The microbiome,microbial-generated proinflammato y neurotoxins, and Alzheimer’s disease
2016-02-05WalterLukiw
Walter J.Lukiw
LSU Neuroscience Center and Departments of Neurology and Ophthalmology,Louisiana State University Health Science Center,New Orleans,LA 70112,USA
The microbiome,microbial-generated proinflammato y neurotoxins, and Alzheimer’s disease
Walter J.Lukiw
LSU Neuroscience Center and Departments of Neurology and Ophthalmology,Louisiana State University Health Science Center,New Orleans,LA 70112,USA
1.Overview
Genomic sequencing studies indicate that the human gastrointestinal(GI)tract is a dynamic repository of about~1014micro-organisms that outnumber host cells by at least 100 to 1.1–6Collectively,these~1014microbes contain about 1017genes;the number of microbial genes is at least 150-fold greater than the total number of human genes.3,6–10About 98% of the microbiota in the human GI tract consists of anaerobic bacteria,with archaebacteria,fungi,protozoa,prions,viruses of plant and animal origin,viroids and other micro-organisms,and other free nucleic acids,such as microRNAs,making up the remainder.Why evolution selected just 2 of the 54 recognized major divisions of bacteriaBacteroidetesandFirmicutesto populate the human GI tract is both enigmatic and open to speculation—these 2 major phyla represent the“bacterial core”of our microbiome.The ability ofBacteroidetesandFurmicutesto coexist symbiotically,to generate essential vitamins and cofactors,and to process dietary constituents such as fibe may have made them particularly suitable to support human immunity,physiology,biochemistry,and neurochemistry.3,9–12Of these 2 phyla,theBacteroidetes,the largest class of obligate anaerobic gram-negative(G-)bacteria of the human GI tract,release extraordinarily complex mixtures of amyloids,lipopolysaccharides(LPSs),enterotoxins,and neurotoxins.Microbiome-derived exudates appear to be noxious to multiple aspects of microbiome–host interactions, including(1)GI tract and blood–brain barrier structure and integrity;(2)systemic,central nervous system(CNS),and peripheral nervous system(PNS)homeostasis and equilibrium; and(3)progressive inflammato y degeneration within the human nervous system.Interestingly,unique LPS toxins of the microbiome-abundantBacteroides fragilis(BF-LPS)and theB.fragilistoxin(BFT)fragilysin are among the most barrier-disruptive and proinflammato y neurotoxins known.
This paper overviews some current research and emerging concepts published over the past 6 months on(1)the toxic array of substances generated byB.fragilis;(2)the potential contribution of microbiome-generated factors such as BF-LPS in driving proinflammato y signaling both systemically and within the nervous system;and(3)the recent recognition of the beneficia effects of dietary fibe on the growth and proliferation ofB.fragilisand other prolifi microbial species in the human GI tract microbiome,with specifi reference to Alzheimer’s disease(AD)neuropathology wherever possible.
2.BF-LPS amyloids,LPSs,and enterotoxins
A priori,we would like to note that in this paper we are using as the principal illustration a major anaerobic microbial species of the GI tract,B.fragilis,as a prime example of an abundant, G-bacteria of the microbiome;it should be kept in mind that at least 1000 additional species of GI tract bacteria have both a dynamic and opportunistic potential to similarly contribute to (1)the homeostatic maintenance of the GI tract–CNS axis in health and(2)the development of both systemic and inflam matory neurodegenerative disease(Fig.1).First,B.fragilisproduces 3 major classes of toxic secretory products:amyloid,LPS,and enterotoxins.They are briefl define here.
