Total soy saponins improve the antioxidant capacity of the myocardium and exercise ability in exhausted rats
2016-02-05ZhigngLiuYunLiuZhengyingXiongYueFengWenkunTng
Zhigng Liu*,Yun Liu,Zhengying Xiong,Yue Feng,Wenkun Tng
aSchool of Sport,Yuxi Normal University,Yuxi 653100,China
bFaculty of Science,Yuxi Normal University,Yuxi 653100,China
cSchool of Sport,Shaanxi Normal University,Xi’an 710062,China
dRehabilitation College,Beijing Sport University,Beijing 100084,China
Total soy saponins improve the antioxidant capacity of the myocardium and exercise ability in exhausted rats
Zhigang Liua,*,Yun Liub,Zhengying Xiongc,Yue Fengd,Wenkun Tanga
aSchool of Sport,Yuxi Normal University,Yuxi 653100,China
bFaculty of Science,Yuxi Normal University,Yuxi 653100,China
cSchool of Sport,Shaanxi Normal University,Xi’an 710062,China
dRehabilitation College,Beijing Sport University,Beijing 100084,China
Purpose:The aim of the present study was to investigate the impact of total soy saponins(TS)on the myocardial antioxidant capacity in rats exercised to exhaustion.
Methods:The one-time exhausted treadmill model was used.All rats were divided into 4 groups:the control group,the TS group,the exhausted group,and the TS exhausted group.The TS and TS exhausted groups were fed TS at a dosage of 20 mg/kg body weight,once a day,for 2 weeks. The exhausted group was given a placebo,and the control group was not given any treatment.The treadmill speed was set at 30 m/min,and the rats(exhausted and TS exhausted groups)were trained at this speed until exhausted.The rats were decapitated and anatomized immediately after exhausted.A 10%homogenate of the myocardial tissue was prepared.
Results:TS significant y increased the exercise time by 20.62%(p<0.05).As compared with the control group,the enzyme activities for catalase (CAT),glutathione peroxidase(GSH-Px),and glutathione reductase(GR)were significant y enhanced in the TS group(p<0.01);GR and GSH-Px activity was significant y enhanced in the TS exhausted group(p<0.01);malondialdehyde(MDA)levels were significant y decreased in the TS exhausted group(p<0.05).As compared with the exhausted group,the GSH-Px activity was significant y enhanced in the TS exhausted group (p<0.01);CAT,GSH-Px,and GR activities were significant y enhanced in the TS group(p<0.01).As compared with the TS group,the CAT and GR activity in the TS exhausted group was significant y decreased(p<0.01).
Conclusion:TS can improve the exercised rats’antioxidant activity in their cardiac muscle to varying degrees,decrease MDA and serum AST and LDH levels,increase the exercise time,and delay the occurrence of sports fatigue.
©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Antioxidant;Cardiac tissue;Exercise exhaustive rat;Total soy saponins
1.Introduction
Total soy saponins(TS)are a subset of pentacyclic triterpenoid glycosides with a variety of biological activities. According to the different sapogenins,TS can be divided into 4 groups:A group,B group,E group,and 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one(DDMP)group(Figs.1 and 2).The A group can be divided into Aa–Ah;the B group can be divided into Ba,Bb,Bc,Bb′,and Bc′;the E group canbe divided into Bd and Be;and the DDMP group can be divided intoαg,βg,βa,γg,andγa subgroups.
There were 2 free radical(FR)defense systems in the human body.One type is an enzymatic defense system such as superoxide dismutase(SOD),glutathione peroxidase(GSH-Px), catalase(CAT),and glutathione reductase(GR).The other is a non-enzymatic defense system such as vitamin C,vitamin E, and glutathione(GSH).Typically,the body keeps a dynamic balance between the generation and removal of FR.However, under the condition of exhausted exercise,FR in the body increases significant y.When the level of lipid peroxidation exceeds the body’s antioxidant capacity,this results in the occurrence of oxidative stress,directly causes biofil injuries,the degeneration of intracellular proteins,and leads to cell death,apoptosis,tissue damage,and disease.1
Fig.1.Total soy saponin structure of the A group.
