Effects of aerobic training on serum paraoxonase activity and its relationship with PON1-192 phenotypes in women
2016-02-05GulinRudrliNlknRnVrolFrukTurgyMesutNlknZekiOzkolSOguzKrmizrk
Gulin Rudrli Nlkn*,S.Rn VrolFruk TurgyMesut Nlkn, M.Zeki OzkolS.Oguz Krmizrk
aCoaching Education Department,School of Physical Education and Sports,Ege University,Izmir 35100,Turkey
bEsrefpasa Hospital,Izmir 35520,Turkey
cMedical Faculty,Sports Medicine Department,Ege University,Izmir 35100,Turkey
Effects of aerobic training on serum paraoxonase activity and its relationship with PON1-192 phenotypes in women
Gulbin Rudarli Nalcakana,*,S.Rana Varola,Faruk Turgaya,Mesut Nalcakanb, M.Zeki Ozkola,S.Oguz Karamizrakc
aCoaching Education Department,School of Physical Education and Sports,Ege University,Izmir 35100,Turkey
bEsrefpasa Hospital,Izmir 35520,Turkey
cMedical Faculty,Sports Medicine Department,Ege University,Izmir 35100,Turkey
Background:Paraoxonase 1(PON1)is an antioxidant enzyme that protects high-density lipoprotein(HDL)and low-density lipoprotein against oxidation.Limited studies have addressed the influenc of exercise on PON1 activity and its relationship with PON1 phenotypes.We investigated relationships between PON1-192 phenotypes,PON1 activity,aerobic exercise,and blood lipid and lipoprotein concentrations in middle-aged women.Methods:An exercise group(n=50)engaging in regular aerobic exercise and a control group(n=41)were selected from a subset of 300 Caucasian women that met the inclusion criteria.Serum PON1,salt-stimulated PON1(SSPON1),and arylesterase(ARE)activities;cholesterol levelsand ARE activities of total HDL and HDL subgroups(HDLs)(supernatants obtained by polyethylene glycol);and blood lipid and lipoprotein concentrations were determined by standardized enzymatic methods.PON1-192 QQ(low activity),QR(moderate activity),and RR(high activity)phenotype groups were define using serum SSPON1/ARE activity ratios.The R-carries(RC)phenotype group consisted of the QR and RR groups combined.Results:All lipid and lipoprotein concentrations were greater in the exercise group than in the control group.Regardless of phenotype,no significan differences were observed between the exercise and control groups in terms of serum PON1,SSPON1,orARE activity associated with HDLs(p>0.05),whereas PON1 activities in QQ-phenotyped women in the exercise group were significant y higher than those in the control group (p<0.01),but not the RC group.A statistically significan interaction between PON1 phenotypes(QQ and RC groups)and exercise(exercise and control groups)on PON1 activity was found.
Aerobic exercise program;Arylesterase;Lipids;Lipoproteins;Paraoxonase;PON1-192 phenotype;Women
1.Introduction
Atherosclerosis is the main cause of death in developed countries.Regular aerobic exercise program reduces the risk of developing cardiovascular disease(CVD)by increasing serum high-density lipoprotein cholesterol(HDL-C)levels.1Paraoxonase 1(PON1)prevents the oxidation of both HDL and low-density lipoprotein(LDL),impeding the development of atherosclerosis.The anti-oxidative property of HDL has been partly attributed to serum PON1 enzyme.2
PON1 enzyme hydrolyzes both paraoxon and phenylacetate substrates,which are referred to in the literature as paraoxonase and arylesterase(ARE)activities,respectively.PON1 can hydrolyze the oxidized lipids of LDL and HDL,which are known to promote atherosclerosis.PON1 enzyme is produced mainly in the liver and is released into the blood.2,3HDL facilitates the secretion of PON1 from the liver into the blood and stabilizes the enzyme.PON1 is located on HDL and its subfractions(HDLs: HDL2and HDL3)were found in the blood.4
PON1 activity can be affected by genetic factors,life style choices,disease status,and HDL-C levels;5however,its activity is mainly determined by polymorphisms in thePON1gene.ThePON1gene has several genetic polymorphisms,1 set of which occurs in codon 192(referred to as PON1-192 polymorphisms),whose Q and R alleles are associated with low and high PON1 activity,respectively.6
It was reported that PON1 activity,HDL2-C,andHDL3-Care related to CVD5–7and that both serum PON17,8and ARE activities8,9are lower in individuals with CVD than in controls. Similarly,it was found that blood HDL-ARE activities are lower in women with CVD than in controls and that these parameters are more predictive for CVD than HDL-C levels.10In addition, ARE activity was shown to be polymorphism-independent.11
Exercise is another major factor that affects PON1 activity.9,12,13Therefore,to protect against CVD,it is important to monitor one’s serum PON1 and HDLs-ARE activities as well as cholesterol levels,with the goal of improving these activities through an appropriate exercise program.
