有机污染物的被动采样材料-水分配系数的QSAR研究
2016-03-17杨蕾罗翔王雅张亚南陈景文
杨蕾,罗翔,王雅,张亚南,陈景文
工业生态与环境工程教育部重点实验室,大连理工大学环境学院,大连 116024
有机污染物的被动采样材料-水分配系数的QSAR研究
杨蕾,罗翔,王雅,张亚南,陈景文*
工业生态与环境工程教育部重点实验室,大连理工大学环境学院,大连 116024
被动采样材料-水分配系数;聚乙烯;聚丙烯酸酯;硅橡胶;定量构效关系
Received 15 April 2016 accepted 26 May 2016
近年来,被动采样技术在水中痕量有机污染物的监测领域得到了广泛应用。半透膜采样装置(SPMDs)[1]、聚乙烯被动采样器(PE)[2]和梯度扩散薄膜技术(DGT)[3]等被动采样技术被用于测定污染物的自由溶解态的浓度,对于生物有效性评价具有重要意义。在被动采样过程中,被动采样器通过吸附作用将水中的污染物富集到采样材料上,从而得到污染物的时间加权平均浓度[4]。有机污染物的被动采样材料-水分配系数(KPW),是衡量被动采样器性能和进行优化的一个重要指标[5]。目前,大部分污染物的KPW值都是通过实验测定获得,但实验方法费时费力[6],难以满足数量庞大且与日俱增的有机污染物的监测需求,需要发展预测方法来获得有机污染物的KPW值。
定量构效关系(QSAR)是一种可以有效预测有机污染物理化性质、环境行为和毒理学效应参数的方法[7]。许多研究曾采用有机化合物的正辛醇-水分配系数(logKOW)、正十六烷-水分配系数(logKHW)或水溶解度(logSW)对其KPW进行预测[8-16]。Lohmann[8]分别用logKHW和logSW预测了100种化合物的聚乙烯-水分配系数,相关系数(R2)分别为0.86和0.92;用logKOW预测79种化合物的聚乙烯-水分配系数,R2达到0.91。Hale等[9]分别用logKHW和logKOW预测了34种化合物的聚乙烯-水分配系数,R2分别为0.85和0.53。另外,有些研究基于线性溶解能关系(LSER)预测KPW。Endo等[5]构建了79种化合物的聚丙烯酸酯-水分配系数的LSER模型,R2达到0.97。然而这些模型所采用的预测变量通常也是需要实验测定的经验性参数,导致模型的适用范围有限。本研究针对3类常用的被动采样材料,即聚乙烯(PE)、聚丙烯酸酯(PA)和硅橡胶(SR),遵循经济合作与发展组织(OECD)发布的QSAR构建与验证导则[17],构建KPW的QSAR预测模型,并对模型进行表征和机理解释。
1 材料与方法(Materials and methods)
1.1 数据来源
考虑7种被动采样材料(聚乙烯、聚丙烯和5种不同的硅橡胶),有机污染物在19 ~ 26 ℃下的KPW实测数据均来自文献报道[2,5-6,8-9,18-27],包括:215种有机物的聚乙烯-水分配系数(此处为区分不同采样材料记为logKPE),数值范围为2.3 ~ 7.8;107种有机物的聚丙烯酸酯-水分配系数(logKPA),数值范围为0.0 ~ 6.0;67种有机物的Silastic A型硅橡胶-水分配系数(logKSR1),数值范围为3.0 ~ 7.6;67种有机物的SR batch 0型硅橡胶-水分配系数(logKSR2),数值范围为2.8 ~ 7.4;93种有机物的AlteSil型硅橡胶-水分配系数(logKSR3),数值范围为3.0 ~ 7.8;67种有机物的SR-RED型硅橡胶-水分配系数(logKSR4),数值范围为3.0 ~ 7.6;67种有机物的SR-TF型硅橡胶-水分配系数(logKSR5),数值范围为2.9 ~ 7.4。有机物涵盖烷烃、烯烃、芳香类、醇类、酮类、酯类、醚类等多种类别。
将各个数据集以4∶1的比例随机拆分为训练集和验证集,其中,训练集中的化合物用于构建模型,验证集中的化合物用于模型验证。
1.2 分子结构描述符的计算
采用ChemBio3D Ultra (Version 12.0)软件中MOPAC 2012模块的PM7算法[28],对化合物结构进行优化并获得稳定构型。同时,基于化合物优化后的稳定结构,由Dragon (Version 6.0)软件计算得到分子结构描述符。
1.3 模型的建立

(1)
(2)
(3)

1.4 应用域表征
采用基于标准残差(δ)对leverage值(hi)的Williams图对模型的应用域进行表征[30]。δ和hi及其预警值(h*)的计算公式如下:
(4)
hi= xiT(XTX)-1xi
(5)
h*= 3(k + 1)/n
(6)