Fig.2.Total soy saponin(TS)structure of the B group,E group,and 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one(DDMP)group.
TS have a variety of biological activities,such as antioxidant2and immune-enhancing activity.3,4They can also improve the rats’macrophage phagocytic capacity5and humoral and cellular immunity.3By inhibiting the activity of α-glucosidase6and α-amylase,7TS significant y reduced the level of blood sugar in diabetic rats and significant y improved glucose tolerance in both diabetic and healthy rats.8TS also have significan effects for anti-aging9and the inhibition of tumor cell DNA,10Herpes simplex virus(HSV-1),human cytomegalovirus(HCMV),polio virus,influenz virus,measles virus,mumps virus,and Coxsackie virus.11–14Further anti-aging studies on human embryonic lung diploid fibro lastsin vitroconfi med that cells treated with TS can grow to 80 generations,whereas the longest survival time of the control group was only 51 generations.15TS also have significan anti-lipid peroxidation activity on plasma lipoproteins.16They can prevent low-density lipoprotein(LDL) from oxidizing and decrease their susceptibility to oxidation, thus hindering the conversion of LDL to oxidized LDL,which is the most important risk factor for atherosclerosis.TS protected not only the heart but also the vascular smooth muscle.TS can significant y reduce the generation of lipid peroxides,protect endothelial integrity,and maintain normal cardiovascular function.
2.Methods
2.1.Experimental animals
Thirty-two Sprague-Dawley(SD)healthy male rats were used(weight 190–210 g,2 months old)and were provided by the Experimental Animal Center of the Medical School, Xi’an Jiaotong University(animal certificat number:Shannxi MedicalAnimal No.08-005).The study was performed according to the international,national,and institutional rules considering animal experiments,clinical studies and biodiversity rights,and had been approved by Xijing Hospital Ethic Committee in Fourth Military Medical University.
2.2.Main instruments
A 721B spectrophotometer(Shanghai Jingke;Shanghai Precision&Scientifi Instrument Co.,Ltd.,Shanghai,China),a 752B spectrophotometer(Shanghai Jingke),a Hitachi 7060 automatic biochemical analyzer(Hitachi Corporation of Japan, Tokyo,Japan),LabStar 2.5(Beijing Zhifang Technology Development Co.Ltd.,Beijing,China),aTGL-16G refrigerated centrifuge(Flying Pigeon;Shanghai Anting Scientifi Instrument Factory,Shanghai,China),a DK-98-1A water bath(Taisite; Tianjin City Taisite Instrument Co.,Ltd.,Tianjin,China),and a DSPT-202 treadmill(Duanshi;Shanghai Xinruan Information Technology Co.,Ltd.,Hangzhou,China)were used.
2.3.Experimental procedure
2.3.1.Animal groups
All rats were randomly divided into 4 groups:control group, TS group,exhausted group,and TS exhausted group.Eight rats from each group were fed in divided cages.The temperature varied from 22°C to 28°C,the relative humidity was 45%–65%, the cages were illuminated by natural light,the ambient noise was no higher than 45 dB,and all rats had free access to water and basic rodent chow.
2.3.2.Supplement dosing
TS were provided by North China Pharmaceutical Co.,Ltd. (Shijiazhuang,China)with a purity of 90% and 10% ash.The rats were started on TS gavage after 3 days of adaptation to the environment.Each rat in the supplement group(TS group and TS exhausted group)was fed a 2 mL aliquot of TS dissolved in normal saline at a fi ed time 9:00–9:30 a.m.,once a day,for 2 weeks.All rats in the supplement groups were fed TS at adosage of 20 mg/kg body weight.During the supplement gavage,the rats were weighed every 3 days,and the dosage was adjusted according to the body weight.The exhausted group was fed the same volume of normal saline vehicle.The control group received no treatments.

