由砷钼酸盐和镍配合物构筑而成的有机-无机杂化的化合物
2016-03-21田淑芳
李 杰,田淑芳,刘 云
(河南大学化学化工学院,河南开封475004)
由砷钼酸盐和镍配合物构筑而成的有机-无机杂化的化合物
李杰*,田淑芳,刘云
(河南大学化学化工学院,河南开封475004)
摘要:利用水热合成法得到了一个结构新颖的砷钼酸盐[Ni( dien)2]2[As6NiMo6O30]·2H2O ( 1) ( dien =二乙烯三胺),并用红外光谱、元素分析、热重分析和X射线单晶衍射对之进行了表征.化合物1属于单斜晶系,P2( 1) /n空间群.化合物1是由[As6NiMo6O30]4-阴离子簇和[Ni( dien)2]2+配合物构成.
关键词:多金属氧酸盐;水热合成;晶体结构;砷钼酸盐
The polyoxomolybdates ( POMs) are of interest,because of their unusual structural chemistry[1-2]and properties that make them attractive for applications in catalysis,materials science,photochemical and electrochemical[3-5].In this field,arsenatomolybdates show a great variety of unique structures and properties owing to the redox-active nature of both molybdenum and arsenic[2,6].Such arsenatomolybdates could be molecularly fine-tuned and provide potential new types of catalyst systems,as well as interesting functionalized materials with other properties.Arsenatomolybdate fragments have a large number of terminal and bridge oxygen atoms,so arsenatomolybdate fragments not only could coordinate with metal ion by their terminal and bridge oxygen atoms,but also can act as inorganic ligands multiply to bind several transition-metal complexes ( TMCs).So far the reports on arsenatomolybdates have been mainly concentrated on several Keggin-type polyanions[7-9],Dawson-type polyanions[10],sandwich-like polyanions[11]and other types of polyanions[12-16].However,in contrast to the rich information on inorganic arsenatomolybdates,the organic-inorganic hybrid arsenatomolybdates remain relatively undeveloped[17-20].Herein,we report the synthesis and structural characterization of a new organic-inorganic hybrid arsenatomolybdate,[Ni( dien)2]2[As6Ni-Mo6O30]·2H2O ( 1) ( dien = diethylenetriamine).
1 Experiment
1.1 Materials and physical measurements
All reagents were purchased commercially and used without further purification.C,H and N elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer.Inductively coupled plasma ( ICP) analysis was performed on a Perkin-Elmer Optima 2000 ICP-OES spectrometer.Infrared spectra were recorded on a Bruker VERTEX 70 IR spectrometer using KBr pellets from 400 to 4 000 cm-1.Thermogravimetric analyses were performed using a Perkin-Elmer7 thermal analyzer from 25 to 800℃under a dry nitrogen atmosphere with a heating rate of 10℃/min.
1.2 Synthesis of compound 1
Hydrothermal treatment of the mixture of Na2MoO4·2H2O ( 0.48 g,2.0 mmol),As2O3( 0.12 g,0.6 mmol),Ni( NO3)2·6H2O ( 0.36 g,1.2 mmol),dien ( 0.20 mL,1.86 mmol),imidazole ( 0.02 g,0.33 mmol) and 18 mL H2O at 160℃for 5 d with pH = 5.87 gave rise to a quantity of purple block crystals,in about 36% yield ( base on Mo).Elemental analysis ( %) calculated for C16H52As6Mo6N12Ni3O32: C 9.04,H 2.47 and N 7.90; found: C 8.87,H 2.65 and N 7.62.
1.3 Single-crystal X-ray diffraction ( XRD)
A purple block crystal with dimensions 0.32 mm× 0.23 mm×0.21 mm for 1 was stuck on a glass fiber and intensity data were collected at 296( 2) K on a Bruker Smart Apex-II CCD diffractometer with graphite-monochromated Mo Kαradiation (λ= 0.071 073 nm).The structure was solved by direct methods and refined by the full-matrix least-squares method on F2using the SHELXTL-97 package[21].Intensity data was corrected for Lorentz and polarization effects,as well as for multi-scan absorption.All of the non-hydrogen atoms were refined anisotropically.The crystallographic data are listed in Table 1.The atomic coordinates and other parameters of structure have been deposited with the Cambridge Crystallographic Data Centre ( No.1 415278 ; deposit@ ccdc.cam.ac.uk).