式中,k为自变量的个数,xi是第i个化合物的描述符矢量,X是描述符矩阵。将|δ| > 3的化合物视为离群点。
2 结果(Results)
2.1 最优QSAR模型
得到7种被动采样材料的最优QSAR模型如下:
(1) 聚乙烯-水分配系数(logKPE)
logKPE= 0.015Vx- 0.034TPSA(NO) + 0.110nBM + 0.137nCl - 0.841
(2) 聚丙烯酸酯-水分配系数(logKPA)
logKPA= 0.010Vx+ 0.154nCl + 0.078nBM - 0.026TPSA(NO)+ 0.940NddsN - 0.778nROH + 0.701
(3) Silastic A型硅橡胶-水分配系数(logKSR1)
logKSR1= 0.024Vx- 0.117Rperim - 0.088
(4) SR batch 0型硅橡胶-水分配系数(logKSR2)
logKSR2= 0.024Vx- 0.139Rperim - 0.088
(5) AlteSil型硅橡胶-水分配系数(logKSR3)
logKSR3= 0.021Vx- 0.824
(6) SR-RED型硅橡胶-水分配系数(logKSR4)
logKSR4= 0.017Vx+ 0.140nCl
(7) SR-TF型硅橡胶-水分配系数(logKSR5)
logKSR5= 0.023Vx- 0.144Rperim + 0.327
2.2 模型的应用域表征
7个模型的应用域表征结果如图2所示。训练集和验证集所有化合物的|δ| < 3,模型无离群点,表明训练集化合物具有很好的代表性。logKPE模型中训练集的4种化合物和logKPA模型中训练集的1种化合物,hi> h*但|δ| < 3,说明这些化合物增加了模型的稳定性和准确性。logKPE模型中验证集的1种化合物,hi> h*但|δ| < 3,落在描述符域外,但其预测效果较好,说明模型适用于远离描述符中心的化合物,进一步推论出模型具有一定的延展能力和外推性。因此,建立的模型可用于预测应用域内其他化合物的logKPW值。
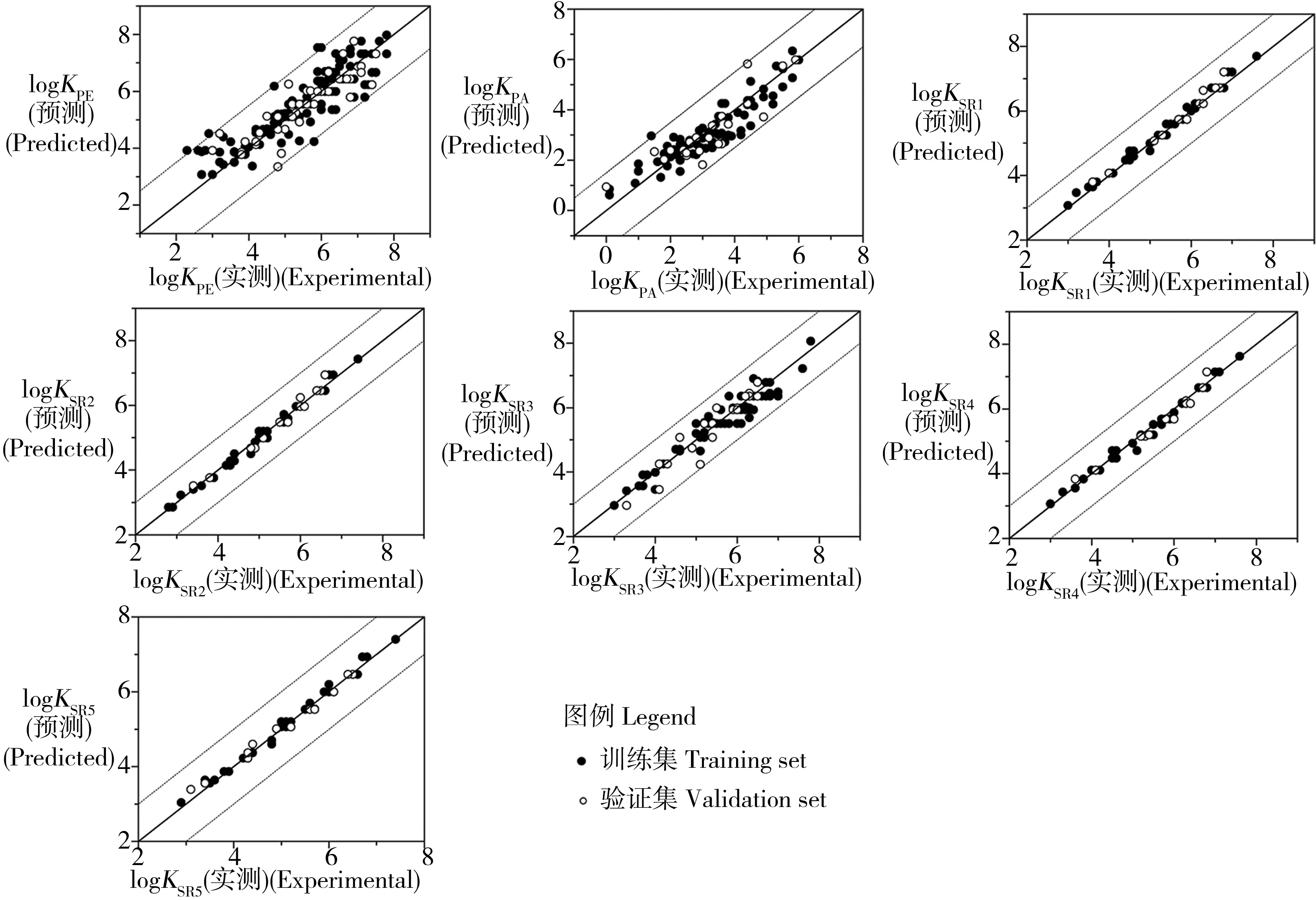
图1 logKPW的实测值与预测值拟合关系图Fig. 1 Plot of the predicted versus experimental logKPW values

图2 logKPW模型的Williams应用域表征图Fig. 2 Williams plot of logKPW models
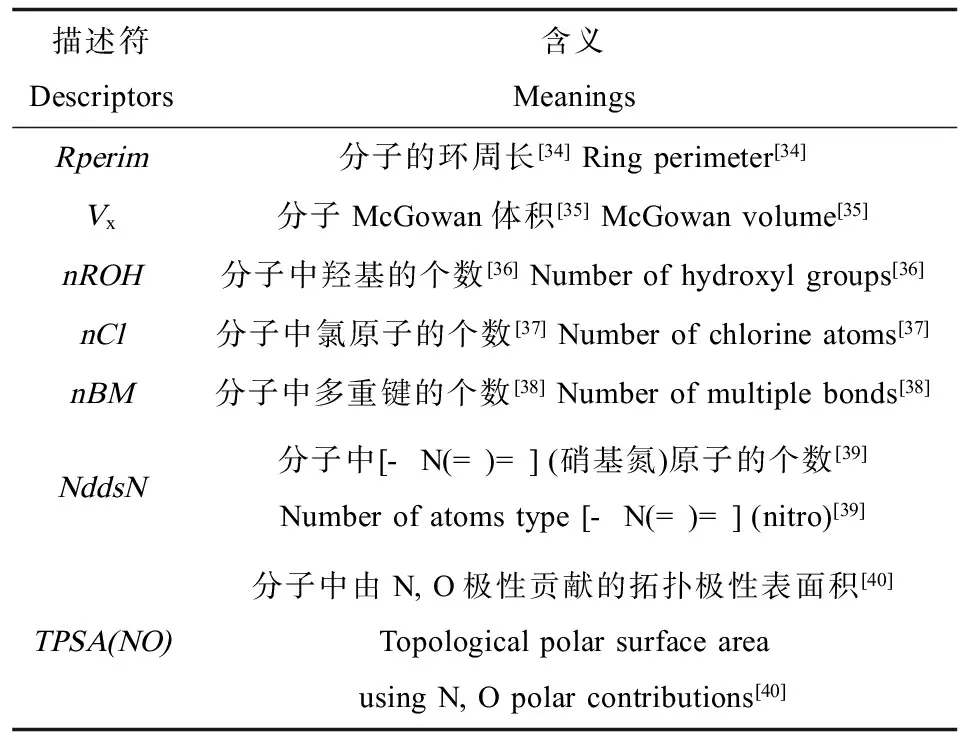
表1 本研究构建的预测模型中分子结构描述符的含义Table 1 Definitions of the molecular structural descriptors involved in the developed models
3 讨论(Discussion)
3.1 机理解释
7个模型中,共包含7个描述符(如表1所示),其中,Vx, nBM, nCl和NddsN与logKPW呈正相关;TPSA(NO), nROH和Rperim与logKPW呈负相关。在所有模型中,Vx的贡献最大,是影响KPW的最主要因素。Vx是分子McGowan体积,表征空穴形成作用。由于水分子排列高度有序且凝聚性强[31],在水中形成空穴所需能量远大于其在被动采样材料中所需能量,因此化合物分子更容易通过空穴形成作用分配到被动采样材料相中。具有较大Vx值的化合物,其logKPW值越大。nCl是氯原子的个数。有研究表明,logKOW与卤素原子个数有关[32],可用化合物的卤素原子数表征其疏水作用,nCl值越大的化合物疏水作用越强,因而越容易分配到采样材料中。TPSA(NO)是由N, O极性贡献的拓扑极性表面积,nROH是羟基的个数。这些亲水结构中的氮和氧原子具有孤对电子,易形成氢键,增加了与水的氢键相互作用[33],使化合物更不易被采样材料吸附,从而具有更小的logKPW值。Rperim是环的周长,化合物的环周长越大,空间位阻越大,越难进入到被动采样材料相中,从而具有更小的logKPW值。此外,nBM和NddsN表明logKPW还与化合物多重键和[-N(=)=] (硝基氮)原子的个数有关。
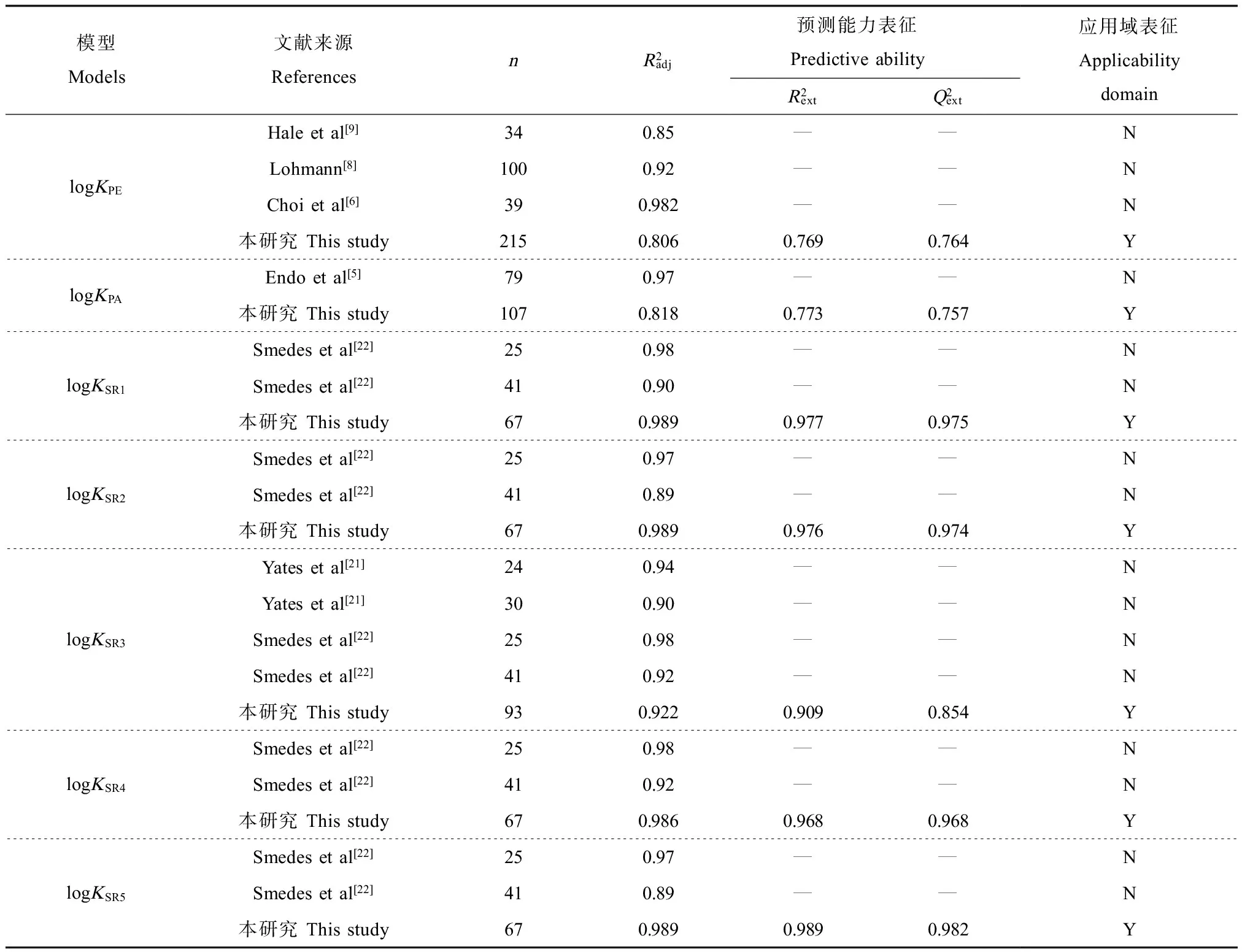
表2 本研究模型和前人相关模型的比较Table 2 Comparison of KPW prediction models from the current study and previous studies
注:N表示无应用域表征,Y表示有应用域表征;— 表示未报道。