Tomás and coworkers13reported that acute and 4-month exercise programs did not affect salt-stimulated PON1 (SSPON1)activity in a group of healthy Spanish men and women athletes,regardless of PON1-192 polymorphism. However,when PON1-192 polymorphism was considered,both types of exercise increased SSPON1 activity,and the effects of exercise on SSPON1 activity were modulated by PON1-192 polymorphism.Results from a separate study showed that PON1 activity increased significant y after maximal exercise independently of PON1-192 polymorphisms in rugby players, but ARE activity was not affected by exercise.12In a crosssectional study,blood SSPON1 and ARE activities,as well as the distribution of PON1-192 genotypes,did not differ between endurance athletes and controls.14Conflictin effects of genotype and exercise on PON1 activity and their relationships with PON1-192 polymorphisms were seen among athletes.Moreover,conflictin results were also observed with non-athlete women and men with CVD.9The reasons underlying such discrepancies are unclear,as only a single study investigating the influenc of PON1-192 phenotypes on PON1 activity is found in the literature.14
In addition,in both humans and mice,it was shown that PON1 activity is greater in women than in men,9,15and it was reported that PON1 is regulated at the mRNA level in a gender-specifi manner by some pro-inflammato y and anti-inflammato y substances.15
In parallel,it was also reported that estradiol may regulate the specifi activity and/or stability of cell surface PON1.16In most studies,8,9,14blood ARE activities has been measured, which are known to be independent of PON1-192 polymorphisms.Thus,ARE and PON1 measurement methods and gender differences may influenc the interpretation of results when examining the effects of PON1-192 phenotypes.
PON1 activity varies widely between individuals within the same genotype group and across ethnic variations.5,17Therefore,similar studies may yield different results with healthy women and Turkish populations with a different ethnic group, although no such study has been published yet.
Furthermore,it is reported that the effect of physical activity on HDL-C concentration is related to PON1-192 polymorphisms.18However,some studies have failed to demonstrate associations between PON1-192 phenotype and lipoprotein changes,19,20while Hegele and coworkers21found significan associations between PON1-192 genetic variants and plasma HDL-C and triglyceride levels.Thus,the relationships between PON1phenotypes and all blood lipid and lipoprotein concentrations are unclear,and these relationships have not been reported for Turkish subjects.Our hypothesis was that a regular aerobic exercise program can increase PON1 activity and lipid and lipoprotein concentrations depending on PON1-192 phenotype in middle-aged Turkish women.To test our hypothesis,we planned this cross-sectional study to investigate the relationships between PON1-192 phenotypes and regular aerobic exercise, PON1 and ARE activities,and all lipid and lipoprotein concentrations in middle-aged women.
2.Methods
2.1.Subjects
Three hundred women volunteers completed an anamnesis questionnaire providing information regarding their basic demographics,medical history,medication usage,frequency of smoking and alcohol consumption,and amount of physical activity.One hundred and sixty women met the inclusion criteria and were selected for medical examination.These criteria included(a)being≥32 years of age and having regular menstrual cycles;(b)not being anemic or actively infected;(c)being free of illnesses predisposing to CVD;(d)not being a smoker and/or an alcohol user;(e)not taking medication affecting lipid,lipoprotein,or antioxidant metabolism;and(f)agreeing to participate in an exercise group(EG,n=50)for 1 h for 3 days per week for at least 3 months,involving a supervised aerobic exercise program (step aerobics).The control group(CG,n=41)included habitually active women(exercising less than 1 h/week)that were not engaged in a structured training program,nor had they been so engaged for at least 3 months prior to the study.Following medical history inquiries,physical examinations,and blood testing,91 women met the inclusion criteria(Table 1).
Participants were informed about the details of the study and the probable risks and all provided written informed consent. The study was approved by the Ege University Medical Faculty’s Ethics Committee.
