three QTL, qGP1a, qGE1a, and qVI1a, were detected in marker interval umc1044−phi339017. qGE5a and qGI5a were both detected in the same marker interval, bnlg1287−umc1221. qGP2a and qGI2a were associated with GP and GI, explaining 10.96% and 9.73% of trait phenotypic variance, respectively. QTL qVI4a contributed 11.53% of total phenotypic variance for VI.
When the RILs seeds were artificially aged for 2 days, eight QTL were identified for the four seed aging parameters. These QTL were responsible for 8.37–18.04% of the trait phenotypic variance. However, no QTLs were identified in the same marker interval for different seed vigor traits. For VI, qVI3, qVI4a, and qVI4b were detected with a total contribution of 46.72% of phenotypic variance. Two GE-associated QTL were derived from the parent Huang C with a contribution of 18.07% of total phenotypic variance. For GI, two QTL were identified with the additive effects from the parent Xu 178, contributing to 10.38% and 15.37% of the total phenotypic variance, respectively. Only one GP-associated QTL, qGP2b, was detected with a contribution of 12.43% of the total phenotypic variance.
After artificial aging treatment for 3 days, six QTL were identified that explained 10.09–15.87% of the trait phenotypic variance. Only one QTL was responsible for GP, with a contribution of 15.87% of total phenotypic variance. For GE, two QTL were identified with a cumulative contribution of 25.52% of phenotypic variance. Two GI-associated QTL, qGI1 and qGI10a, were identified, explaining, respectively, 10.28% and 10.09% of total phenotypic variance. qVI6a was identified for VI, explaining 12.95% of phenotypic variance.
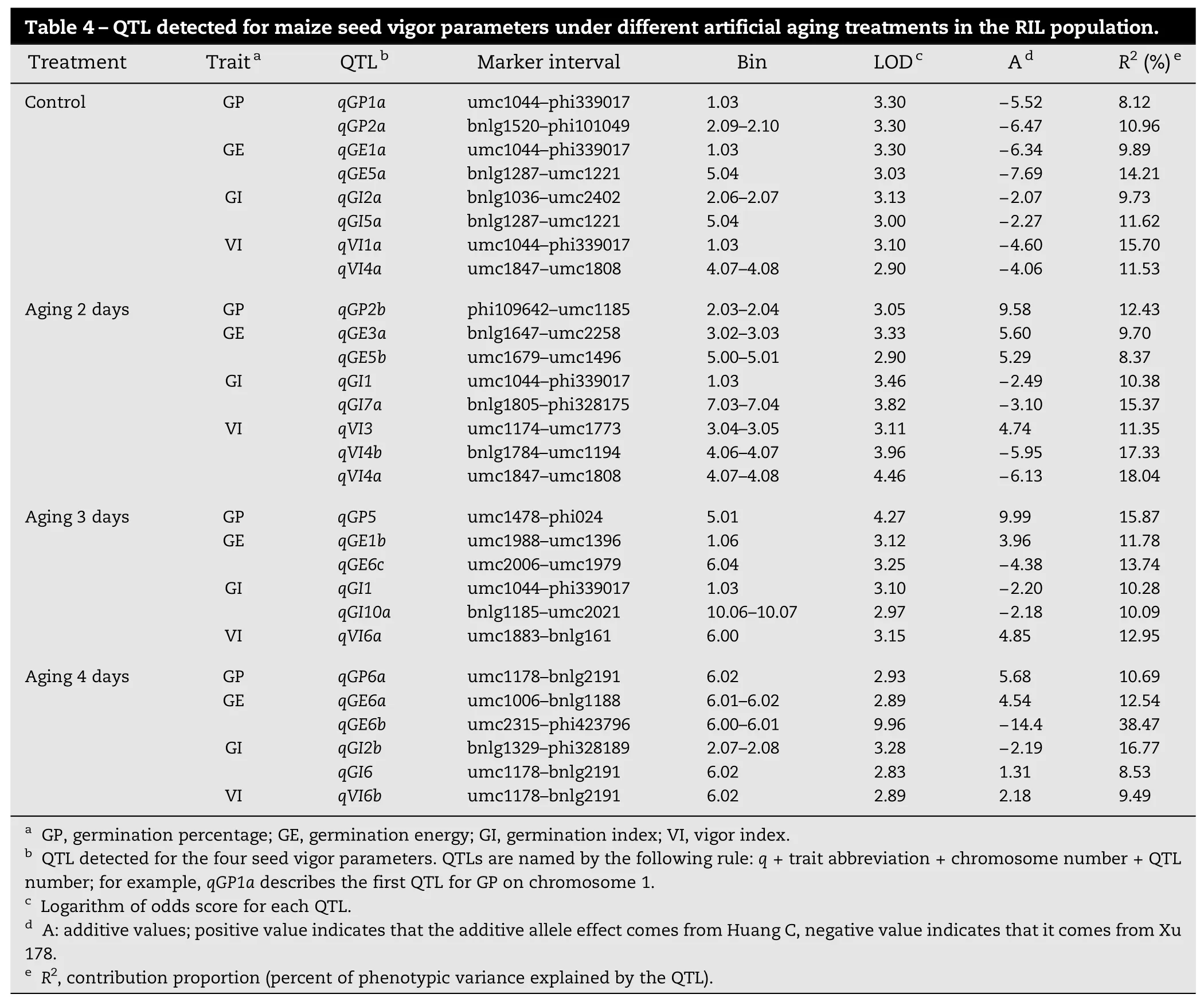
Table 4–QTL detected for maize seed vigor parameters under different artificial aging treatments in the RIL population. Treatment Traita QTLb Marker interval Bin LODc Ad R2(%)eControl GP qGP1a umc1044–phi339017 1.03 3.30 −5.52 8.12 qGP2a bnlg1520–phi101049 2.09–2.10 3.30 −6.47 10.96 GE qGE1a umc1044–phi339017 1.03 3.30 −6.34 9.89 qGE5a bnlg1287–umc1221 5.04 3.03 −7.69 14.21 GI qGI2a bnlg1036–umc2402 2.06–2.07 3.13 −2.07 9.73 qGI5a bnlg1287–umc1221 5.04 3.00 −2.27 11.62 VI qVI1a umc1044–phi339017 1.03 3.10 −4.60 15.70 