Note: N, applicability domain was not characterized; Y, applicability domain was characterized; —, unreported.
3.2 模型比较
将本研究构建的模型与前人的一些代表性模型进行比较,见表2。本研究logKPE, logKPA和logKSR模型与前人模型相比,化合物种类更丰富且数量更多,而且所采用的分子结构参数均可通过计算获得,不依赖于实验测定。此外,本研究将数据集划分为训练集和验证集,利用MLR方法建立模型,所有模型均进行了外部验证和应用域的表征,并进行了机理解释。
综上,本研究遵循OECD关于QSAR模型构建和验证的导则,构建了3类共7种被动采样材料的KPW的QSAR预测模型。模型具有良好的拟合优度、稳健性和预测能力,能够用于预测含有>C=C<, -OH, -O-, >C=O, -C=O(O), -C6H5, -NO2, -NH2, -NH-, -X(F, Cl, Br, I)等多种结构官能团的有机污染物的logKPW值,可为快速获取有机污染物的KPW值以及为被动采样器的应用提供基础数据。
辅助信息:化合物logKPW实测值、预测值以及模型中包含的分子结构描述符值,需要者请和通讯作者联系。
[1] Booij K, van Bommel R, van Aken H M, et al. Passive sampling of nonpolar contaminants at three deep-ocean sites [J]. Environmental Pollution, 2014, 195: 101-108
[2] Sacks V P, Lohmann R. Development and use of polyethylene passive samplers to detect triclosans and alkylphenols in an urban estuary [J]. Environmental Science & Technology, 2011, 45(6): 2270-2277
[3] Chen C E, Zhang H, Ying G G, et al. Evidence and recommendations to support the use of a novel passive water sampler to quantify antibiotics in wastewaters [J]. Environmental Science & Technology, 2013, 47(23): 13587-13593
[4] Alvarez D A, Cranor W L, Perkins S D, et al. Chemical and toxicologic assessment of organic contaminants in surface water using passive samplers [J]. Journal of Environmental Quality, 2008, 37(3): 1024-1033
[5] Endo S, Droge S T J, Goss K U. Polyparameter linear free energy models for polyacrylate fiber-water partition coefficients to evaluate the efficiency of solid-phase microextraction [J]. Analytical Chemistry, 2011, 83(4): 1394-1400
[6] Choi Y, Cho Y M, Luthy R G. Polyethylene-water partitioning coefficients for parent-and alkylated-polycyclic aromatic hydrocarbons and polychlorinated biphenyls [J]. Environmental Science & Technology, 2013, 47(13): 6943-6950
[7] 陈景文, 全燮. 环境化学[M]. 大连: 大连理工大学出版社, 2009: 260
Chen J W, Quan X. Environmental Chemistry [M]. Dalian: Dalian University of Technology Press, 2009: 260 (in Chinese)