2.2.Physical and physiological measurements
Physical examination consisted of anamnesis,ECG,anthropometry(height,weight,and body mass index(BMI)),and percentage of body fat(%BF).Body density and%BF values were calculated using widely recognized equations included in the book of Ratames and American College of Sports Medicine.22
Physiological measurements included resting heart rate (HR),blood pressure(BP),and maximum oxygen consumption (VO2max).VO2maxwas determined by respiratory gas analysis (Quark b2;COSMED,Rome,Italy)on a cycle ergometer (Monark,Varberg,Sweden),using gradually increasing loads. VO2maxmeasurements were confi med when 3 or more of the following criteria were met:(1)a plateau in VO2despite an increase in workload,(2)a respiratory exchange ratio higher than 1.2,(3)a peak HR at least equal to 90%of the agepredicted maximum,and/or(4)visible exhaustion.23
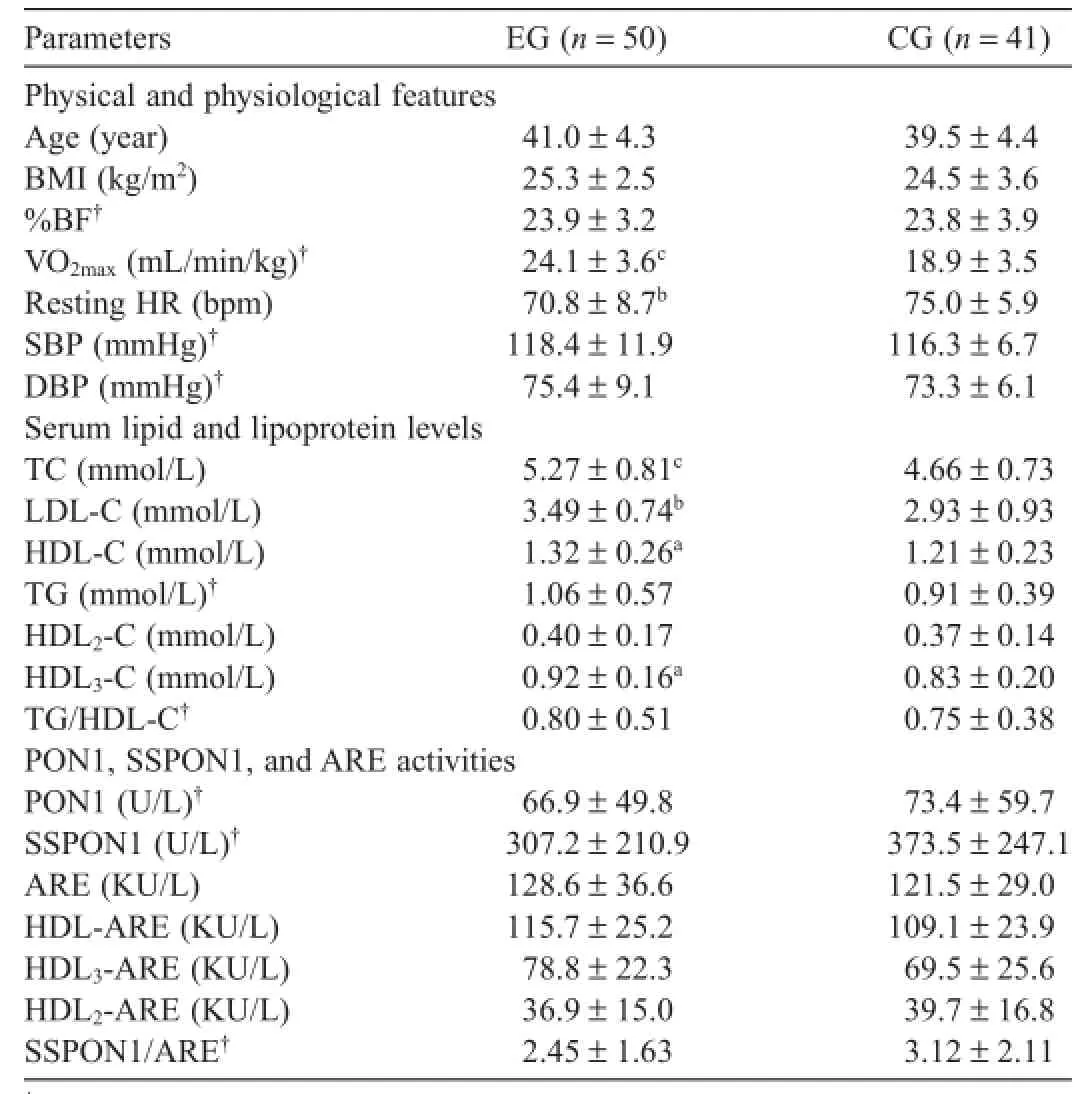
Table 1 Physical and physiological features,serum lipid and lipoprotein levels,PON1, SSPON1,and ARE activities of exercise and control groups(mean±SD).
2.3.Blood sampling and analysis
Participants were instructed not to exercise,not to change their diet,and not to take any medication or supplements for at least 48 h prior to blood sampling.Overnight-fasting venous blood samples were drawn between 08:30 a.m.and 10:00 a.m. The sample-collection day was selected according to the women’s menstrual cycles.
Blood samples were allowed to clot at 25°C for 30 min and centrifuged at 2000gfor 10 min.Hematological analyses were performed with a hematology analyzer(BC-3000 Plus; Mindray,Shenzhen,China).Serum samples were stored at -82°C until analysis.The analyses were performed within 1 month.