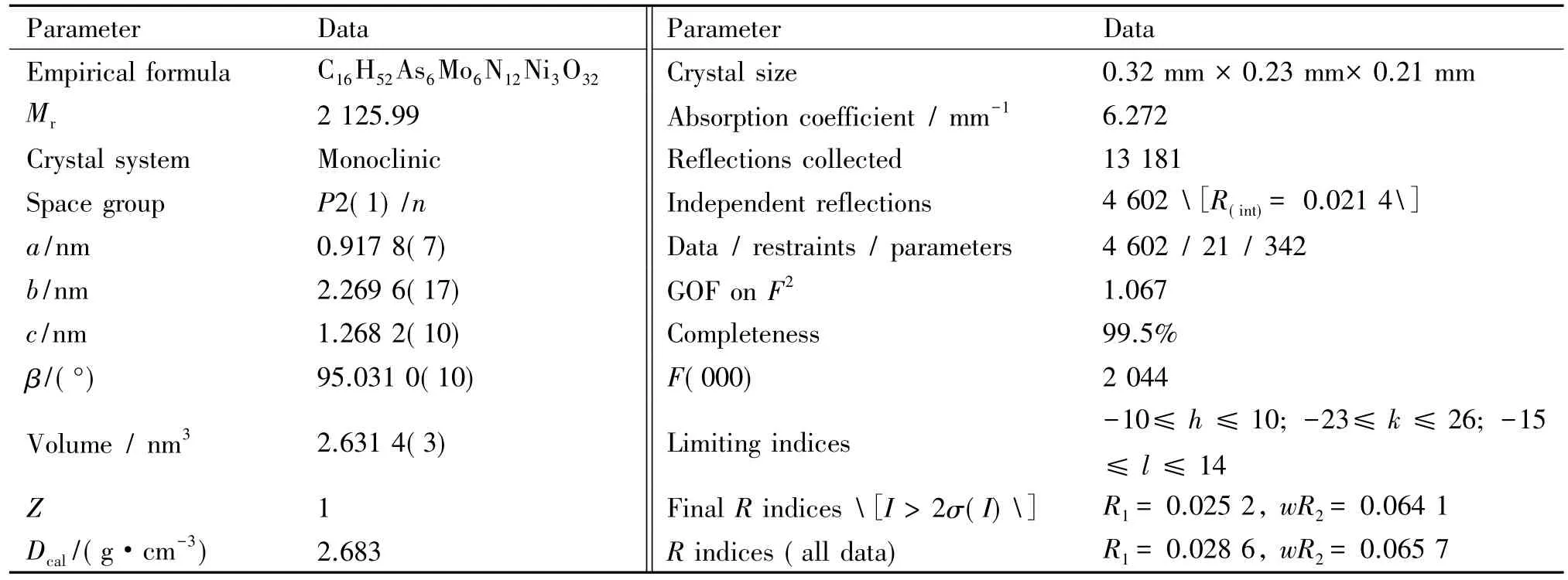
Table 1 Crystal data and structural refinements for compound 1
2 Results and discussion
2.1 Syntheses
In our case,the pH value of a solution is an important factor for the formation of arsenomolybdate.A lot of parallel experiments indicated the most suitable pH value was 5.87.At lower pH values,the N-ligand groups tend to be protonated and fail to bond to the metal ions.
2.2 Crystal structure
Compound 1 has been synthesized under hydrothermal conditions and crystallizes in the monoclinic space group P2( 1) /n.The single-crystal X-ray analysis reveals that compound 1 is constructed from [As6NiMo6O30]4-anionic clusters and [Ni( dien)2]2+complex subunits ( see Fig.1).

Fig.1 Polyhedral/ball-and-stick representation of the molecular structural unit of compound 1.The hydrogen atoms and lattice water molecules are omitted for clarity
The anion [As6NiMo6O30]4-is composed of the well-known A-type Anderson anion [NiO6Mo6O18]10-,in which a central { NiO6} octahedron is coordinated with six { MoO6} octahedra hexagonally arranged by sharing their edges in a plane.The cyclic As3O6trimers are capped on opposite faces of the Anderson-type anion plane.Each As3O6group consists of three AsO3pyramids linked in a triangular arrangement by sharing corners and bonded to the central NiO6octahedron and two MoO6octahedra via μ3-oxo groups.This kind of polyoxoanion architecture was reported for the first time by JEANNIN et al.for the cobalt( II) -containing arsenomolybdate [Co( H2O)6]K2[As6CoMo6O30][16].Later,it is exploited that the polyoxoanions [( MO6) ( As3O3)2Mo6O18]4-( M = Co,Zn,Cu) have been used as inorganic building blocks to construct extended and modified structures[6a,20,22-23].Coordination sphere of crystallographically unique Ni in the nickel dien complexes is defined by six nitrogen atoms from two dien ligands ( Ni2-N1 = 0.211 9( 4) nm,Ni2-N2 = 0.215 6( 4) nm,Ni2-N3 = 0.213 8 ( 4) nm; Ni2-N4 = 0.213 2 ( 4) nm,Ni2-N5 = 0.216 3( 4) nm,Ni2-N6 = 0.212 4( 4) nm).Every anionic cluster [As6NiMo6O30]4-is surrounded by six centrosymmetric complex subunits [Ni ( dien)2]2+along a axis ( see Fig.2).