[8] Lohmann R. Critical review of low-density polyethylene’s partitioning and diffusion coefficients for trace organic contaminants and implications for its use as a passive sampler [J]. Environmental Science & Technology, 2011, 46(2): 606-618
[9] Hale S E, Martin T J, Goss K U, et al. Partitioning of organochlorine pesticides from water to polyethylene passive samplers [J]. Environmental Pollution, 2010, 158(7): 2511-2517
[10] Josefsson S, Arp H P H, Kleja D B, et al. Determination of polyoxymethylene (POM)-water partition coefficients for oxy-PAHs and PAHs [J]. Chemosphere, 2015, 119: 1268-1274
[11] Perron M M, Burgess R M, Suuberg E M, et al. Performance of passive samplers for monitoring estuarine water column concentrations: 1. Contaminants of concern [J]. Environmental Toxicology and Chemistry, 2013, 32(10): 2182-2189
[12] Reitsma P J, Adelman D, Lohmann R. Challenges of using polyethylene passive samplers to determine dissolved concentrations of parent and alkylated PAHs under cold and saline conditions [J]. Environmental Science & Technology, 2013, 47(18): 10429-10437
[13] Sacks V P, Lohmann R. Freely dissolved PBDEs in water and porewater of an urban estuary [J]. Environmental Pollution, 2012, 162: 287-293
[14] Kwon J H, Wuethrich T, Mayer P, et al. Dynamic permeation method to determine partition coefficients of highly hydrophobic chemicals between poly (dimethylsiloxane) and water [J]. Analytical Chemistry, 2007, 79(17): 6816-6822
[15] Booij K, Smedes F, van Weerlee E M, et al. Environmental monitoring of hydrophobic organic contaminants: The case of mussels versus semipermeable membrane devices [J]. Environmental Science & Technology, 2006, 40(12): 3893-3900
[16] Hawthorne S B, Miller D J, Grabanski C B. Measuring low picogram per liter concentrations of freely dissolved polychlorinated biphenyls in sediment pore water using passive sampling with polyoxymethylene [J]. Analytical Chemistry, 2009, 81(22): 9472-9480