2.3.1.Assay of HDL,HDL2,and HDL3supernatants(HDLs)
Supernatants of HDL and HDL3were separated from serum samples using polyethylene glycol(Ma:20.000;Merck,Darmstadt,Germany)according to the differential precipitation method.24Serum baseline PON1,SSPON1,andARE activities, as well as cholesterol and ARE activities of HDLs were analyzed on the same day by the following methods.The cholesterol and ARE activity levels of HDL2were estimated by calculating the difference between HDL and HDL3.
2.3.2.Analysis of cholesterol and triglyceride levels
The cholesterol contents of HDLs(HDL-C,HDL2-C,and HDL3-C),serum total cholesterol(TC)and serum triglycerides (TG)levels were determined using standardized enzymatic methods with commercial kits(Dialab GmbH,Wien,Austria) with an autoanalyzer(Modular DP;Roche Diagnostics,Tokyo, Japan).LDL-C was calculated as described by Friedewald et al.25
2.3.3.Analysis of PON1 activities
Serum PON1 and SSPON1 activities were determined using paraoxon(diethylp-nitrophenylphosphate;Sigma Chemical Co.,St.Louis,MO,USA)as the substrate with an autoanalyzer (Modular DP),as described previously.11,26Paraoxon hydrolysis rates were determined by recording the absorbance at 412 nm and 37°C,which provided a measurement ofp-nitrophenol release.PON1 activities were measured in 800 μL assay mixtures containing 1 mmol/L paraoxon,1 mmol/L CaCl2,and 5 μL of serum in 50 mmol/L Tris–HCl buffer(pH 7.4),as described.11One unit of paraoxonase activity was define as 1 μmolp-nitrophenol formed per minute under the above assay conditions.
For SSPON1 assays,26the 800 μL assay mixture consisted of 1 mmol/L paraoxon,1 mmol/L CaCl2,5 μL of serum,and 1 mol/L NaCl in 50 mmol/L glycine buffer(pH 10.5).Results are expressed as U/L for both PON1 and SSPON1.The withinrun variation constants(CVs)were<1.5%for both enzyme assays.
2.3.4.Analysis of ARE
ARE activities associated with serum ARE and HDLs (HDL-ARE and HDL3-ARE) were determined using phenylacetate substrate (Merck-Schuchardt, Darmstadt, Germany)by measuring phenol concentrations at 270 nm and 37°C with a UV–visible spectrophotometer(UV160A; Shimadzu,Kyoto,Japan),as described previously.11The 3 mL assay mixture contained 1 mmol/L phenylacetate,0.9 mmol/L CaCl2,and 5 μL of serum in 9 mmol/LTris–HCl buffer(pH 8). One unit of ARE hydrolyzes 1 mmol of substrate per minute is represented as(KU/L)ofARE.AllARE activity measurements were performed within the same assay.Within-run variation constants(CVs)were<5.5%.
2.3.5.Distribution of PON1-192 phenotypes
The paraoxonase phenotype distribution among the subjects was determined using a dual-substrate (paraoxon and phenylacetate)method.11SSPON1/ARE activity ratios were used to classify the phenotypes of each participant as EG,CG, QQ(low paraoxonase activity),QR(moderate paraoxonase activity),or RR(high paraoxonase activity)phenotypes.The RR and QR groups together were define as R-carriers(RC).
2.4.Statistical analyses
Data were analyzed using the SPSS program Version 15.0(SPSS Inc.,Chicago,IL,USA),following normality(Kolmogorov–Smirnov Test)and homogeneity(Levene Test) testing.Natural logarithmic transformation was performed on dependent variables to normalize the non-normal data and meet the homogeneity of variance assumption for parametric tests. The interactions between PON1-192 phenotype and exercise for dependent variables were assessed using a 2×2(main group×phenotype subgroups)2-way analysis of variance (ANOVA).One-way ANOVA with thepost hocleast signifi cance difference(LSD)test was used to compare dependent variables between the EG phenotype subgroups(EQQ,EQR, ERR,and ERC)and CG phenotype subgroups(CQQ,CQR, CRR,and CRC).Differences in the dependent variables investigated between the EG and CG were assessed by performing an unpaired Student’sttest.In the case of non-normal distributions,nonparametric versions of the Kruskal–WallisHtest and Mann–WhitneyUtest were used for statistical analysis.Dependent variables analyzed with non-parametric tests were identifie using the“†”symbol in the tables.Relationship levels between the variables investigated were determined either by calculating Pearson Product-Moment Correlation Coefficient (raw data were used for variables having normal distributions and log transformed data were used for variables that met the normality assumption after log-transformation)or Spearman’s Rank Correlation Coefficien(raw data were used for nonnormal variables).
