Biomarker-guided classificatio scheme of neurodegenerative diseases
2016-02-05FilippoBldcciSimoneListFrncescoGrciUbldoBonuccelliNicolToschiHrldHmpel
Filippo Bldcci,Simone List,Frncesco Grci,Ubldo Bonuccelli, Nicol Toschi,Hrld Hmpel*
aDepartment of Clinical and Experimental Medicine,University of Pisa,Pisa 56126,Italy
bInstitute of Memory and Alzheimer’s Disease(IM2A),Brain and Spine Institute(ICM)UMR S 1127,Department of Neurology, Pitié-Saltrire Hospital,Paris 75651,France
cSorbonne Universities,Pierre and Marie Curie University,Paris VI,Paris 75005,France
dAXA Research Fund and Pierre and Marie Curie University Chair,Paris 75005,France
eIHU-A-ICM—Paris Institute of Translational Neuroscience,Pitié-Salpêtrière Hospital,Paris 75651,France
fDepartment of Biomedicine and Prevention,Faculty of Medicine,University of Rome Tor Vergata,Rome 00173,Italy
gSan Raffaele Cassino Nursing Home,Cassino(FR)03043,Italy
hDepartment of Radiology,Athinoula A.Martinos Center for Biomedical Imaging,Massachusetts General Hospital and Harvard Medical School,
Biomarker-guided classificatio scheme of neurodegenerative diseases
Filippo Baldaccia,b,c,Simone Listad,e,Francesco Garacif,g,Ubaldo Bonuccellia, Nicola Toschif,h,Harald Hampelb,c,d,*
aDepartment of Clinical and Experimental Medicine,University of Pisa,Pisa 56126,Italy
bInstitute of Memory and Alzheimer’s Disease(IM2A),Brain and Spine Institute(ICM)UMR S 1127,Department of Neurology, Pitié-Saltrire Hospital,Paris 75651,France
cSorbonne Universities,Pierre and Marie Curie University,Paris VI,Paris 75005,France
dAXA Research Fund and Pierre and Marie Curie University Chair,Paris 75005,France
eIHU-A-ICM—Paris Institute of Translational Neuroscience,Pitié-Salpêtrière Hospital,Paris 75651,France
fDepartment of Biomedicine and Prevention,Faculty of Medicine,University of Rome Tor Vergata,Rome 00173,Italy
gSan Raffaele Cassino Nursing Home,Cassino(FR)03043,Italy
hDepartment of Radiology,Athinoula A.Martinos Center for Biomedical Imaging,Massachusetts General Hospital and Harvard Medical School,
Charlestown,02129 MA,USA
1.The complex spectrum of neurodegenerative diseases
Epidemiologic data indicate that neurodegenerative diseases (NDDs)show high prevalence with a trend of a progressively growing incidence,especially in aging societies.This presents an increasing social and economic burden.In individuals>60 years of age,the prevalence of Alzheimer’s disease(AD)is estimated to be 40 of 1000 persons with an annual incidence of 34 per 1000 persons.1Parkinson’s disease(PD)is considered the second most common NDD after AD,2and,similarly to AD, its prevalence consistently increases with age.
The pathologic termneurodegenerationrefers to a heterogeneous group of progressively evolving central nervous system (CNS)and brain diseases.It is an“umbrella”term indicating gradual structural neuronal loss with functional consequences due to the abnormal accumulation of misfolded and dysfunctional proteins within the complex nervous system.Therefore, NDDs are better classifie as protein misfolding disorders or proteinopathies of the CNS and the brain.For consistency reasons,however,we will use the traditional term of NDDs throughout this perspective.
NDDs may be either monogenic(i.e.,characterized by mutations)like Huntington disease,with more-or-less monolinear mechanistic pathophysiology,or complex polygenic(e.g.,sporadic late-onset AD),that is,involving several pathophysiological and nonlinear progressive biological mechanisms.3Lifestyle, environmental risk factors,and variability of individual genetic polymorphisms contribute to the onset and progression of the idiopathic and polygenic forms of NDDs.In this perspective,we focus on the complex multifactorial NDDs that are most relevant and represent the major clinical phenotypes,including cognitive impairment and dementia in the general aging population.