Table 1 Impact of total soy saponins(TS)on the exhaustion time in exhausted rats (n=8 in each group,mean±SD).
2.3.3.Exhaustive exercise protocol
An acute exhaustive exercise protocol was applied.The rats were not given any prior training.The exhausted and TS exhausted group rats underwent the acute exhaustive exercise on the treadmill only before dissection.The treadmill was horizontal and gradually increased to the predetermined exercise intensity(30 m/min)within 3 min.The treadmill speed was set at 10 m/min for the firs minute,20 m/min for the second minute,and 30 m/min for the third minute.The exercise time and exercise distances of each rat were then recorded.We judged whether the rats exercised to exhaustion according to the following criteria:the rats could not maintain a predetermined movement speed,squatted against the back wall of the treadmill lane on its buttocks,and both the current stimulus and the brush driving could not force the rats to continue exercising.The exhausted behavior was characterized by shortness of breath, mental fatigue,and a prone nutation.
2.3.4.Dissection and index test
The rats were anesthetized with ether after exhaustion and killed by decapitation.The blood was collected,and the serum was separated after blood coagulation.The heart was removed immediately,and the blood was washed away with 4°C normal saline and then placed in a clean culture dish marked according to each group.The weight of the myocardial tissue was measured,and the heart was ground in 4°C normal saline.Myocardial tissue homogenates of 10%mass concentration were prepared and the supernatant was separated after centrifugation (7.99×g,5 min).Finally,the antioxidant indicators were assayed accordingly.
2.4.Methods of testing
The antioxidant indicators were tested with reagent kits provided by Nanjing Jiancheng Bioengineering Institute(Nanjing, China).SOD was tested by the xanthine oxidase method; malondialdehyde(MDA)was tested by the thiobarbituric method(TBA method);CAT was tested by the ultraviolet spectroscopy method;GSH-Px,GR,and GSH were tested by the dithiobis nitrobenzoic acid method;total antioxidant capacity (T-AOC)was tested by a spectrophotometry method.The serum aspartate aminotransferase (AST)lactate dehydrogenase (LDH)levels were tested by an automatic biochemistry analyzer(Hitachi 7060).
2.5.Data processing
The experimental data were processed with statistical software SigmaStat(Version 3.5;SYSTAT Software Inc.,San Jose, CA,USA),and the results were shown as means±SD.Ap<0.05 orp<0.01 was considered statistically significan after a 1-way analysis of variance Student–Newman–Keuls test (S-N-K test).The exhaustive time was processed by attest,and the results of thettest were measured by Cohen’sdvalue.
3.Results
3.1.The exhaustion time of the rats
3.1.1.Antioxidant and serum enzyme indices
As shown in Table 1,TS can significant y increase exhaustion time of the rats by 20.62%(p<0.05,Cohen’sd=1.07). According to the Cohen standards,thettest has a small effect size,medium effect size,and large effect size when Cohen’sdvalue=0.2,0.5,and>0.8.The Cohen’sdvalue of presentttest was 1.07>0.8,which indicated that the presentttest was trustworthy.
Table 2 shows that TS can improve rats’antioxidant capacity in their cardiac muscle to varying degrees.