qVI4a umc1847–umc1808 4.07–4.08 2.90 −4.06 11.53 Aging 2 days GP qGP2b phi109642–umc1185 2.03–2.04 3.05 9.58 12.43 GE qGE3a bnlg1647–umc2258 3.02–3.03 3.33 5.60 9.70 qGE5b umc1679–umc1496 5.00–5.01 2.90 5.29 8.37 GI qGI1 umc1044–phi339017 1.03 3.46 −2.49 10.38 qGI7a bnlg1805–phi328175 7.03–7.04 3.82 −3.10 15.37 VI qVI3 umc1174–umc1773 3.04–3.05 3.11 4.74 11.35 qVI4b bnlg1784–umc1194 4.06–4.07 3.96 −5.95 17.33 qVI4a umc1847–umc1808 4.07–4.08 4.46 −6.13 18.04 Aging 3 days GP qGP5 umc1478–phi024 5.01 4.27 9.99 15.87 GE qGE1b umc1988–umc1396 1.06 3.12 3.96 11.78 qGE6c umc2006–umc1979 6.04 3.25 −4.38 13.74 GI qGI1 umc1044–phi339017 1.03 3.10 −2.20 10.28 qGI10a bnlg1185–umc2021 10.06–10.07 2.97 −2.18 10.09 VI qVI6a umc1883–bnlg161 6.00 3.15 4.85 12.95 Aging 4 days GP qGP6a umc1178–bnlg2191 6.02 2.93 5.68 10.69 GE qGE6a umc1006–bnlg1188 6.01–6.02 2.89 4.54 12.54 qGE6b umc2315–phi423796 6.00–6.01 9.96 −14.4 38.47 GI qGI2b bnlg1329–phi328189 2.07–2.08 3.28 −2.19 16.77 qGI6 umc1178–bnlg2191 6.02 2.83 1.31 8.53 VI qVI6b umc1178–bnlg2191 6.02 2.89 2.18 9.49aGP, germination percentage; GE, germination energy; GI, germination index; VI, vigor index.bQTL detected for the four seed vigor parameters. QTLs are named by the following rule: q + trait abbreviation + chromosome number + QTL number; for example, qGP1a describes the first QTL for GP on chromosome 1.cLogarithm of odds score for each QTL.dA: additive values; positive value indicates that the additive allele effect comes from Huang C, negative value indicates that it comes from Xu 178.eR2, contribution proportion (percent of phenotypic variance explained by the QTL).
After artificial aging for 4 days, six QTL were identified for the four measured traits of seed vigor with contributions ranging from 8.53% to 38.47% of total phenotypic variance. Of these, three QTL were detected in marker interval umc1178−bnlg2191 for GP, GI, and VI, with contributions of 10.69%, 8.53%, and 9.49%, respectively. For GE, we identified two QTL on chromosome 6 that explained 51.01% of GE phenotypic variance; in particular, qGE6b explained 38.47% of totalphenotypic variance. Another GI-associated QTL, qGI2b, was identified with a contribution of 16.77% of total phenotypic variance.