Among emerging theories,it has been postulated that a specifi misfolded protein characteristic of the pathobiochemistry in a particular NDD can spread from one cell to another(both transcellularly and synaptically)in a prionlike manner,thus potentially inducing complex neural network alterations,which are structural and functional system substrates of clinical symptomatic progression at advanced stages of NDDs.4,5Notably,these aberrantly altered proteins represent candidate targets for therapy development and/or may be utilized as specifi diagnostic and prognostic biomarkers of disease.The neurodegenerative process is hypothesized to initiate focally and then propagate strategically across wider brain regions through complex networks,in line with specifi dynamic progression patterns likely define by genetic drivers and triggers during the initiation stages of the proteinopathy.4,6Hence,as a consequence,the emerging pathophysiological mechanisms,the subsequent neuropathology,and both the time of onset and dynamics of progression through time and space and the emergence of the late-stage clinical phenotype of NDDs can vary substantially between postulated clinical disease groups,subsets of disease,and individuals grouped into those operationalized categories.For instance,“typical AD”—that is,the hippocampal variant of AD according to the International Working Group-2 diagnostic criteria7—shows,at the preclinical asymptomatic stage,a marked alteration and subsequent disruption of the default mode network,6whereas primaryprogressive aphasia displays a pattern of network dysfunction mainly localized in language brain areas.4However,several cases of amyloid beta(Aβ)pathophysiology may also exhibit atypical patterns of network change leading to disconnection and disintegration.7The atypical variants—namely,frontal, posterior,and logopenic—present behavioral,visual,or language symptoms,which from a clinical point of view overlap those involved in other NDDs.These include behavioral frontotemporal dementia(FTD),generally categorized as a tauopathy;dementia with Lewy bodies(DLB),which is linked to synucleinopathies;and primary progressive aphasia, classifie as transactive-response DNA-binding protein 43 proteinopathy or,alternatively,as a tauopathy.As a result,distinct proteinopathies may induce different patterns of early functional and anatomic alteration of brain networks in NDDs. Moreover,it remains unclear how misfolded proteins are translating into distinct patterns of complex network impairment over longer periods of years and decades and,ultimately,result in very different clinical motor and/or cognitive syndromes.
2.An outdated vision
On this basis of an inherent genetic and biological heterogeneity,determination of an early and accurate diagnosis of NDDs merely from descriptive clinical symptoms and syndromes is most challenging and questionable.In the past 30 years,international diagnostic consensus criteria for diagnosing NDDs in the clinical setting scenario have been formulated more or less slowly,refine and updated to ameliorate the phenotypical characterization of disease categories associated and compared with fina postmortem pathologic examination. However,in spite of intense efforts,the clinical criteria for diagnosing NDDs still remain largely variable in terms of delineation between categories and their subsets,diagnostic and classificato y accuracy,and clinical practicality.7–13Postmortem neuropathologic studies confi m that the clinical diagnostic criteria of PD are very good,in terms of both sensitivity(90%)and specificit (around 100%),at distinguishing PD from other forms of Parkinsonism,provided that they are assessed in expert centers highly specialized in the diagnosis of movement disorders.10For AD,the neuropathologic“gold standards”14have shown that purely clinical diagnostic criteria15exhibit correlative accuracy in terms of sensitivity(81%)and specificit (70%),mostly in specialist settings.The clinical diagnostic criteria for other NDDs exhibit high specificit but low sensitivity;in this respect,the clinical diagnostic criteria for DLB have very good specificit(95%)but poor sensitivity(around 30%),16although according to the third report of the DLB Consortium,13,16the sensitivity has recently improved.Similarly,the criteria for progressive supranuclear palsy display a specificit of 100%and a sensitivity of 50%.12Moreover, pathologic examination9has shown that the accuracy of the clinical diagnostic criteria for corticobasal degeneration,a rare form of atypical Parkinsonism,is unsatisfactory.This applies to the clinical diagnostic criteria of FTD11as well;indeed,as opposed to a specifi disease category,FTD is considered a disease spectrum in itself clinically characterized by progressive behavioral,executive,and language impairment,encompassing symptoms typical of motor neuron diseases(i.e.,amyotrophic lateral sclerosis),and Parkinsonism.17Notably, the overlapping impact of cerebrovascular alterations in neurodegenerative processes should also be carefully considered in the clinicopathologic interpretation of any NDDs.18In conclusion,in most cases,a classificatio system based purely on a clinical descriptive diagnosis is largely heterogeneous, practically highly unreliable,and not very helpful as a basis of therapy development across the NDD spectrum.The traditional clinicopathologic diagnosis scheme is largely unable to specifi cally consider the diverse genetic underpinnings and multiple pathophysiological mechanisms underlying the complex dimensional spectrum of NDDs.