As compared with the control group,the enzyme activities of CAT,GSH-Px,and GR were all significant y enhanced in the TS group(p<0.01);GR and GSH-Px activity was enhanced significant y(p<0.01),and the MDA levels decreased significantly in the TS exhausted group(p<0.05).The SOD activity increased in the TS,exhausted,and TS exhausted group,and the MDA levels decreased in the TS and exhausted groups,but this was not statistically significant
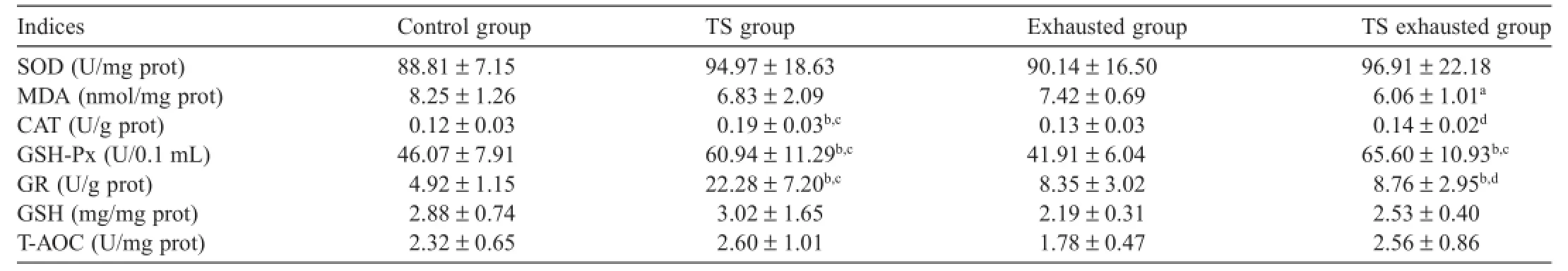
Table 2 Impact of TS on the antioxidant capacity of the myocardium in exercised rats(n=8 in each group,mean±SD).

Table 3 Impact of total soy saponins(TS)on the serum aspartate aminotransferase(AST)and lactate dehydrogenase(LDH)activity in exercised rats(n=8 in each group, mean±SD).
As compared with the exhausted group,the GSH-Px activity was significant y enhanced in the TS exhausted group (p<0.01).The CAT,GSH-Px,and GR activities were all significant y enhanced in the TS group(p<0.01).SOD,CAT,GR, GSH,and T-AOC activities showed a tendency to increase (whereas MDA decreased)in the TS exhausted group,but again this was not statistically significant
As compared with the TS group,the CAT and GR activity in the TS exhausted group was significant y decreased(p<0.01). SOD and GSH-Px activities showed a tendency to increase,but a tendency to decrease was observed for MDA,GSH,and T-AOC in the TS exhausted group(not statistically significant)
Table 3 shows that TS can decrease rats’serum AST and LDH levels to varying degrees.As compared with the control and TS groups,the serum AST levels were significant y increased in the exhausted group(p<0.05).As compared with the TS group,the serum LDH levels were increased in the exhausted group(p<0.05).
4.Discussion
The workability of the cardiac muscle was an important factor in limiting the exercise capabilities of these rats.Under the condition of exhausted exercise,the cardiac muscle was probably injured by ischemia–reperfusion,calcium overload, myocardial stunning,and FR.17These factors can reinforce each other and create a vicious circle,and finaly result in a weakness of heart contractility and increase the permeability of the rat myocardium cell membrane.The present results showed that TS can decrease the rats’serum AST and LDH levels in both the TS and TS exhausted groups.Since serum AST and LDH are typical markers of myocardial damage,this result indicated that TS can significant y protect the myocardial muscle in both the resting state and working state.
As compared with the control group,the experimental data showed that MDA tended to decrease,whereas SOD,CAT,and GR activities tended to increase in the exhausted group.This result can be explained as follows:under the condition of exhausted exercise,the rats’FR metabolism increased and stimulated to the emergence of a compensatory reaction to eliminate these FR.As a result,the antioxidant enzyme activity increased,and this led to the rise in the FR elimination rate and followed by the reduction in MDA formation.This was due to the body’s self-regulation via negative feedback,and probably was one of the reasons why exercise shows an anti-aging effect.18
As compared with the exhausted group,GSH-Px increased significant y in the TS exhausted group,and SOD,CAT,GR, GSH,and T-AOC also showed a tendency to increase.This indicated that,under the TS intervention,the antioxidant compensatory mechanism of the body was reinforced,and thus the antioxidant enzymes activity increased and MDA levels decreased.