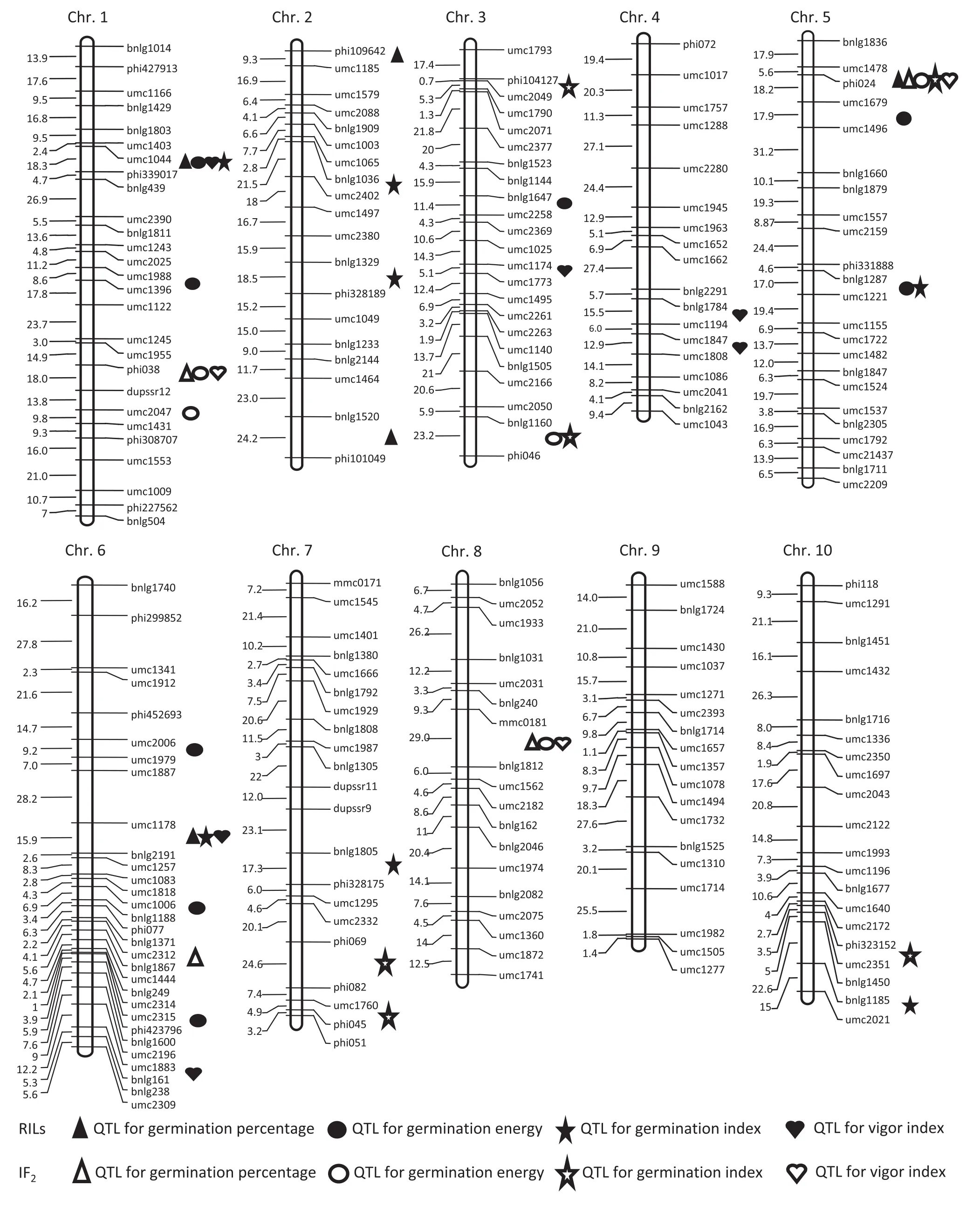
Fig. 1–Mapping QTL detectedfor maizeseed vigorparametersin the RIL and IF2populations ona genetic linkage map containing 217 SSRs.
In the control, the QTL detected for the four measured traits were located mainly on chromosomes 1, 2, and 5. The QTL were distributed mainly on chromosome 6 after artificial aging for 3 and 4 days. The QTL detected in the control for GP, GE, and VI were located in the marker interval umc1044−phi339017, as were those for GI after 2 days of artificial aging treatment. qVI4a was detected in the control and after 2 days of artificial aging.
3.3. QTL identification for seed vigor in the IF2population under different aging treatments
We performed QTL mapping for the four seed vigor traits in the IF2population, 21 QTL were identified on seven chromosomes (Table 5; Fig. 1). In the control, five QTL were detected for the four seed vigor parameters, with contributions ranging from 5.54% to 18.65% of total phenotypic variance. Four of these were detected in markers interval umc1478−phi024 on chromosome 5 with additive effects from the parent Xu 178, explaining 11.44%, 5.54%, 11.55%, and 6.68% of the total phenotypic variance, respectively. Another QTL, qGE1c, explained 18.65% of the phenotypic variance.