Fig.2 Each anionic cluster [As6NiMo6O30]4-lies in the centre of six centrosymmetric complex subunits [Ni( dien)2]2+in compound 1 along the a axis.The hydrogen atoms and lattice water molecules are omitted for clarity
On the basis of valence sum (∑s) calculations[24],the oxidation states of all three Mo atoms are +6 (∑s = 5.95-5.98),the three As atoms are + 3 (∑s = 3.00-3.03),and the oxidation states of the two Ni atoms are +2 (∑s = 2.04-2.10) in 1.The oxidation states of the Mo,As and Ni atoms are consistent with the formula of compound 1.
2.3 IR spectrum
For 1,three groups of characteristic vibration absorption bands are observed at 925,881,804 and 661 cm-1,which are attributed to the ν( Mo-Ot),ν( Mo-Ob),ν( As-O),and ν( Mo-Oc) vibrations,respectively[21,25].The signals appearing at 3 322 and 2 899 cm-1for 1 are attributable to the ν( NH2) and ν ( CH2) stretching vibrations,while the resonances centered at 1 589 and 1 465 cm-1for 1 are assigned to the δ( NH2) and δ( CH2) bending vibration,respectively.The occurrence of these resonance signals confirms the presence of organic amine groups in 1,which are consistent with the single-crystal structural analyses.
2.4 Thermal analyses
According to the TG curve of compound 1 ( in Fig.3),we can deduce that the thermal decomposition process of the compound is approximately divided into two steps.There is a continuous decrease in the range of 25-372℃; the weight loss of 22.9% is comparable with the calculated value of 20.9%,corresponding to the loss of two lattice water molecules and 4 molecules of dien.The second weight loss of 22% is between 372 and 673℃due to the sublimation of most As2O3molecules.
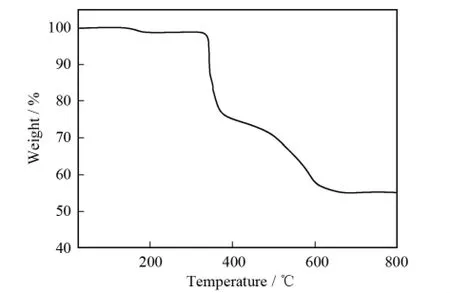
Fig.3 TG curve of compound 1
References:
[1]POPE M T.Heteropoly and isopoly oxometalates [M ].New York: Springer,1983: 1-426.
[2]MÜLLER A,RETERS F,POPE M T,et al.Polyoxometalates: Very large clusters nanoscale magnets [J ].Chem Rev,1998,98 ( 1) : 239-272.
[3]CUI X B,XU J Q,MENG H,et al.A novel chainlike As-V-O polymer based on a transition metal complex and a dimeric polyoxoanion [J ].Inorg Chem,2004,43 ( 25) : 8005-8009.
[4]WANG Y,YU J H,GUO M,et al.[{ Zn2( HPO4)4} { Co( dien)2} ]·H3O: A zinc phosphate with multidirectional intersecting helical channels [J].Angew Chem Int Ed,2003,42 ( 34) : 4089-4092.
[5]KATSOULIS D E.A survey of applications of polyoxometalates [J].Chem Rev,1998,98 ( 1) : 359-388.
[6]( a) HE Q L,WANG E B.Hydrothermal synthesis and crystal structure of a new molybdenum( VI) arsenate( III),CoIII( en)3H3O[( CoIIO6) MoIO18( AsIIO3)2]·2H2O[J].Inorg Chim Acta,1999,295( 2) : 244-247.( b) MIZUNO N,MISONO M.Heterogeneous catalysis [J].Chem Rev,1998,98( 1) : 199-218.( c) FRUCHART J M,HERVE G,LAUNAY J P,et al.Electronic spectra of mixed valence reduced heteropolyanions [J].Inorg Nucl Chem,1976,38( 9) : 1627-1634.( d) BU X,GIER T E,STUCKY G D.Novel lamella hydrated sodium zinc arsenate with 4-connected two-dimensional nets [J].Chem Commun,1997: 2271-2272.( e) WIGGIN S B,WELLER M T.A chiral,16-ring channel framework and a layered caesium zincoarsenate [J].Chem Commun,2006: 1100-1102.
[7]SANCHEZ C,LIVAGE J,LAUNAY J P,et al.Electron delocalization in mixed-valence molybdenum polyanions [J].J Am Chem Soc,1982,104( 11) : 3194-3202.
[8]KHAN M I,CHEN Q,ZUBIETA J.Hydrothermal synthesis and structure of molybdoarsenate [H4AsIIAsVMoMoIO40]-,a bicapped,reduced Keggin species [J].Inorg Chem,1993,32( 13) : 2924-2928.