As compared with the control group,the CAT,GSH-Px,and GR activities increased significant y in the TS group,and SOD, GSH,and T-AOC showed a tendency to increase,whereas the MDA levels showed a tendency to decrease.This indicated that, in the resting state,TS can improve the antioxidant capacity of the rats’myocardial muscle.The antioxidant effect of TS may be related to its chemical structure.The TS parent nuclear structure,which was rich in phenolic hydroxyls,can combine with FR and form a stable semiquinone and hence break the chain reaction of FR and directly clear FR.It has been reported that the TS monomer AI can significant y inhibit the activities of myocardial calcium channels T,L,and B,reduce its opening rate and time,alleviate the cell damage caused by calcium overload,and significant y attenuate FR levels induced by xanthine–xanthine oxidase.19Sun et al.20reported that TS also has a strong total antioxidant capacity and an anti-active oxygen capacityin vitro.It can inhibit lipid peroxidation in liver tissue and alleviate the swelling of liver mitochondria,20inhibit erythrocyte membrane lipid peroxidation and reduce the hemolysis of red blood cells.21
During exhausted exercise,the body’s oxygen uptake increased substantially,of which approximately 2%is converted into FR.22Therefore,during exhausted exercise,FR and especially oxygen FR levels increased significant y;this constituted one of the major factors of body injury and prompted the occurrence of exercise fatigue.Researchers have shown that exhausted exercise can weaken heart contractility,which can probably recover by 24 h after exercise.23It was also shown that the recovery process was significant y correlated to the myocardial metabolism of FR.17FR can cause an excitation–contraction coupling disorder by damaging the functions of the endoplasmic reticulum,which resulted in systolic dysfunction and contractility decline.FR also caused calcium overload by influencin intracellular calcium ion transport in myocardial cells,and thus the calcium overload also contributed to the decline in myocardial contractility.17Other studies have also shown that myocardial contractility was negatively correlated to the rise of myocardial intracellular Ca2+.17FR attack biofilm containing a large amount of unsaturated fatty acids,caused biofil lipid peroxidation and membrane potential instability. Membrane potential abnormalities can in turn affect the action potential,which led to systolic dysfunction.24
Along with myocardial ischemia–reperfusion injury,FR can increase in various ways such as via mitochondrial electronic leakage,xanthine oxidase in vascular endothelial cells,neutrophil respiratory bursts,and catecholamine oxidation.24These mechanisms can lead to increased fluidit and permeability of the myocardial cell biofil and subcellular organelles,and therefore destroy the integrity and functions of the cell.Myocardial ischemia–reperfusion injury also damages the arterial endothelium.25As a result,it interferes with prostaglandin I2(PGI2)synthesis,which occurs mainly in the coronary vascular endothelial cells,and platelets adhered to the endothelial collagen tissue,leading to a further release of vasoconstrictors, predominately thromboxane A2(TXA2).TXA2can be available as a Ca2+carrier and directly promotes Ca2+influ and Ca2+release from the dense tubular system,thereby promoting platelet aggregation and local vasoconstriction,and thus increasing endothelium injury.Therefore,the imbalance between TXA2and PGI2can be one of the main causes of myocardial ischemia and myocardial necrosis,26and this further affected cardiac contractility.The present study also showed that the serum AST and LDH levels increased after exhausted exercise,which indicated that the myocardial cells were injured and supported the hypothesis above.
SOD,CAT,and GSH-Px are common antioxidant enzymes which can eliminate FR and reduce their hazards.Under normal physiological conditions and an appropriate exercise load,the antioxidant enzymes system of the body,by way of their respective roles,keep a dynamic balance between FR generation and elimination.However,under the condition of exhaustive exercise,the generation rate of FR was far greater than the body’s ability to clear,and the balance was broken,which resulted in FR accumulation in the body.This in turn caused lipid peroxidation,then caused lipid peroxidation injury,DNA breakage,protein denaturation,and eventually led to sports fatigue.27The interaction between the FR and the body was a negative feedback process:exercise intensity↑→FR level↑→body compensatory reaction↑→antioxidant enzyme activity↑→FR level↓.Although the FR levels decreased finaly,the body had already been injured by the FR before they were cleared.This inference was consistent with the present experimental data.