Given the substantial discrepancies commonly found between clinical and pathologic diagnoses,the idea of an existing biunivocal relationship between a definit clinical phenotype and its corresponding neuropathology appears misleading and unrealistic.In addition,proteinopathies may largely appear in combination with effects associated with brain aging and cerebrovascular disease,thus arranging a complex multidimensional and not categorical clinicopathologiccontinuumexhibiting mixed patterns of partly converging neurodegenerative mechanisms with overlapping and converging comorbidities.3,7,8
Currently,based on the pathologic deposition of misfolded proteins in the CNS,NDDs may be categorized as follows: cerebral amyloidosis(AD),tauopathies(progressive supranuclear palsy,corticobasal degeneration,behavioral variant of FTD,progressive nonfluen aphasia of FTD),synucleinopathies (PD,DLB,multiple system atrophy),and transactive response DNA-binding protein 43 proteinopathies(semantic variant of FTD,behavioral FTD,amyotrophic lateral sclerosis).5
However,postmortem neuropathologic examination frequently reveals the deposition of multiple misfolded proteins, reflectin a mixed pattern of proteinopathies in an examined patient.DLB and PD with dementia often present overlapping Aβ-related pathology.Several studies have reported the accumulation of α-synuclein within Lewy body inclusions in the brains of AD patients.8In addition,minor strokes,small vessel disease,and cerebral amyloid angiopathy,alone or in combination,are associated with high prevalence of progressive neurodegeneration,thus making it additionally challenging to precisely label and dissect any pathologic categorization.18In this regard,a community-based neuropathologic study has reported a consistent variability in the expression of several combinations of multiple proteinopathies within the CNS of elderly subjects.This demonstrates that the coexistence of mixed neuropathologies is commonly observed in the clinical setting19and increasingly during aging.
3.Forward an adaptive perspective
In view of this,the way forward is to expand the scope of detection and diagnosis in definin biological criteria of NDDs, as in other disease fields Discovery,development,and validation of distinct biological/pathophysiological biomarkers and genetic drivers/triggers of NDDs is the key to step up progress ofin vivodiagnostic,prognostic,and therapeutic(biomarkerguided therapy)technologies.In particular,(1)postmortemexamination is not sufficien or practical for early detection and diagnosis purposes;(2)postmortem examination represents not an early dynamicin vivoassessment but rather a late-stage result of visible traces of several pathophysiological mechanisms potentially leading to different dynamics of onset and progression;(3)asymptomatic and cognitively healthy elderly subjects are likely to display postmortem neuropathologic deposits of misfolded protein;(4)multiple misfolded deposits of proteins detected within the CNS may coexist in the same clinical phenotype or in the same patient;(5)a clinical phenotype may exhibit more than 1 aberrant process of protein deposition;and(6)a general agreement on the definitio of pathologic vascular processes and dementia is still missing.8,18
To overcome stagnation in effective early disease detection and drug development for NDDs,a paradigm shift is evolving from clinically and clinicopathologic descriptive phenotypes to clinicobiological definitions toward the ultimate solutions of a true“precision medicine”.