[9 ]HE Q L,WANG E B,YOU W,et al.Hydrothermal synthesis and structure of [AsIIIAsVMoO34]6-,a monocapped,trivacant Keggin species [J ].J Mol Struct,1999,508: 217-221.
[10]WANG E B,HU C W,ZHOU Y X,et al.The synthesis and characterization of dawson structure molybdoarsenic acid and salts [J].Acta Chim Sin,1990,48( 8) : 790-796.
[11]LI L L,SHEN Q,XUE G L,et al.Two sandwich arsenomolybdates based on the new building block As( III) Mo7O279-: [Cr2( AsMo7O27)2]12-and [Cu2( AsMo7O27)2]14-[J ].J Chem Soc Dalton Trans,2008,42: 5698-5700.
[12]HSU K F,WANG S L.Cs5Mo8O24( OH)2AsO4·2H2O and Cs7Mo8O26AsO4: two novel molybdenum( VI) arsenates containing heteropolyanions [AsMo8O30H2]5-and[AsMo8O30]7-[J ].Inorg Chem,1997,36( 14) : 3049-3054.
[13]WANG S L,HSU K F,NIEH Y P.Hydrothermal synthesis and structural characterization of the molybdenum ( VI) arsenate ( C5H5NH)2( Mo2O5)-( HAsO4)2·H2O [J].J Chem Soc Dalton Trans Inorg Chem,1994,11: 1681-1684.
[14]KHAN M I,CHEN Q,ZUBIETA J.Hydrothermal synthesis and crystal and molecular structure of a reduced,arsenic rich heteropolyanion,Na4[Mo4As6O20( OH)2]· 9H2O [J ].J Chem Soc Chem Commun,1993: 356-357.
[15]MARTIN-FRÈRE J,JEANNIN Y,ROBERT F,et al.
Synthesis and x-ray structures of two unprecedented heteropolymetalates [As3M3O15]3-( M = Mo,W) and [As6CoMo6O30]4-.First examples of linear triarsenate( III) and cyclic triarsenate( III) [J].Inorg Chem,1991,30( 19) : 3635-3639.
[16]HE Q L,WANG E B.Hydrothermal synthesis and crystal structure of a new copper( II) molybdenum( VI) arsenate( III),( C5H5NH)2( H3O)2[( CuO6) Mo6O18( As3O3)2][J].Inorg Chem Commun,1999,2( 9) : 399-402.
[17]HE Q L,WANG E B.Hydrothermal synthesis and crystal structure of an As( III) -Mo( V) -O cluster decorated with Cu( II) -en groups [J ].Inorg Chim Acta,2000,298( 2) : 235-238.
[18]KORTZ U,SAVELIEFF M G,ABOU GHALI F Y,et al.Heteropolymolybdates of AsIII,SbIII,BiIII,SeIV,and TeIVfunctionalized by amino acids [J].Angew Chem Int Ed,2002,41( 21) : 4070-4073.
[19]ZHAO Z F,ZHOU B B,SU Z H,et al.A new [As3Mo3O15]3-fragment decorated with Cu( I) -imi ( imi = imidazole) complexes: Synthesis,structure and electrochemical properties [J ].Inorg Chem Commun,2008,11( 6) : 648-651.
[20]SUN C Y,LI Y G,WANG E B,et al.A series of new organic-inorganic molybdenum arsenate complexes based on[( ZnO6) ( As3O3)2Mo6O18]4-and [HxAs2Mo6O26]( 6-x)-clusters as SBUs [J ].Inorg Chem,2007,46 ( 5) : 1563-1574.
[21 ]SHELDRICK G M.SHELXL 97: Program for refinement crystal structure [CP ].Göttingen: University of Göttingen,1997.
[22]LI L L,LIU B,XUE G L,et al.Three hybrid organicinorganic assemblies based on different arsenatomolybdates and CuII-organic units [J].Cryst Growth Des,2009,9 ( 12) : 5206-5212.
[23]ZHAO J W,ZHANG J L,LI Y Z,et al.Novel one-dimensional organic-inorganic polyoxometalatehybrids constructed from heteropolymolybdate units and copper-aminoacid complexes [J].Cryst Growth Des,2014,14: 1467 -1475.
[24]BRESE N E,O'KEEFFE M.Bond-valence parameters for solids [J].Acta Cryst,1991,B47: 192-197.
[25]HASENKNOPF B,DELMONT R,GOUZERH P.Anderson-type heteropolymolybdates containing tris( alkoxo) ligands: synthesis and structural characterization [J ].Eur J Inorg Chem,2002,2002( 5) : 1081-1087.
[责任编辑:吴文鹏]