After artificial aging treatment for 2 days, five QTL for the four measured traits were identified. Of these, Xu 178 QTL responsible for GP, GE, and VI were detected in marker interval phi038−dupssr12, explaining 7.29%, 7.25%, and 8.64% of the corresponding trait total phenotypic variance, respectively. Another GP-associated QTL was located on chromosome 6 with a contribution of 9.42% of phenotypic variance. For GI, only qGI3a was identified, explaining 16.19% of phenotypic variance.
Five QTL were detected after 3 days of artificial aging treatment, and six QTL were identified after artificial aging treatment for 4 days (a total of 11 QTL). Among them, eight QTL were derived from the parent Xu 178, and all explained relatively small proportions of phenotypic variance. QTL derived from the parent Xu 178 in marker interval mmc0181−bnlg1812 were detected for GP, GE, and VI after 3 and 4 days of artificial aging. After aging treatment for 3 days, two QTL were detected for GI on chromosomes 7 and 10, explaining 30.06% of total phenotypic variance. qGE3b and qGI3b were identified for GE and GI after aging treatment for 4 days, contributing 6.41% and 17.19% of the variance in the respective traits. qGI7c was identified on chromosome 7 and explained 5.04% of total phenotypic variance.

Table 5–QTL detected for maize seed vigor parameters under different artificial aging treatments in the IF2population. Treatment Traita QTLb Marker interval Bin LODc Ad Dd Effecte R2(%)fControl GP qGP5 umc1478–phi024 5.01 3.73 −4.55 9.73 OD 11.44 GE qGE1c umc2047–umc1431 1.09 4.08 9.78 −8.37 D 18.65 qGE5c umc1478–phi024 5.01 3.84 −5.09 8.64 OD 5.54 GI qGI5b umc1478–phi024 5.01 3.80 −1.41 3.35 OD 11.55 VI qVI5 umc1478–phi024 5.01 3.60 −4.32 8.52 OD 6.68 Aging 2 days GP qGP1b phi038–dupssr12 1.08 4.26 −1.25 −11.66 OD 7.29 qGP6b umc2312–bnlg1867 6.01 3.90 9.99 −15.37 OD 9.42 GE qGE1d phi038–dupssr12 1.08 3.91 −0.54 −5.02 OD 7.25 GI qGI3a phi104127–umc2049 3.01 4.40 −1.64 −1.96 D 16.19 VI qVI1b phi038–dupssr12 1.08 4.54 −0.51 −6.13 OD 8.64 Aging 3 days GP qGP8 mmc0181–bnlg1812 8.05–8.06 5.89 −6.96 −13.95 OD 5.28 GE qGE8 mmc0181–bnlg1812 8.05–8.06 7.72 −3.87 −7.51 OD 5.02 GI qGI7b umc1760–phi045 7.05 5.07 −3.46 0.37 A 12.64 qGI10b phi323152–umc2351 10.05–10.07 4.60 3.62 −2.12 PD 17.42 VI qVI8 mmc0181–bnlg1812 8.05–8.06 5.14 −2.10 −6.40 OD 9.01 Aging 4 days GP qGP8 mmc0181–bnlg1812 8.05–8.06 6.11 −2.80 −10.07 OD 5.12 GE qGE8 mmc0181–bnlg1812 8.05–8.06 8.68 −2.12 −3.79 OD 6.03 qGE3b bnlg1160–phi046 3.06–3.08 7.92 1.26 −1.63 OD 6.41 GI qGI3b bnlg1160–phi046 3.06–3.08 5.92 3.13 −0.47 A 17.19 qGI7c phi069–phi082 7.05 5.98 −1.66 1.51 D 5.04 VI qVI8 mmc0181–bnlg1812 8.05−8.06 4.95 −1.18 −5.66 OD 9.49aAs in Table 3.bAs in Table 3.cAs in Table 3.dA: additive values; positive value indicates that additive effect comes from Huang C, negative value indicate that additive effect comes from Xu 178; D: dominance values.eEffect of each QTL; A, additive; PD, partial dominance; D, dominance; OD, overdominance.fR2, contribution proportion (percent of phenotypic variance explained by that QTL).
The QTL detected in the control and after 2 days of artificial aging treatment were distributed mainly on chromosomes 5 and 1. During artificial aging treatment, most QTL identified were distributed on chromosomes 3, 7, and 8. Of the 21 QTL identified, strikingly, 18 QTL showed OD or D in thefour treatments, and only three QTL detected in artificial aging treatments for 3 or 4 days exhibited A or PD effects.