1.Amyloid.The termamyloidis generic for any insoluble, lipoprotein-enriched molecule exhibiting β-pleated sheet structures oriented perpendicular to its fibrilla axis;a remarkably wide variety of microbiome-resident species, particularly bacteria and fungi,generate significan quantities of functional amyloids and related microbial exudates.6–10Interestingly,well over half of all known proteins contain“unstructured”regions of amino acids that are intrinsically amyloidogenic.7–11For example,the amyloids that characterize AD consist largely of“perivascular”amyloid deposits enriched in the 40-amino acid Aβ40 peptide;“parenchymal”amyloid,enriched in the 42-amino acid Aβ42 peptide;and“nuclear”amyloids, which contain highly complex mixtures of lipoprotein fibril and dense amyloid aggregates.9–12Interestingly,common tertiary protein structures or pathogenicassociated molecular patterns between microbial and host amyloids(1)may be involved in propagation or acceleration of amyloidogenesis through“molecular mimicry” and(2)may be important in the priming of the host innate immune system and/or microglial cell activation by microbiota,a factor that may enhance inflammato y and immunologic responses to AD amyloid.9–16
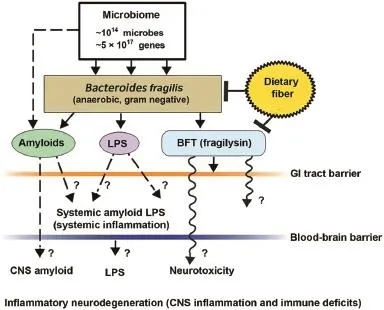
Fig.1.Schematic representation of the potential contribution of gastrointestinal(GI)tract microbiome-derived amyloids,lipopolysaccharides (LPS),and endotoxins to systemic inflammatio and/or to central nervous system(CNS)neurotoxicity and immune deficits One of the major microbial species in the human GI tract isBacteroides fragilis.In concert with other microbiome components,this anaerobic gram-negative bacillus generates complex mixtures of amyloid,LPS,and/or potent neurotoxins such asB.fragilistoxin(BFT;fragilysin),one of the most potent proinflammato y molecules known.20–23Fragilysin is recognized to both(1)increase the paracellular permeability of the intestinal epithelium largely via the dissolution of tight junctions in epithelial cells16–19and(2)at low,physiologically realistic (nanomolar)concentrations,induce robust inflammato y signaling(such as activation of nuclear factor kappa B(NF-κB)-DNA binding)in human brain cells in primary culture20–24(unpublished observations).The presence ofB. fragilisand/orB.fragilis-derived amyloids,LPSs,or endotoxins such as BFT in the bloodstream during systemic inflammatio(bacteremia)is more common than any for any other anaerobe of the microbiome.15–23In combination with other facultative/obligate anaerobic microbes,their secretory exudates are extremely powerful proinflammato y and innate immune system activators in the CNS once they pass GI tract and blood–brain barriers.These actions would further induce vascular permeability,trigger host immunogenicity,and induce the generation of reactive oxygen species and NF-κB signaling.For example, (1)these neuropathogenic signals further promote amyloid aggregation and inflammato y degeneration characteristic of age-related neurologic diseases such as AD and other neurologic disorders that exhibit defective Aβ42 peptide clearance mechanisms and progressive amyloidogenesis,9,24,25and(2)B.fragilis-derived toxins are also responsible for the majority of localized abscesses within the cranium.24,25As a major component of the human microbiome,GI tract microbial sources of amyloid,LPS,and/or other microbial-derived endotoxins have a remarkable potential to contribute to both systemic amyloid and CNS amyloid burden in their respective CNS compartments.This contribution of noxious,proinflammato y molecules from the GI tract microbiome may be increasingly important during the course of aging,when both the GI tract and blood–brain barriers become significanty more permeable.14–23Interestingly,high intake of dietary fibe is a strong inhibitor ofB.fragilisabundance and proliferation in the human GI tract and as such is a potent inhibitor of the neurotoxicB.fragilis-derived amyloids,LPSs,and enterotoxins.Hence dietary fibe-mediated suppression ofB.fragilisabundance may be beneficia for both human GI tract and CNS health.14,21–26