[17] OECD. Guidance document on the validation of (Quantitative) Structure-Activity Relationships [(Q)SAR] models [R]. Paris: OECD, 2007
[18] Booij K, Hofmans H E, Fischer C V, et al. Temperature-dependent uptake rates of nonpolar organic compounds by semipermeable membrane devices and low-density polyethylene membranes [J]. Environmental Science & Technology, 2003, 37(2): 361-366
[19] Adams R G, Lohmann R, Fernandez L A, et al. Polyethylene devices: Passive samplers for measuring dissolved hydrophobic organic compounds in aquatic environments [J]. Environmental Science & Technology, 2007, 41(4): 1317-1323
[20] Paschke A, Popp P. Solid-phase microextraction fibre-water distribution constants of more hydrophobic organic compounds and their correlations with octanol-water partition coefficients [J]. Journal of Chromatography A, 2003, 999(1): 35-42
[21] Yates K, Davies I, Webster L, et al. Passive sampling: Partition coefficients for a silicone rubber reference phase [J]. Journal of Environmental Monitoring, 2007, 9(10): 1116-1121
[22] Smedes F, Geertsma R W, Zande T, et al. Polymer-water partition coefficients of hydrophobic compounds for passive sampling: Application of cosolvent models for validation [J]. Environmental Science & Technology, 2009, 43(18): 7047-7054
[23] Thompson J M, Hsieh C H, Luthy R G. Modeling uptake of hydrophobic organic contaminants into polyethylene passive samplers [J]. Environmental Science & Technology, 2015, 49(4): 2270-2277
[24] Hale S E, Tomaszewski J E, Luthy R G, et al. Sorption of dichlorodiphenyltrichloroethane (DDT) and its metabolites by activated carbon in clean water and sediment slurries [J]. Water Research, 2009, 43(17): 4336-4346
[25] Cornelissen G, Pettersen A, Broman D, et al. Field testing of equilibrium passive samplers to determine freely dissolved native polycyclic aromatic hydrocarbon concentrations [J]. Environmental Toxicology and Chemistry, 2008, 27(3): 499-508