4. Discussion
4.1. Differences in seed aging between the IF2population and RILs
In maize, seed vigor and aging resistance are important for high yield and seed storage. In the present study, maize seeds of RIL and IF2populations were aged for 2, 3, and 4 days at 45°C and 85% relative humidity. This method for accelerating seed aging under high temperature and relative humidity has been generally used for investigating seed longevity and aging response in several species [4,26,40]. However, most of these studies were conducted using inbred lines or inbred populations and few in heterozygous populations. In the present study, an IF2population was adopted to elucidate the genetic basis of seed aging response in maize. During the artificial aging process, the seed viability of the inbred lines (the two parents of Nongda 108 and the RIL population) and heterozygous materials (Nongda 108 and IF2population) showed significant decreases. However, different trends of seed viability decrease were observed between the inbred lines and heterozygous materials. In the RIL population, the seed vigor decrease showed a marked slowdown in aging for 2 days and aging for 3 days treatments. In contrast, seed vigor showed a sharp decrease at the corresponding aging stage in the IF2population. After artificial aging treatment for 4 days, the hybrid and IF2crosses showed higher viability than inbred lines and RILs, showing that the heterozygous materials had higher aging resistance. Of the 49 QTL detected, only qGP5 was simultaneously detected in chromosome bin 5.00–5.01 in both the RIL and IF2populations. These results show that seed response to aging is complex and that different genetic mechanisms regulate seed aging in inbred lines and heterozygous materials.
Two main hypotheses, dominance [41,42] and overdominance [43], have been proposed to explain the genetic basis of heterosis. Strong trait heterosis was associated with more QTLorbinsshowing OD/D and fewer QTLor bins showing PD/A [44,45]. In the present study, our phenotypic analysis showed that heterozygous materials had more stable seed vigor than did inbred lines throughout an artificial aging process (Table 1). The four seed vigor parameters of IF2population showed heterosis in aging for 4 days, but not in aging for 3 days. Most of the QTL identified in the IF2population exhibited D or OD gene action, high percentage of D/OD gene action seems to be important for heterosis in artificial aging. Two QTL showed A or PD effects on aging for 3 days, possibly the reason why the IF2showed no heterosis for this treatment.
4.2. Major QTL responsible for seed aging between the two populations
In the present study, a total of 49 QTL (28 in the RILs and 21 in the IF2population) for four measured seed vigor traits were identified by QTL mapping under different artificial aging treatments. In the IF2population, qGP5, qGE5c, qGI5b, and qVI5 were detected in marker interval umc1478−phi024 on chromosome 5 in the control, whereas qGP8, qGE8, and qVI8 were detected in marker interval mmc0181−bnlg1812 after artificial aging treatment for 3 and 4 days. qGI10b was identified for GP, GE, and GI in the same chromosomal region by Liu et al. [11] in a RIL population. These common QTL may correspond to major genes associated with seed germination or seed aging. In contrast, in the RIL population, several QTL were detected in marker interval umc1044−phi339017 on chromosome 1 and bnlg1287−umc1221 on chromosome 5 for multiple seed vigor traits in the control. This finding suggests that there are important genes for seed vigor with additive effects in these two markerintervals.Duringtheagingtreatments,qGI1wasdetected after 2 and 3 days of aging treatment in the umc1044−phi339017 markerintervalonchromosome1,andthree QTLwereidentified in the umc1178−bnlg2191 marker interval for GP, GI, and VI after 4 days of artificial aging treatment. The two chromosomal regions may contain important genes responsible for seed aging metabolism. qGE6b was located in the marker interval umc2315−phi423796andexplained38.47%ofthephenotypicvariancein GE in the RIL population. In the study of Liu et al. [11], qGE3a was located in the same chromosomal region as the QTL responsible for GE, GI, and VI on chromosome 3. In these chromosomal regions of importance for seed aging and vigor in the B73 genome, there are numerous genes associated with energy metabolism, stress response, signal transduction, and protein degradation pathway. These metabolic activities are involved in seed germination and aging. qVI4b and qGE3a detected in the RIL population were co-located with genes ZmLOX1 and ZmPLD1 in the same chromosomal regions, respectively [21,22,28]. In seed production, these identified major QTL can be used to map and clone seed anti-aging genes, which can be used in breeding or genetic engineering strategies to extend the maize seed storage period. The identification of anti-aging genes will allow maize breeding programs to use marker-assisted selection to breed varieties with better storage properties.