2.LPS.Distinguishing components of the outer leafle of the outer membrane of G-bacteria shed into the extracellular space,LPSs have historically been thought to play some host-pathogen immune-evasion strategy useful to bacterial survival while eliciting strong immune and proinflammato y responses within the host.8,10,17–20Although LPSs contain large and hypervariable polysaccharide/oligosaccharide regions,a relatively conserved lipid region(known as the“lipid A”core)is the endotoxic and biologically active moiety that is responsible for the induction of systemic inflammatio and ensuing septic shock.9–12,14–18Interestingly,it has been recently shown by several independent groups that (1)LPS strongly promotes amyloid aggregation,9,10,20and(2)BF-LPS is one of the most potent inducers of nuclear factor kappa B(NF-κB)activation in primary human neuronal-glial cocultures known.17–20
3.Enterotoxins:fragilysin.This is also known as BFT,a Zn2+-requiring metalloprotease highly cytopathic to intestinal epithelial cells with the following characteristics:(1) possesses broad proteolytic specificit of hydrolytic cleavage adjacent to leucines;17–19(2)hydrolyzes gelatin, fibrin gen,and extracellular matrix proteins such as the extracellular domain of type 1 transmembrane proteins such as E-cadherin;15,19(3)rapidly degrades intracellular proteins such as actin and myosin;15,16,20and(4)disrupts the tight-junction zona occludens-1 protein of the intestinal epithelial cell.16–21Taken together,these recent find ings indicate that fragilysin’s pathogenic actions(1) interrupt the integrity of intercellular adhesion;15,18,19(2) increase mucosal permeability and the permeability of the intestinal epithelium to GI tract contents;14–17and(3) induce epithelial cell–cell detachment,laying the morphologic basis for a compromised and“leaky”GI tract barrier.17–23
Clinically,the presence of BF-LPS in the blood serum signifie(1)a breach of epithelial cell–GI tract barriers;(2)an early,major,and contributing factor to the initiation and propagation of systemic inflammato y disease;and(3)the firs major biophysical barrier crossed for complex mixtures of proinflammato y toxins of the GI tract contents to gain access to the systemic circulation,and onward to the blood–brain barrier of the CNS.It would be surprising if BF-LPS were not similarly effective in weakening tight junctions of the blood–brain barrier,and recent data support this concept.17,19,20Furthermore,in primary human neuronal-glial cocultures BF-LPS is(1)an unusually potent inducer of the proinflammato y transcription factor NF-κB(p50/p65 complex)and hence proinflammato y gene expression programs and(2)recognized by toll-like receptors 2 and 4(TLR2,TLR4)and/or cluster of differentiation 14(CD14)microglial cell receptors,as are the amyloid peptides that characterize AD neuropathology.9,10,20
Of related interest is thatB.fragilisand its toxins,such as BF-LPS and fragilysin,also appear to play critical roles in the postmortem microbiome,when at the point of death the human microbiome rapidly transforms into the“thanato-microbiome”(thanatos-,Greek,death)that plays a primary role in the rapid decomposition of host tissues.9,21
3.Proliferation of BF-LPS in the absence of dietary fber
The trillions of micro-organisms that constitute the human GI tract microbiome are reliant on sufficien sources of complex dietary fiber(sometimes called“roughage”)to promote and maintain their dynamism and diversity,which support efficien microbiome–host functions.Indeed,microbiome-accessible carbohydrates found in dietary fibe provide a critical contribution to shaping the microbial ecosystem of the GI tract;their individual distributions are notably altered in high-fat,highcholesterol“Western diets”(high in fat and processed carbohydrates and low in fiber compared with more traditional“Paleolithic diets”(moderate in fat and processed carbohydrates and high in fiber)16–18Several recent studies indicate that in high-fat,high-cholesterol diets deprived of sufficien dietary fibe,there is an upset in the balance of normal bacterial stoichiometry conducive to human health and a proliferation inB. fragilisand similar opportunistic anaerobic bacteria.14–20It is perhaps not too surprising that diets low in the fibe provided by complex carbohydrates that favorB.fragilisproliferation are also associated with increased amyloid,LPS,and endotoxins such as fragilysin,which are systemically detrimental to health, supporting leaky barrier functions,which are ultimately proinflammato y toward the PNS and CNS in a variety of ways.