The precision medicine paradigm aims at targeting the issue of genetic,molecular,and clinical heterogeneity by identifying a person’s comprehensive and specifi pattern of risk,reflecte by polygenetic as well as epigenetic20variants,such as high-tomedium risk mutations,structural,functional,and metabolic imaging indicators,and flui biomarkers,then detecting and delineating the array of molecular pathophysiological mechanisms underlying disease processes and finaly administering a preventive or therapeutic intervention that is specifi(i.e.,“tailored or customized”)to the identifie molecular pattern and biological disease progression pattern in time and space. This paradigm is clearly in contrast with the traditional idea that NDDs are homogeneous clinicopathologic and clinicobiological entities and can be treated with the resulting, largely inadequate“one-drug-fits-all approach.21Notably,the scientificaly advanced,more matured translational research field of oncology or rheumatology have already begun the implementation and practical use of precision medicine and could serve as an inspirational model for neuroscience,neurology,and psychiatry.This approach is promising breakthroughs in the future of medicine,including the almost isolated and detached fiel of complex psychiatric disorders.21Thanks to the emergence of systems theory and the complex neural network paradigm,the implementation of large-scale biological databases,and the development of high-throughput screening methods—the“-omic”tools—which can aid in characterizing disease biomarkers,precision medicine is likely to be practically applied broadly across a vast number of human diseases. Such an integrative and interdisciplinary approach is possible through the maturing fiel of systems biology.22
Translating innovative concepts from theory to practice is undoubtedly challenging,especially in the multifaceted and still largely unexplored fiel of neurologic and psychiatric brain diseases.In summary,we need an innovative,practical model allowing patient stratificatio in a way that is pertinent to clinical trials.To this end,for patients showing cognitive impairment supposedly associated with a proteinopathy or neurodegeneration,we propose a practical diagnostic approach structured on 3 different assessment levels:(1)traditional,clinical descriptive;(2)transitional,both clinical and biomarker based;and(3)an unbiased biomarker-guideda posterioriaggregation scheme.Our initial aim will be to compare the 3 levels of stratificatio to overcome traditional reductionistic and obsolete classificatio models.The firs level will be based on a purely clinical diagnostic categorization,in which we will study the diagnostic accuracy of core biomarkers(i.e.,Aβ1–42,p-tau, t-tau)as well as novel emerging candidate biomarkers in discriminating betweena prioridefine clinical groups(for instance,typical AD dementia patientsvs.probable DLB (dementia stage)patients).The second level will encompass a combined clinical- and a biomarker-driven diagnostic approach.In detail,we will test the performance of the array of emerging and novel candidate biomarkers to differentiate subgroups of patients within the dementia and mild cognitive impairment categories as well as in asymptomatic subjects stratifie according to the unbiased descriptive“A/T/N”system for characterizing the AD spectrum,recently proposed by Jack and colleagues.23In brief,this unbiased classificatio scheme—theoretically appropriate for any NDD—is based on 3 binary biomarker categories that reflec the core pathophysiological mechanisms of AD:in particular,“A”refers to Aβ pathology,“T”to hyperphosphorilated tau(p-tau)pathology,and“N”to total tau(t-tau)or other biomarkers of neurodegeneration.23In the third level of analysis,we will preserve the conceptual distinction of the severity of cognitive impairment by identifying 3“macroclinical”categories of subjects/patients:(1) asymptomatic subjects,(2)prodromal or mild cognitive impairment subjects,and(3)syndromic demented patients.Successively,within these categorical diagnostic macrogroups,we will employ advanced machine learning techniques to defin novel patient subsets in an unbiaseda posterioriclustering process based on a combination of genetic polymorphisms,blood-and cerebrospinalflui (CSF)-based flui biomarkers,and multimodal neuroimaging data.We will focus on unsupervised pattern recognition methods with the objective of detecting higher-order,nonlinear associations within the multidimensional data point represented by each patient in the highly heterogeneous space constituted by genetic,imaging,and biohumoral data.This will result in novel innovative,unbiased categorizations based on effective degrees of freedom present in a subject’s enlarged biomarker panel,hence independent of the large variance clinical phenotypes,and these degrees of freedom will defin and span the effective dimensions of the biological continuum of NDDs.In turn,this could form the basis for formulating mechanistic(rather than purely datadriven)integrative models,with the goal of explaining disease endophenotypes and phenotypes in an individualized fashion.24The parameters estimated by these models could aid in early detection,diagnosis,and prognosis and eventually function as so-calledpreventive biomarkers(i.e.,aid in stratifying the population into risk classes).24As a fina result of this articulated process,a set of optimal cutoffs and,hence,an unbiased descriptive panel displaying a sequence of categorizations for NDDs may be developed(Fig.1).