4.3. Approaches to seed vigor parameter evaluation in maize
In maize, several QTL analyses have been performed during seed germination and early seedling stages under field conditions [11,46,47] or in growth chambers [48–50]. These analyses have been performed in RIL or F2:3populations. For accurately evaluating agronomic traits, crop field experiments for QTL analysis are usually conducted in multiple environments or planted in growth chambers to minimize environmental effects. It is undoubted that multiple-environment experiments are beneficial for accurately evaluating trait phenotypes. In the present study, however, the field seed germination experiment was performed in a single environment in three replicates for estimating maize seed vigor. Of the detected QTL in this RIL population, several QTL were consistent with those identified in several previous studies performed in growth chambers. The QTL located in chromosome bins 1.02–1.03 and 1.06 in the present study were consistent with the QTL for shoot dry weight reported by Trachsel et al. (2010) [50]. Numerous GP- and GI-related QTL identified in chromosome bin 4.08, 5.04–5.05, and 6.02 byHund et al. (2004) [49] were consistent with qVI4a, qGE5a, qGI5a, qGP6a, qGE6a, qGI6, and qVI6b in the present study. The high QTL consistency in RIL showed that seed vigor can be estimated accurately under controlled climate conditions. In this study, suitable environment conditions and three replicates ensured the accurate evaluation of seed vigor characters. Seed vigor parameters in the field can be evaluated in a short period of 20 days, during which time the effects of environmental factors are limited. Thus, such crop traits as seed vigor parameters can be evaluated accurately in a single environment. However, a single-environment trial cannot estimate the heritability of the four seed vigor parameters, a reality that will limit the application of the identified QTL via molecular marker-assisted selection.
4.4. Applications of seed aging-related QTL for marker-assisted selection in maize
Maize hybrids have spread around the world, and maize seed production has become an industrial system. As the fundamental factor in maize production, maize seed with high seed vigor will readily lead to high grain yield. Maize seeds also comprise maize grain yield. Thus, improvement of seed anti-aging ability will not only bring large economic benefits to the maize seed industrial production system but also promote the improvement of grain quality. In the present study, we have conducted a comparative QTL analysis between an inbred population and a heterozygous population for detecting the genetic basis of seed vigor regulation and aging effects. We identified major QTL responsible for seed vigor and seed aging in the two populations. The major QTL identified in the RIL population could be used for screening for elite inbred lines with high seed vigor and high seed anti-aging ability via marker-assisted selection. In contrast, the major QTL detected in the IF2population could be used in a breeding program to screen promising hybrids.
Acknowledgments
This study was supported by the National Key Technology R&D Program of China (2011BAD35B00) and the National Nature Science Foundation of China (31271732). The authors thank Dr. Sachin Teotia for critical review of the manuscript.
R E F E R E N C E S
[1] E. Nambara, H. Nonogaki, Seed biology in the 21st century: perspectivesandnewdirections,Plant Cell Physiol.53(2012)1–4.
[2] M.L. Gupta, D.L. George, I.G.M.A. Parwata, Effect of harvest time and drying on supersweet sweet corn seed quality, Seed Sci. Technol. 33 (2005) 167–176.
[3] A.B. Rodo, F.J. Marcos, Onion seed vigor in relation to plant growth and yield, Hortic. Bras. 31 (2003) 220-206.
[4] M.B. McDonald, Seed deterioration: physiology, repair and assessment, Seed Sci. Technol. 27 (1999) 177–237.
[5] L. Ventura, M. Dona, A. Macovei, D. Carbonera, A. Buttafava, A. Mondoni, G. Rossi, A. Balestrazzi, Understanding the molecular pathways associated with seed vigor, Plant Physiol. Biochem. 60 (2012) 196–206.
[6] R.H. Ellis, E.H. Roberts, Improved equations for the prediction of seed longevity, Ann. Bot. 45 (1980) 13–30.
[7] M. Horbowicz, R.L. Obendorf, Seed desiccation tolerance and storability: Dependence on flatulence-producing oligosaccharides and cyclitols-review and survey, Seed Sci. Res. 4 (1994) 385–400.
[8] D.A. Priestley, Seed Ageing, Cornell University Press, Ithaca, New York, 1986.
[9] H.W. Pritchard, J.B. Dickie, Predicting seed longevity: the use and abuse of seed viability equations, in: R.D. Smith, J.B. Dickie, S.H. Linington, H.W. Pritchard, R.J. Probert (Eds.), Seed conservation: turning science into practice, Royal Botanic Gardens, Kew, London 2003, pp. 655–721.
[10] C. Walters, L.M. Wheeler, J.M. Grotenhuis, Longevity of seeds stored in a genebank: species characteristics, Seed Sci. Res. 15 (2005) 1–21.
[11] J. Liu, Z. Fu, H. Xie, Y. Hu, Z. Liu, L. Duan, S. Xu, J. Tang, Identificationof QTLsformaizeseedvigoratthreestagesofseed maturity using a RIL population, Euphytica 178 (2010) 127–135.
[12] S.P. Groot, A.A. Surki, R.C. de Vos, J. Kodde, Seed storage at elevated partial pressure of oxygen, a fast method for analysing seed ageing under dry conditions, Ann. Bot. 110 (2012) 1149–1159.