4.Concluding remarks
The microbiome represents a dynamic ecosystem in which structure and function are influence by multiple interactive factors including age,maternal influences antibiotic or drug presence,environment,exercise,metabolism,and stress. Microbial amyloids,LPSs,and endotoxins of abundant bacterial species such asB.fragilismay be the most obvious examples of microbiome-resident micro-organisms that can affect GI tract and blood–brain barrier function,stimulate proinflammato y signaling systemically,and promote inflam matory neurodegeneration of the PNS and CNS.It has been known for some time that the functional optimization of the microbiome–GI tract–CNS axis,via studies on GI tract“microbial imbalance”or“dysbiosis”in germ-free animals,the administration of probiotics,and bacterial infections with enteric pathogens have strong effects that can ultimately modulate cognitive behavior,learning,memory,and healthy brain aging.22,23Recent studies underscore the concept that just as exercise requires the replenishment of efficien and sufficien energy stores,diets that support optimal CNS and cognitive health require both a healthy dietary intake and sufficien dietary fibe to ensure the“best possible performance”of our microbiome,14–20leading to the optimization of microbial speciation and stoichiometry,functional symbiosis,and communication along the microbiome–GI tract–CNS axis.
Acknowledgments
This work was presented in part at the Society for Neuroscience Annual Meeting,October 17–21,2015,Chicago,IL, USA;at the Association for Research in Vision and Ophthalmology Annual Conference,May 1–5,2016,Seattle,WA, USA;and at the Scientifi Committee of Aging and Antiaging,Gerontological Society of China,May 6–8,2016, Shanghai,China.Sincere thanks are extended to the late Dr. James M.Hill and Drs.Cristoph Eicken,Chris Hebel,Yuhai Zhao,and Vivian Jaber for LPS extracts,BFT,and other microbiome-derived proinflammato y factors,DNA array, and RNA sequencing data and initial data interpretation, and to Darlene Guillot and Aileen Ivy Pogue,the latter for expert technical assistance in the preparation of this manuscript.Research on microRNA and messenger RNA in the Lukiw laboratory involving the innate immune response, neuroinflammation amyloidogenesis,and the microbiome in AD,prion disease,age-related macular degeneration,and other neurologic or retinal diseases was supported through an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness;the Louisiana Biotechnology Research Network,and National Institutes of Health grants NEI EY006311,NIA AG18031,and NIA AG038834.
Competing interests
The author declares no competing financia interests.
1.Hamady M,Knight R.Microbial community profilin for human microbiome projects:tools,techniques,and challenges.Genome Res2009;19:1141–52.
2.Turnbaugh PJ,Ley RE,Hamady M,Fraser-Liggett CM,Knight R,Gordon JI.The human microbiome project.Nature2007;449:804–10.
3.Zhu B,Wang X,Li L.Human gut microbiome:the second genome of human body.Protein Cell2010;1:718–25.
4.Jandhyala SM,Talukdar R,Subramanyam C,Vuyyuru H,Sasikala M, Nageshwar Reddy D.Role of the normal gut microbiota.World J Gastroenterol2015;21:8787–803.
5.Sankar SA,Lagier JC,Pontarotti P,Raoult D,Fournier PE.The human gut microbiome,a taxonomic conundrum.Syst Appl Microbiol2015;38: 276–86.
6.Hugon P,Dufour JC,Colson P,Fournier PE,Sallah K,Raoult D.A comprehensive repertoire of prokaryotic species identifie in human beings.Lancet Infect Dis2015;15:1211–9.
7.Buxbaum JN,Linke RP.A molecular history of the amyloidoses.J Mol Biol2012;421:142–59.
8.Blanco LP,Evans ML,Smith DR,Badtke MP,Chapman MR.Diversity, biogenesis and function of microbial amyloids.Trends Microbiol2012;20: 66–73.
9.Zhao Y,Dua P,Lukiw WJ.Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer’s disease(AD).J Alzheimers Dis Parkinsonism2015;5:177.doi:10.4172/2161-0460.1000177
10.Hill JM,Lukiw WJ.Microbial-generated amyloids and Alzheimer’s disease (AD).Front AgingNeurosci2015;7:9.doi:10.3389/fnagi. 2015.00009
11.Sampson TR,Mazmanian SK.Control of brain development,function, and behavior by the microbiome cell host microbe.Cell Host Microbe2015;17:565–76.
12.von Mikecz A.Pathology and function of nuclear amyloid.Protein homeostasis matters.Nucleus2014;5:311–7.