3.Results
Participants with SSPON1/ARE ratios≤1.93,between 2.45 and 4.89,or≥5.48 were define as individuals having QQ(low activity),QR(moderate activity),or RR(high activity)phenotypes,respectively.Thus,46 participants(50.5%of all participants)had the QQ(low activity)phenotype,10(11.0%)had the RR(high activity)phenotype,and 35(38.5%)belonged to the QR(moderate activity)phenotype group.The RC phenotype group consisted of QR and RR groups combined,to obtain statistically reliable results.The trimodal frequency distribution of participants in this study was similar to that observed in other Caucasian groups.1,2,27
3.1.Physical and physiological characteristics of participants
EG and CG subjects displayed similar anthropometric characteristics.The mean training experience for the EG was 3.12±3.29 years.VO2max(p<0.001)and resting HR(p<0.01) values were statistically different between EG and CG(Table 1).Considering phenotyping,VO2maxwas significant y higher in EQQ(p<0.001)and ERC(p<0.001)than those in CQQ and CRC,respectively;resting HR was significant y lower in EQQ (p<0.05)and ERC(p<0.05)than those in CQQ and CRC, respectively(Table 2).
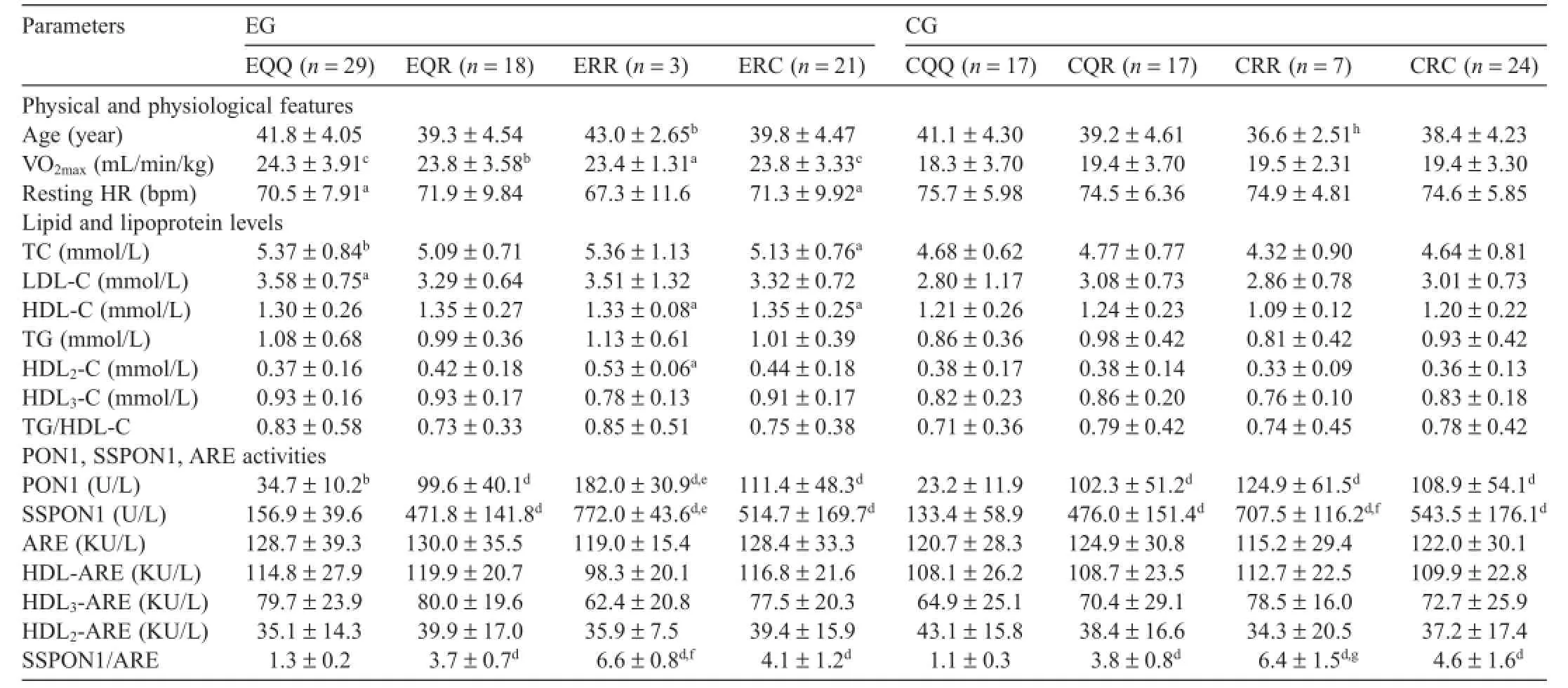
Table 2 Serum PON1,SSPON1,ARE activities,and lipid and lipoprotein levels in exercise and control groups according to PON1-192 phenotyping(mean±SD).