[13] L. Oge, C. Broyart, B. Collet, B. Godin, D. Jallet, G. Bourdais, D. Job, P. Grappin, Protein damage and repair controlling seed vigor and longevity, Methods Mol. Biol. 773 (2011) 369–384.
[14] A. Banerjee, M.M. Choudhuri, B. Ghosh, Changes in nucleotide content and histone phosphorylation of aging rice seeds, Z. Pflanzenphysiol. 102 (1981) 33–36.
[15] C. Walters, Understanding the mechanisms and kinetics of seed ageing, Seed Sci. Res. 8 (1998) 223–244.
[16] U.M. Murthy, P.P. Kumar, W. Sun, Mechanisms of seed ageing under different storage conditions for Vigna radiata (L.) Wilczek: lipid peroxidation, sugar hydrolysis, Maillard reactions and their relationship to glass state transition, J. Exp. Bot. 54 (2003) 1057–1067.
[17] ISTA, International rules for seed testing 2012, The International Seed testing Association (ISTA), Bassersdorf, Switzerland, 2012.
[18] C.M. McDonough, C.D. Floyd, R.D. Waniska, L.W. Rooney, Effect of accelerated aging on maize, sorghum, and sorghum meal, J. Cereal Sci. 39 (2004) 351–361.
[19] H. Sveinsdottir, F. Yan, Y. Zhu, T. Peiter-Volk, S. Schubert, Seed ageing-induced inhibition of germination and post-germination root growth is related to lower activity of plasma membrane H+-ATPase in maize roots, J. Plant Physiol. 166 (2009) 128–135.
[20] K. Miura, Y. Lin, M. Yano, T. Nagamine, Mapping quantitative trait loci controlling seed longevity in rice (Oryza sativa L.), Theor. Appl. Genet. 104 (2002) 981–986.
[21] S.P. Devaiah, X. Pan, Y. Hong, M. Roth, R. Welti, X. Wang, Enhancing seed quality and viability by suppressing phospholipase D in Arabidopsis, Plant J. 50 (2007) 950–957.
[22] J. Li, Y. Zhang, Z. Yu, Y. Wang, Y. Yang, Z. Liu, J. Jiang, M. Song, Y. Wu, Superior storage stability in low lipoxygenase maize varieties, J. Stored Prod. Res. 43 (2007) 530–534.
[23] S. Landjeva, U. Lohwasser, A. Börner, Genetic mapping within the wheat D genome reveals QTL for germination, seed vigour and longevity, and early seedling growth, Euphytica 171 (2009) 129–143.
[24] Z. Wang, J. Wang, Y. Bao, F. Wang, H. Zhang, Quantitative trait loci analysis for rice seed vigor during the germination stage, J. Zhejiang Univ. Sci. B 11 (2010) 958–964 (in Chinese with English abstract).
[25] X.Wu, H.Liu,W.Wang, S.Chen,X.Hu,C.Li, Proteomicanalysis of seed viabilityin maize, Acta Physiol. Plant. 33 (2010) 181–191.
[26] X. Xin, X. Lin, Y. Zhou, X. Chen, X. Liu, X. Lu, Proteome analysis of maize seeds: the effect of artificial ageing, Physiol. Plant. 143 (2011) 126–138.
[27] H. Chen, P. Chu, Y. Zhou, Y. Li, J. Liu, Y. Ding, E.W. Tsang, L. Jiang, K. Wu, S. Huang, Overexpression of AtOGG1, a DNA glycosylase/AP lyase, enhances seed longevity and abiotic stress tolerance in Arabidopsis, J. Exp. Bot. 63 (2012) 4107–4121.
[28] J. Lee, R. Welti, M. Roth, W.T. Schapaugh, J. Li, H.N. Trick, Enhanced seed viability and lipid compositional changes during natural ageing by suppressing phospholipase Dalpha in soybean seed, Plant Biotechnol. J. 10 (2012) 164–173.
[29] K. Nakabayashi, M. Bartsch, Y. Xiang, E. Miatton, S. Pellengahr, R. Yano, M. Seo, W.J. Soppe, The time required for dormancy release in Arabidopsis is determined by DELAY OF GERMINATION1 protein levels in freshly harvested seeds, Plant Cell 24 (2012) 2826–2838.
[30] T. Presterl, M. Ouzunova, W. Schmidt, E.M. Moller, F.K. Rober, C. Knaak, K. Ernst, P. Westhoff, H.H. Geiger, Quantitative trait loci for early plant vigour of maize grown in chilly environments, Theor. Appl. Genet. 114 (2007) 1059–1070.
[31] M.A. Gore, J.M. Chia, R.J. Elshire, Q. Sun, E.S. Ersoz, B.L. Hurwitz, J.A. Peiffer, M.D. McMullen, G.S. Grills, J. Ross-Ibarra, D.H. Ware, E.S. Buckler, A first-generation haplotype map of maize, Science 326 (2009) 1115–1117.