13.Maldonado RF,Sá-Correia I,Valvano MA.Lipopolysaccharide modificatio in Gram-negative bacteria during chronic infection.FEMS Microbiol Rev2016;40:480–93.
14.Barczynska R,Slizewska K,Litwin M,Szalecki M,Kapusniak J.Effects of dietary fibe preparations made from maize starch on the growth and activity of selected bacteria from the Firmicutes,Bacteroidetes,and Actinobacteria phyla in fecal samples from obese children.Acta Biochim Pol2016;63:261–6.
15.Choi VM,Herrou J,Hecht AL,Teoh WP,Turner JR,Crosson S,et al. Activation ofBacteroides fragilistoxin by a novel bacterial protease contributes to anaerobic sepsis in mice.Nat Med2016;22:563–7.
16.Sonnenburg ED,Smits SA,Tikhonov M,Higginbottom SK,Wingreen NS, Sonnenburg JL.Diet-induced extinction in gut microbiota compound over generations.Nature2016;529:212–5.
17.Sears CL,Geis AL,Housseau F.Bacteroides fragilissubverts mucosal biology:from symbiont to colon carcinogenesis.J Clin Invest2014;124: 4166–72.
18.Vines RR,Perdue SS,Moncrief JS,Sentz DR,Barroso LA,Wright RL, et al.Fragilysin,the enterotoxin fromBacteroides fragilis,enhances the serum antibody response to antigen co-administered by the intranasal route.Vaccine2000;19:655–60.
19.Varatharaj A,Galea I.The blood-brain barrier in systemic inflammationBrain Behav Immun2016;doi:10.1016/j.bbi.2016.03.010;[Epub ahead of print].
20.Lukiw WJ.Bacteroidetes fragilislipopolysaccharide(BF-LPS)and inflammato y signaling in Alzheimer’s disease(AD).Front Microbiol2016;7:1544.doi:10.3389/fmicb.2016.01544
21.Javan GT,Finley SJ,Abidin Z,Mulle JG.The Thanatomicrobiome: a missing piece of the microbial puzzle of death.Front Microbiol2016;7:225.doi:10.3389/fmicb.2016.00225
22.Zhan LS,Davies SS.Microbial metabolism of dietary components to bioactive metabolites:opportunities for new therapeutic interventions.Genome Med2016;8:46.doi:10.1186/s13073-016-0296-x
23.Troletti CD,de Goede P,Kamermans A,de Vries HE.Molecular alterations of the blood-brain barrier under inflammato y conditions:the role of endothelial to mesenchymal transition.Biochim Biophys Acta2016;1862:452–60.
24.Greenlee JE.Brain abscess.Available at:http://www.merckmanuals.com/ professional/neurologic-disorders/brain-infections/brain-abscess; 2016 [accessed 15.08.2016].
25.Clement C,Hill JM,Dua P,Culicchia F,Lukiw WJ.Analysis of RNA from Alzheimer’s disease post-mortem brain tissues.Mol Neurobiol2016;53:1322–8.
26.Zhao Y,Jaber V,Lukiw WJ.Over-expressed pathogenic miRNAs in Alzheimer’s disease and prion disease drive deficit in TREM2-mediated Aβ42 peptide clearance.Front Aging Neurosci2016;8:140.doi:10.3389/ fnagi.2016.00140
Received 20 June 2016;accepted 22 June 2016
Available online 24 August 2016
Peer review under responsibility of Shanghai University of Sport.
E-mail address:wlukiw@lsuhsc.edu.
http://dx.doi.org/10.1016/j.jshs.2016.08.008
2095-2546/©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Journal of Sport and Health Science的其它文章
- Biomarker-guided classificatio scheme of neurodegenerative diseases
- Mechanism of neurodegeneration through tau and therapy for Alzheimer’s disease
- Examining the relationship between sport and health among USA women: An analysis of the Behavioral Risk Factor Surveillance System
- Longitudinal trajectories of physical activity in women using latent class growth analysis:The WIN Study
- Evidence of a conservative gait strategy in athletes with a history of concussions
- Total soy saponins improve the antioxidant capacity of the myocardium and exercise ability in exhausted rats