3.2.Measurement of all lipid and lipoprotein concentrations
HDL-C and HDL3-C(p<0.05),TC(p<0.001),and LDL-C (p<0.01)levels were significant y greater in the EG than in the CG(Table 1).TC(p<0.01)and LDL-C(p<0.05)levels were higher in EQQ phenotype group than in those of the CQQ phenotype group.The ERC phenotype group had greater TC (p<0.05)and HDL-C(p<0.05)levels,as compared to the CRC group(Table 2).However,no differences were found in all lipid and lipoprotein concentrations between the CQQ and CRC phenotype groups(Table 2).
3.3.Serum PON1,SSPON1,and ARE activities
No significan difference was found between the EG and CG in terms of serum PON1 and SSPON1 activities,ARE, and HDLs-ARE activities(Table 1).Evaluation of the effects of exercise according to PON1-192 phenotypes revealed that serum PON1 activity of the EQQ group was greater than that in the CQQ group(p<0.01).Although HDL3-ARE activities in the EQQ group were 23%higher than those in the CQQ group,this difference was not statistically significantARE activities were also not significant y different among the phenotype groups(Table 2).As expected, serum PON1 and SSPON1 levels,as well as SSPON1/ARE ratios were significant y greater among QR,RR,and RC phenotyped individuals than those with the QQ phenotype (p<0.001),with or without taking into account of exercise status.
Two-way ANOVA showed a significan interaction between PON1 phenotypes(QQ and RC groups)and exercise (exercise and control groups)on PON1 enzymatic activity (F(1,87)=4.01;η2=0.044;p=0.048).Significan positive correlations were found between serum PON1 and SSPON1, SSPON1/ARE ratio,HDL3-ARE activities(p<0.01),and HDLARE activities(p<0.05),and between HDL-C and HDL-ARE, HDL2-C,HDL3-C(p<0.01),HDL2-ARE,HDL3-ARE,andARE (p<0.05).
4.Discussion
The main findin of the present study was that serum PON1 activity in the EQQ phenotype group was greater than that in the CQQ group(p<0.01),but not the ERC group.Moreover, a significan interaction was found between regular aerobic exercise and PON1-192 phenotype on PON1,whereas no similar interaction was found for all lipid and lipoprotein concentrations.The results show that there are the beneficia effects of regular aerobic exercise training on PON1 activity and that this effect is related to PON1-192 phenotype,but not to all lipid and lipoprotein concentrations.Although HDL3-ARE activity was 23%greater in the EQQ group compared to the CQQ group,this was not a statistically significan difference.This findin supports also the beneficia effects of regular exercise on PON1 activity.No apparent PON1 phenotype effects on ARE activities or HDLs-ARE activities were observed in the present study,suggesting that ARE activity is not related to phenotype.
4.1.The effects of regular exercise on all lipid and lipoprotein concentrations
Regardless of phenotyping,HDL-C(p<0.05),HDL3-C (p<0.05),TC(p<0.001)and LDL-C(p<0.01)levels were greater significant y in the EG than CG.Considering phenotyping,while EQQ subjects had greater serum lipid and lipoprotein concentrations than their controls,ERC subjects had higherTC and higher HDL-C levels.In fact,exercise intensity,frequency,6duration of the exercise program,weekly exercise energy expenditures surpassing 2000 kcal,27and the need for more vigorous physical activity compared with men28were found to be important factors in attaining improved lipid profile in women.Therefore,exercise-linked energy expenditure can be thought of as insufficien for improving all lipid and lipoprotein concentrations in the present study.
It has been shown that PON1-192 QQ genotype group members have elevated antiatherosclerotic lipid and lipoprotein concentration profile compared to RC in the highest tertile of physical activity and that the effect on HDL concentration levels is modifieby PON1-192 polymorphisms.18Similar to previous studies,19,20we did not observe a significan difference in all lipid and lipoprotein concentrations between the CQQ and CRC phenotype groups.Differences among these studies may be mainly due to differences in the ethnic groups studied and the frequencies of various PON1-192 polymorphisms in the groups.The present study shows that the PON1 phenotype is not related to any lipid and lipoprotein concentrations notwithstanding the effects of regular exercise.
4.2.The effects of regular exercise on PON1 and ARE activity
The beneficia effects of exercise on PON1 enzyme activities observed in this study did not parallel the observations of Brites et al.14with highly trained male endurance athletes in a crosssectional study(the only published study similar to our own measurement methods).Brites et al.14did not detect any effects of PON1 phenotype or exercise on serum PON1 and ARE activities in men.Tomás et al.13determined that SSPON1 activities were decreased in RC,increased in QQ homozygous youths of both genders who trained aerobically,and that these effects were dependent on PON1-192 polymorphisms.Another study in athletes showed that the increase in PON1 activity following maximal exercise does not depend on the PON1-192 polymorphism,12in contrast to results presented by Tomás et al.13In addition,in a population study,Ferré et al.29also found that the lifestyle factors as physical activity do not play significan role on PON1 activity except PON1-192 polymorphism.Although the studies by Otocka-Kmiecik et al.,12Tomás et al.,13and Ferré et al.29were polymorphism studies unlike the present study,these results showed that the PON1-192 genotype groups may give difference answers on PON1 activity to exercise.The differences between these studies may due to differences in ethnicity,age,gender,and methods for measuring PON1 activity in these studies.