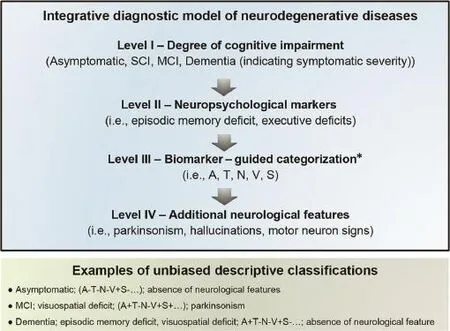
Fig.1.Unbiased descriptive classificatio system of neurodegenerative dementia integrating clinical and biomarker information.Level I indicates the severity of symptomatic impairment ranging from asymptomatic to dementia.Level II indicates differentiated neuropsychological characterization.Level III indicates the evolving array of topographical and pathophysiological biomarkers.Level IV indicates additional neurologic signs and symptoms useful for categorization,such as extrapyramidal signs,cerebellar ataxia,etc.*Evolving array of validated pathophysiological and topographic biomarkers(genetic,biochemical,neuroimaging). A=Aβ amyloid;MCI=mild cognitive impairment;N=t-tau;S=α-synuclein;SCI=subjective cognitive impairment;T=p-tau;V=vascular.
During the past decade,conceptual shifts have occurred in the fielof NDDs,and these diseases are now considered as partly overlapping,dynamic,nonlinear progressive“dimensions”that reside among a wide spectrum of brain proteinopathies.Future breakthrough discoveries are likely to happen through transfertilization from more advanced research fields such as oncology,by means of the innovative precision medicine and systems biology paradigms.This reintegration of neuroscience and neurology into biological medicine will be characterized by advancing biomarker research objectively reflectin the multifaceted pathophysiological profil of sporadic and polygenic NDDs.This will allow the characterization and identificatio of disease even at the earliest asymptomatic preclinical stage through population-based screening efforts facilitated by the Precision Medicine Initiative.In this context, given that early biomarker-guided intervention may offer the best chance of therapeutic success,the preclinical stage of AD and other NDDs has become a major research focus.To date, very little evidence has been established on this uncharted“silent stage”.25A clarificatio is needed about the definition and lexicon,the limits,the natural history,the markers of risk and progression,and the ethical consequence of detecting the disease at this asymptomatic stage and how to effectively operationalize precision medicine.
Acknowledgment
Harald Hampel is supported by the AXA Research Fund, the Fondation Université Pierre et Marie Curie,and the Fondation pour la Recherche sur Alzheimer,Paris,France.The research leading to these results has received funding from the program Investissements d’avenir ANR-10-IAIHU-06 (Agence Nationale de la Recherche-10-IA Agence Institut Hospitalo-Universitaire-6).
Authors’contributions
FB conceived of the study and drafted the manuscript;SL conceived of the study and helped to draft the manuscript;FG reviewed the manuscript;UB reviewed the manuscript;NT reviewed and helped to draft the manuscript;and HH conceived of the study and reviewed the manuscript.All authors have read and approved the fina version of the manuscript and agree with the order of presentation of the authors.