[26] Fernandez L A, MacFarlane J K, Tcaciuc A P, et al. Measurement of freely dissolved PAH concentrations in sediment beds using passive sampling with low-density polyethylene strips [J]. Environmental Science & Technology, 2009, 43(5): 1430-1436
[27] Bao L J, You J, Zeng E Y. Sorption of PBDE in low-density polyethylene film: Implications for bioavailability of BDE-209 [J]. Environmental Toxicology and Chemistry, 2011, 30(8): 1731-1738
[28] Jhin C, Hwang K T. Prediction of radical scavenging activities of anthocyanins applying adaptive neuro-fuzzy inference system (ANFIS) with quantum chemical descriptors [J]. International Journal of Molecular Sciences, 2014, 15(8): 14715-14727
[29] Norušis M J. SPSS Inc. SPSS 7.5 Guide to Data Analysis [M]. Upper Saddle River, New Jersey: Prentice Hall, 1997: 458
[30] Gramatica P. Principles of QSAR models validation: Internal and external [J]. QSAR & Combinatorial Science, 2007, 26(5): 694-701
[31] Vitha M, Carr P W. The chemical interpretation and practice of linear solvation energy relationships in chromatography [J]. Journal of Chromatography A, 2006, 1126(1): 143-194
[32] Li F, Xie Q, Li X H, et al. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-β: In vitro and in silico investigations [J]. Environmental Health Perspectives, 2010, 118(5): 602-606
[33] Schüürmann G, Ebert R U, Kühne R. Prediction of the sorption of organic compounds into soil organic matter from molecular structure [J]. Environmental Science & Technology, 2006, 40(22): 7005-7011
[34] Fernández A, Rallo R, Giralt F. Prioritization of in silico models and molecular descriptors for the assessment of ready biodegradability [J]. Environmental Research, 2015, 142: 161-168
[35] Abraham M H, McGowan J C. The use of characteristic volumes to measure cavity terms in reversed phase liquid chromatography [J]. Chromatographia, 1987, 23(4): 243-246
[36] Wang Y, Chen J W, Yang X H, et al. In silico model for predicting soil organic carbon normalized sorption coefficient (KOC) of organic chemicals [J]. Chemosphere, 2015, 119: 438-444