The most significan differences found in the study were the increased PON1(p<0.01)and HDL3-ARE(23%;p>0.05)activities in the EQQ group,compared with their controls. Because serum ARE activity is also used as an indicator of PON1 enzyme protein levels,30PON1 induction may have played a role in the observed increases.A previous study demonstrated that small HDL3particles have greater PON1 activity than large HDL2particles.31In addition,ARE activity is independent of PON1-192 polymorphisms.Therefore,elevated HDL3-ARE activity in the EQQ subgroup shows an improvement owing to the aerobic exercise program that was independent of the PON1 phenotype.
It has been reported that PON1 activity is related to blood HDL-C levels.5However,PON1 or ARE activities did not correlate with HDL-C levels in the present study,and the difference in HDL-C levels between the EQQ and CQQ groups was not significant Therefore,our study stands in contrast to previous work and suggests that PON1 activity in the EQQ group is unrelated to HDL-C levels.Oxidative stress can inhibit PON1 activity,whereas improvements in the antioxidant system by regular aerobic exercise can improve PON1 activity.2,5,13,14The presence of greater PON1 and HDL3-ARE activity in exercise group can also exhibit the increased antioxidant capacity by exercise training.
A limitation of this cross-sectional study was that it only enabled comparisons with a single environmental effect,which was the participation in an exercise-training program of at least 3 months in duration.Thus,caution should be used in interpreting results,as the starting lipid profil of the subjects might have been biased.Nevertheless,the exclusion and inclusion criteria imposed were designed to reduce the selection bias to an acceptable level.
The attempt to correlate PON1 activity levels with potential PON1-192 phenotypes through an indirect method11,26does not replace the need for genotyping allele frequencies,which themselves account for approximately half of the variation in PON1 activities.Although genotyping was beyond the scope of the current study,analyzing the trimodal frequency distribution of PON1 activities may be promising for future larger scale genotype-environmental effect interaction studies.
5.Conclusion
Participation in the aerobic exercise program significant y improved PON1 activities.The PON1-192 phenotype is related to the beneficia effects of regular aerobic exercise on PON1 activity,but not lipid and lipoprotein levels,in middle-aged women.
Acknowledgment
This study was supported by the Ege University Scientifi Research Projects Directorate(2006-BESYO-004).The authors would like to express thanks to Drs.Zisan Cetinkalp and Ekim Pekunlu at the Ege University School of Physical Education and Sports for their assistance in statistical analysis.
Authors’contributions
GRN carried out all phases of the study;FT executed all the biochemical analysis and drafted the manuscript;SRV conceived of the study and participated in its design and coordination;MZO orginised the determination of participants and carried out the physical and physiological measuremets of the study;MN carried out medical emaninations of the participants and assisted all the physical,physiological and biochemical analysis;SOK participated in biochemical analysis and drafted the manuscript.All authors have read and approved the fina version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financia interests.
1.Durstine JL,Haskell WL.Effects of exercise training on plasma lipids and lipoproteins.Exerc Sport Sci Rev1994;22:477–521.
2.Aviram M,Rosenblat M,Bisgaier CL,Newton RS,Primo-Parmo SL,La Du BN.Paraoxonase inhibits high density lipoprotein oxidation and preserves its functions.A possible peroxidative role for paraoxonase.J Clin Invest1998;101:1581–90.
3.Durrington PN,Mackness B,Mackness MI.Paraoxonase and atherosclerosis.Arterioscler Thromb Vasc Biol2001;21:473–80.
4.Deakin S,Leviev I,Gomaraschi M,Calabresi L,Franceschini G,James RW.Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinit,saturable, desorption mechanism.J Biol Chem2002;277:4301–8.
5.Deakin SP,James RW.Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1.Clin Sci(Lond)2004;107:435–47.
6.Superko HR,Pendyala L,Williams PT,Momary KM,King 3rd SB,Garrett BC.High-density lipoprotein subclassesand their relationship to cardiovascular disease.J Clin Lipidol2012;6:496–523.
7.Mackness B,Durrington P,McElduff P,Yarnell J,Azam N,Watt M,et al. Low paraoxonase activity predicts coronary events in the Caerphilly prospective study.Circulation2003;107:2775–9.
8.Ayub A,Mackness MI,Arrol S,Mackness B,Patel J,Durrington PN. Serum paraoxonase after myocardial infarction.Arterioscler Thromb Vasc Biol1999;19:330–5.