Disclosure statement
Filippo Baldacci,Nicola Toschi,and Francesco Garaci declare no competing financia interests.
Simone Lista has received lecture honoraria from Roche.
Ubaldo Bonuccelli has received fees for consultation from GlaxoSmithKline and Eisai Co.Ltd.and for talks from Novartis,GlaxoSmithKline,and Lundbeck.
Harald Hampel declares no competing financia interests related to the present article.He serves as senior associate editor for the journalAlzheimer’s&Dementiaand has been a scientifi consultant and/or speaker and/or attended scientifi advisory boards of Axovant Sciences Ltd.,Anavex Life Science Corp.,Eli Lilly and Company,GE Healthcare,Cytox Ltd.,Jung Diagnostics,Roche Holding AG,Biogen Inc.,Takeda and Zinfandel Pharmaceuticals,and Oryzon Genomics S.A.He also receives research support from the Association for Alzheimer Research(Paris),Pierre and Marie Curie University(Paris),andPfize and Avid Radiopharmaceuticals(paid to institution)and has patents as coinventor but received no royalties as follows:
·A patentin vitromultiparameter determination method for the diagnosis and early diagnosis of neurodegenerative disorders.Patent number:8916388 Issued.
·A patentin vitroprocedure for diagnosis and early diagnosis of neurodegenerative diseases.Patent number:8298784 Issued.
·A patent neurodegenerative markers for psychiatric conditions.Publication number:20120196300 Issued.
·A patentin vitromultiparameter determination method for the diagnosis and early diagnosis of neurodegenerative disorders.Publication number:20100062463 Issued.
·A patentin vitromethod for the diagnosis and early diagnosis of neurodegenerative disorders.Publication number: 20100035286 Issued.
·A patentin vitroprocedure for diagnosis and early diagnosis of neurodegenerative diseases. Publication number: 20090263822 Issued.
·A patentinvitromethod for the diagnosis of neurodegenerative diseases.Patent number:7547553 Issued.
·A patent CSF diagnostic in vitro method for diagnosis of dementias and neuroinflammato y diseases.Publication number:20080206797 Issued.
·A patentinvitromethod for the diagnosis of neurodegenerative diseases. Publication number: 20080199966 Issued.
·A patent neurodegenerative markers for psychiatric conditions.Publication number:20080131921 Issued.
1.Fiest KM,Roberts JI,Maxwell CJ,Hogan DB,Smith EE,Frolkis A,et al. The prevalence and incidence of dementia due to Alzheimer’s disease:a systematic review and meta-analysis.Can J Neurol Sci2016;43(Suppl.1): S51–82.
2.Kalia LV,Lang AE.Parkinson’s disease.Lancet2015;386:896–912.
3.Scheltens P,Blennow K,Breteler MMB,de Strooper B,Frisoni GB, Salloway S,et al.Alzheimer’s disease.Lancet2016;388:505–17.
4.Warren JD,Rohrer JD,Schott JM,Fox NC,Hardy J,Rossor MN. Molecular nexopathies:a new paradigm of neurodegenerative disease.Trends Neurosci2013;36:561–9.
5.Kovacs GG.Molecular pathological classificatio of neurodegenerative diseases:turning towards precision medicine.Int J Mol Sci2016;17:pii: E189.doi:10.3390/ijms17020189
6.Pievani M,de Haan W,Wu T,Seeley WW,Frisoni GB.Functional network disruption in the degenerative dementias.Lancet Neurol2011;10:829–43.
7.Dubois B,Feldman HH,Jacova C,Hampel H,Molinuevo JL,Blennow K, et al.Advancing research diagnostic criteria for Alzheimer’s disease:the IWG-2 criteria.Lancet Neurol2014;13:614–29.
8.Hyman BT,Phelps CH,Beach TG,Bigio EH,Cairns NJ,Carrillo MC, et al.National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease.Alzheimers Dement2012;8:1–13.
9.Alexander SK,Rittman T,Xuereb JH,Bak TH,Hodges JR,Rowe JB. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration.J Neurol Neurosurg Psychiatry2014;85:925–9.