5.Conclusion
TS can significant y improve the antioxidant capacity of rat myocardial tissue,decrease MDA level and serum AST and LDH levels,protect myocardial muscle,enhance its T-AOC, attenuate FR damage to the myocardial cells and delay sports fatigue.Therefore,this protective effect on myocardial muscle may be the one of mechanisms whereby TS can improve the rats’exercise capability.
Acknowledgment
This study was supported by the National Natural Science Foundation of China(No.11101354).
Authors’contributions
ZL carried out the antioxidant studies,participated in the data analysis and drafted the manuscript;YL performed the statistical analysis;ZL,ZX,andYF conceived of the study and participated in its design;WT helped to draft the manuscript. All authors have read and approved the fina version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financia interests.
1.Huang CC,Lin TJ,Chen CC,Wan TL.Endurance training accelerates exhaustive exercise-induced mitochondrial DNA deletion and apoptosis of left ventricle myocardium in rats.Eur J Appl Physiol2009;107:697–706.
2.Song BJ,Zhao Y,Sun YW.Effects of soysaponins on blood lipid and antioxidation in hyperlipidemia population.Chin Gener Pract2010;13: 3880–1.[in Chinese].
3.Dong WY,Zhang DP,Gao XM,Jin L,Wang JB.Enhancement effect of total soyasaponin on immune function.J Chin Cereals Oils Assoc2001;16:9–11.[in Chinese].
4.Ye BG,Wen CY,Li XB,Zhu QS,Song XS.Effect of soyasaponins on levels of IL-2 and γ-IFN of spleen cell in diabetic rats.Chin J Laborat Diagn2013;17:261–3.[in Chinese].
5.Zhu XJ,Zhou CL,Song BJ,Sun YW.Effect of soyasaponins on immunological function of tumor bearing mice.Progress Mod Biomed2013;13:4847–50.[in Chinese].
6.Quan JS,Yin XZ,Jin M,Shen MH.Study on the Inhibition of alpha-glucosidase by soyasaponins.J Chin Med Mater2003;26:654–6.[in Chinese].
7.Quan JS,Yin XZ,Tanaka M,Kanazawa T.The hypoglycemic effects of soybean hypocotyl extract in diabetic rats and their mechanism.Acta Nutrimenta Sinica2004;26:207–10.[in Chinese].
8.Wang W,Li DM,Li R.Experimental study of soyasponins protective effect on diabetic kidney disease.Chin J Immunol2013;29:1272–5. [in Chinese].
9.Jin L,Gao XM,Wang JB,Dong WY.A study on the anti-aging effect of soyasaponin.II.Experiment of soyasaponin on the anti-aging function.Sci Technol Food Indust1999;20(Suppl.1):31–3.[in Chinese].
10.Huang GQ,Xiao JX,Du DH,Qiu HW.Study on the anti-tumor effect of soyasaponins on H22-bearing mice.Food Res Develop2009;30:52–4.[in Chinese].
11.He ZM,Zhang FX,Deng WD,Wu XX,Li BJ.The inhibitory effects of soybean saponins and soybean trypsin inhibitor on simian immunodeficien y virus.Chin J Appl Environ Biol1998;4:383–5. [in Chinese].
12.Nakashima H,Okubo K,Honda Y,Tamura T,Matsuda S,Yamamoto N. Inhibitory effect of glycosides like saponin from soybean on infectively of HIVin vitro.AIDS1989;3:655–8.
13.Hayashi K,Hayashi H,Hiraoka N.Inhibitory activity of soyasaponin II on virus replicationin vitro.Planta Med1997;63:102–5.
14.Li JB,Hu JS,Chen BH,Wang XQ,An ZY,Wei YD.Inhibitory effect of total soyasaponin of virus replication and its clinical application.Chin Experim Clinic Virol1995;9:111–4.[in Chinese].