[32] USDA Foreign Agricultural Service, World agricultural production: Corn area, yield, and production, http://www.fas. usda.gov/psdonlines/2013.
[33] J. Hua, Y. Xing, C. Xu, X. Sun, S. Yu, Q. Zhang, Genetic dissection of an elite rice hybrid revealed that heterozygotes are not always advantageous for performance, Genetics 162 (2002) 1885–1895.
[34] J. Hua, Y. Xing, W. Wu, C. Xu, X. Sun, S. Yu, Q. Zhang, Single-locus heterotic effects and dominance by dominance interactions can adequately explain the genetic basis of heterosis in an elite rice hybrid, Proc. Natl. Acad. Sci. U. S. A. 100 (2003) 2574–2579.
[35] SPSS Inc, IBM SPSS Statistics 19.0 core system user's guide, IBM Corporation, New York, 2010.
[36] Z. Zeng, Precision mapping of quantitative trait loci, Genetics 136 (1994) 1457–1468.
[37] Z. Liu, H. Ji, Z. Cui, X. Wu, L. Duan, X. Feng, J. Tang, QTL detected for grain-filling rate in maize using a RIL population, Mol. Breed. 27 (2010) 25–36.
[38] J. Tang, J. Yan, X. Ma, W. Teng, W. Wu, J. Dai, B.S. Dhillon, A.E. Melchinger, J. Li, Dissection of the genetic basis of heterosis in an elite maize hybrid by QTL mapping in an immortalized F2population, Theor. Appl. Genet. 120 (2010) 333–340.
[39] C.W. Stuber, M.D. Edwards, J.F. Wendel, Molecular marker-facilitated investigations of quantitative trait loci in maize: II. Factors influencing yield and its component traits, Crop Sci. 27 (1987) 639–648.
[40] R.J. Probert, M.I. Daws, F.R. Hay, Ecological correlates of ex situ seed longevity: a comparative study on 195 species, Ann. Bot. 104 (2009) 57–69.
[41] A.B. Bruce, The Mendelian theory of heredity and the augmentation of vigor, Science 32 (1910) 627–628.
[42] D.F. Jones, Dominance of linked factors as a means of accounting for heterosis, Genetics 2 (1917) 466–479.
[43] E.M. East, Heterosis, Genetics 21 (1936) 375–397.
[44] E. Frascaroli, M.A. Cane, P. Landi, G. Pea, L. Gianfranceschi, M. Villa, M. Morgante, M.E. Pe, Classical genetic and quantitative trait loci analyses of heterosis in a maize hybrid between two elite inbred lines, Genetics 176 (2007) 625–644.
[45] T. Guo, N. Yang, H. Tong, Q. Pan, X. Yang, J. Tang, J. Wang, J. Li, J. Yan, Genetic basis of grain yield heterosis in an “immortalized F2”maize population, Theor. Appl. Genet. 127 (2014) 2149–2158.
[46] C. Jompuk, Y. Fracheboud, P. Stamp, J. Leipner, Mapping of quantitative trait loci associated with chilling tolerance in maize (Zea mays L.) seedlings grown under field conditions, J. Exp. Bot. 56 (2005) 1153–1163.
[47] F. Qiu, Y. Zheng, Z. Zhang, S. Xu, Mapping of QTL associated with waterlogging tolerance during the seedling stage in maize, Ann. Bot. 99 (2007) 1067–1081.
[48] A.M. Limami, C. Rouillon, G. Glevarec, A. Gallais, B. Hirel, Genetic and physiological analysis of germination efficiency in maize in relation to nitrogen metabolism reveals the importance of cytosolic glutamine synthetase, Plant Physiol. 130 (2002) 1860–1870.
[49] A. Hund, Y. Fracheboud, A. Soldati, E. Frascaroli, S. Salvi, P. Stamp, QTL controlling root and shoot traits of maize seedlings under cold stress, Theor. Appl. Genet. 109 (2004) 618–629.
[50] S. Trachsel, R. Messmer, P. Stamp, N. Ruta, A. Hund, QTLs for early vigor of tropical maize, Mol. Breed. 25 (2010) 91–103.
猜你喜欢
杂志排行
The Crop Journal的其它文章
- Single nucleotide polymorphisms linked to quantitative trait loci for grain quality traits in wheat
- Characterization of QTL for unique agronomic traits of new-plant-type rice varieties using introgression lines of IR64
- A multivariate partial least squares approach to joint association analysis for multiple correlated traits
- Analysis of simple sequence repeats in rice bean (Vigna umbellata) using an SSR-enriched library
- Intra-population genetic variance for grain iron and zinc contents and agronomic traits in pearl millet
- Fosmid library construction and screening for the maize mutant gene Vestigial glume 1