10.Hughes AJ,Daniel SE,Ben-Shlomo Y,Lees AJ.The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service.Brain2002;125:861–70.
11.Neary D,Snowden JS,Gustafson L,Passant U,Stuss D,Black S,et al. Frontotemporal lobar degeneration:a consensus on clinical diagnostic criteria.Neurology1998;51:1546–54.
12.Litvan I,Agid Y,Calne D,Campbell G,Dubois B,Duvoisin RC,et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop.Neurology1996;47:1–9.
13.McKeith IG,Dickson DW,Lowe J,Emre M,O’Brien JT,Feldman H,et al. Diagnosis and management of dementia with Lewy bodies:third report of the DLB Consortium.Neurology2005;65:1863–72.
14.Knopman DS,DeKosky ST,Cummings JL,Chui H,Corey-Bloom J, Relkin N,et al.Practice parameter:diagnosis of dementia(an evidence-based review).Report of the Quality Standards Subcommittee of the American Academy of Neurology.Neurology2001;56:1143–53.
15.McKhann G,Drachman D,Folstein M,Katzman R,Price D,Stadlan EM, et al.Clinical diagnosis of Alzheimer’s disease:report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease.Neurology1984;34:939–44.
16.Walker Z,Possin KL,Boeve BF,Aarsland D.Lewy body dementias.Lancet2015;386:1683–97.
17.Bang J,Spina S,Miller BL.Frontotemporal dementia.Lancet2015;386:1672–82.
18.O’Brien JT,Thomas A.Vascular dementia.Lancet2015;386:1698–706.
19.Kovacs GG,Milenkovic I,Wöhrer A,Höftberger R,Gelpi E,Haberler C,et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series.ActaNeuropathol2013;126:365–84.
20.Lista S,Garaci FG,Toschi N,Hampel H.Imaging epigenetics in Alzheimer’s disease.Curr Pharm Des2013;19:6393–415.
21.Reitz C.Toward precision medicine in Alzheimer’s disease.Ann Transl Med2016;4:107.doi:10.21037/atm.2016.03.05
22.Hampel H,Lista S,Khachaturian ZS.Development of biomarkers to chart all Alzheimer’s disease stages:the royal road to cutting the therapeutic Gordian Knot.Alzheimers Dement2012;8:312–36.
23.Jack CR,Bennett DA,Blennow K,Carrillo MC,Feldman HH,Frisoni GB,et al.A/T/N:An unbiased descriptive classificatio scheme for Alzheimer disease biomarkers.Neurology2016;87:539–47.
24.Younesi E,Hofmann-Apitius M.From integrative disease modeling to predictive,preventive,personalized and participatory(P4)medicine.EPMA J2013;4:23.doi:10.1186/1878-5085-4-23
25.Dubois B,Hampel H,Feldman HH,Scheltens P,Aisen P,Andrieu S,et al. Preclinical Alzheimer’s disease:definition natural history,and diagnostic criteria.Alzheimers Dement2016;12:292–323.
Received 10 August 2016;accepted 12 August 2016
Available online 23 August 2016
Peer review under responsibility of Shanghai University of Sport.
*Corresponding author.
E-mail address:harald.hampel@med.uni-muenchen.de(H.Hampel)
http://dx.doi.org/10.1016/j.jshs.2016.08.007
2095-2546/©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Journal of Sport and Health Science的其它文章
- Mechanism of neurodegeneration through tau and therapy for Alzheimer’s disease
- Examining the relationship between sport and health among USA women: An analysis of the Behavioral Risk Factor Surveillance System
- The microbiome,microbial-generated proinflammato y neurotoxins, and Alzheimer’s disease
- Longitudinal trajectories of physical activity in women using latent class growth analysis:The WIN Study
- Evidence of a conservative gait strategy in athletes with a history of concussions
- Total soy saponins improve the antioxidant capacity of the myocardium and exercise ability in exhausted rats