15.Lee SJ,Bae J,Kim S,Jeong S,Choi CY,Choi SP,et al.Saponins from soy bean and mung bean inhibit the antigen specifi activation of helper T cells by blocking cell cycle progression.Biotechnol Lett2013;35:165–73.
16.Tsai CY,Chen YH,Chien YW,Huang WH,Lin SH.Effect of soy saponin on the growth of human colon cancer cells.World J Gastroenterol2010;16:3371–6.
17.Xu SM,Liu TB,Su QS.Study on heavy load training-induced cardiac contractility changes of rats,and the relationship between this changes and the levels of cardiac free radical,calcium ions.China Sport Sci2011;31:73–8.[in Chinese].
18.Wang AL,Chi J,Xiang ZC,Wang N,Song CL.Research on effects of aerobic training on anti-senility(I)—Effects of swimming on free radicalmetabolism in mice of different months old.J Beijing Univ Phys Educ2000;23:474–7.[in Chinese].
19.Zeng QH,Sun XX,Sun CW,Zhan S,Zhong GG.Single channel analysis and ESR spectral study on the myocardial effects of soyasaponin AI.J N Bethune Univ Med Sci1997;23:345–7.[in Chinese].
20.Sun Q,Liu SP,Jin AH,Yin XZ.In vitroantioxidative effects of soyasaponins.Food Sci Technol2010;35:72–5.[in Chinese].
21.Li T,Liu SP,Quan JS.Anti-plasma lipid peroxidative and erythrocyte protective effects of soya-saponins.Soyb Sci2009;28:1067–70.[in Chinese].
22.Xu SM,Shangguan RN,Peng FL,Su QS.Protection effect and its mechanism of exercise preconditioning on myocardial injury-induced by heavy load treadmill training in rat.China Sport Sci2012;32:45–52.[in Chinese].
23.Scharhag J,George K,Shave R,Urhausen A,Kindermann W. Exercise-associated increases in cardiac biomarkers.Med Sci Sports Exerc2008;40:1408–15.
24.Asano G,Takashi E,Ishiwata T,Onda M,Yokoyama M,Naito Z,et al. Pathogenesis and protection of ischemia and reperfusion injury in myocardium.J Nippon Med Sch2003;70:384–92.
25.Zhang L,An GY,Chen K,Li CZ,Zhang WG.Models of spinal cord ischemia/reperfusion injury by blocking lumbar artery versus abdominal aorta.J Med Postgraduates2014;27:124–8.[in Chinese].
26.Neri M,Fineschi V,Di Paolo M,Pomara C,Riezzo I,Turillazzi E,et al. Cardiac oxidative stress and inflammato y cytokines response after myocardial infarction.Curr Vasc Pharmacol2015;13:26–36.
27.Li XX,Chang Z,Yang JX,Bao Y.Antioxidative activity and DNA protected by ethanol extract from pumpkin.J Shaanxi Norm Univ(Natl Sci Ed)2008;36:74–7.[in Chinese].
Received 22 September 2014;revised 30 November 2014;accepted 22 April 2015
Available online 25 September 2015
Peer review under responsibility of Shanghai University of Sport.
*Corresponding author.
E-mail address:xbaili@126.com(Z.Liu).
http://dx.doi.org/10.1016/j.jshs.2015.09.005
2095-2546/©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Journal of Sport and Health Science的其它文章
- Biomarker-guided classificatio scheme of neurodegenerative diseases
- Mechanism of neurodegeneration through tau and therapy for Alzheimer’s disease
- Examining the relationship between sport and health among USA women: An analysis of the Behavioral Risk Factor Surveillance System
- The microbiome,microbial-generated proinflammato y neurotoxins, and Alzheimer’s disease
- Longitudinal trajectories of physical activity in women using latent class growth analysis:The WIN Study
- Evidence of a conservative gait strategy in athletes with a history of concussions