[37] Tang W Z, Wang F. Quantitative structure activity relationship (QSAR) of chlorine effects on ELUMOof disinfection by-product: Chlorinated alkanes [J]. Chemosphere, 2010, 78(7): 914-921
[38] Edraki N, Hemmateenejad B, Miri R, et al. QSAR study of phenoxypyrimidine derivatives as potent inhibitors of p38 kinase using different chemometric tools [J]. Chemical Biology & Drug Design, 2007, 70(6): 530-539
[39] Butina D. Performance of Kier-hall E-state descriptors in quantitative structure activity relationship (QSAR) studies of multifunctional molecules [J]. Molecules, 2004, 9(12): 1004-1009
[40] Patel J R, Prajapati L M. Predictive QSAR modeling on tetrahydropyrimidine-2-one derivatives as HIV-1 protease enzyme inhibitors [J]. Medicinal Chemistry Research, 2013, 22(6): 2795-2801
◆
QSAR Models for Predicting Partition Coefficients of Organic Pollutants between Passive Sampling Materials and Water
Yang Lei, Luo Xiang, Wang Ya, Zhang Ya’nan, Chen Jingwen*
Key Laboratory of Industrial Ecology and Environmental Engineering (MOE), School of Environmental Science and Technology, Dalian University of Technology, Dalian 116024, China
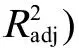
passive sampling materials-water partition coefficients; polyethylene; polyacrylate; silicone rubber; quantitative structure-activity relationships
国家自然科学基金(21325729; 21661142001)
杨蕾(1990-),女,硕士,研究方向为污染生态化学,E-mail: yanglei_dlut@mail.dlut.edu.cn;
*通讯作者(Corresponding author), E-mail: jwchen@dlut.edu.cn
10.7524/AJE.1673-5897.20160415001
2016-04-15 录用日期:2016-05-26
1673-5897(2016)6-053-08
X171.5
A
陈景文(1969-),男,博士,教授,研究方向为污染生态化学、污染控制化学和环境生态技术。
杨蕾, 罗翔, 王雅, 等. 有机污染物的被动采样材料-水分配系数的QSAR研究[J]. 生态毒理学报,2016, 11(6): 53-60
Yang L, Luo X, Wang Y, et al. QSAR models for predicting partition coefficients of organic pollutants between passive sampling materials and water [J]. Asian Journal of Ecotoxicology, 2016, 11(6): 53-60 (in Chinese)