9.Goldhammer E,Ben-Sira D,Zaid G,Biniamini Y,Maor I,Lanir A,et al. Paraoxonase activity following exercise-based cardiac rehabilitation program.J Cardiopulm Rehabil Prev2007;27:151–4.
10.Chemnitius JM,Winkel H,Meyer I,Schirrmacher K,Armstrong VW, Kreuzer H,et al.Age related decrease of high density lipoproteins(HDL) in women after menopause.Quantificatio of HDL with genetically determined HDL arylesterase in women with healthy coronary vessels and in women with angiographically verifie coronary heart disease.Med Klin (Munich)1998;93:137–45.
11.Eckerson HW,Wyte CM,La Du BN.The human serum paraoxonase/arylesterase polymorphism.Am J Hum Genet1983;35: 1126–38.
12.Otocka-Kmiecik A, Bortnik K, Szkudlarek U, Nowak D, Orłowska-Majdak M.Effect of exercise on plasma paraoxonase1 activity in rugby players:dependence on training experience.Redox Rep2013;18: 113–9.
13.Tomás M,Elosua R,Senti M,Molina L,Vila J,Anglada R,et al. Paraoxonase1-192 polymorphism modulates the effects of regular and acute exercise on paraoxonase1 activity.J Lipid Res2002;43:713–20.
14.Brites F,Zago V,Verona J,Muzzio ML,Wikinski R,Schreier L.HDL capacity to inhibit LDL oxidation in well-trained triathletes.Life Sci2006;78:3074–81.
15.Bin Ali A,Zhang Q,Lim YK,Fang D,Retnam L,Lim SK. Expression of major HDL-associated antioxidant PON-1 is gender dependent and regulated during inflammationFree Radic Biol Med2003;34:824–9.
16.Ahmad S,Scott JE.Estradiol enhances cell-associated paraoxonase 1 (PON1)activityin vitrowithout altering PON1 expression.Biochem Biophys Res Commun2010;397:441–6.
17.Gan KN,Smolen A,Eckerson HW,La Du BN.Purificatio of human paraoxonase/arylesterase.Evidence for one esterase catalyzing both activities.Drug Metab Dispos1991;19:100–6.
18.Senti M,Aubó C,Elosua R,Sala J,Tomás M,Marrugat J.Effects of physical activity on lipid levels in a population-based sample of men with and without the Arg192 variant of the human paraoxonase gene.Genet Epidemiol2000;18:276–86.
19.Antikainen M,Murtomäki S,Syvänne M,Pahlman R,Tahvanainen E, Jauhiainen M,et al.The Gln-Arg192 polymorphism of human paraoxonase gene(HUMPONA)is not associated with the risk of coronary heart disease in Finns.J Clin Invest1996;98:883–5.
20.Ombres D,Pannitteri G,Montali A,Candeloro A,Seccareccia F, Campagna F,et al.The Gln-Arg192 polymorphism of human paraoxonase gene is not associated with coronary heart disease in Italian patients.Arterioscler Thromb Vasc Biol1998;18:1611–6.
21.Hegele RA,Brunt JH,Connelly PW.A polymorphism of the paraoxonase gene associated with variation in plasma lipoproteins in a genetic isolate.Arterioscler Thromb Vasc Biol1995;15:89–95.
22.RatamessNA,American College of Sports Medicine.ACSM’s Foundations of Strength TrainingandConditioning.Philadelphia, PA:Wolters Kluwer Health/Lippincott Williams&Wilkins;2012. p.457.
23.Midgley AW,Carroll S.Emergence of the verificatio phase procedure for confi ming‘true’VO2max.Scand J Med Sci Sport2009;19:313–22.
24.Kostner GM,Molinari E,Pichler P.Evaluation of a new HDL2/HDL3 quantitation method based on precipitation with polyethylene glycol.Clin Chim Acta1985;148:139–47.
25.Friedewald WT,Levy RI,Fredrickson DS.Estimation of the concentration of low-density lipoprotein cholesterol in plasma,without use of the preparative ultracentrifuge.Clin Chem1972;18:499–502.
26.Eckerson HW,Romson J,Wyte C,La Du BN.The human serum paraoxonase polymorphism:identificatio of phenotypes by their response to salts.Am J Hum Genet1983;35:214–27.
27.Owens JF,Matthews KA,Wing RR,Kuller LH.Physical activity and cardiovascular risk:a cross-sectional study of middle-aged premenopausal women.Prev Med1990;19:147–57.
28.Haskell WL.Exercise-induced changes in plasma lipids and lipoproteins.Prev Med1984;13:23–36.
29.Ferré N,Camps J,Fernández-Ballart J,Arija V,Murphy MM,Ceruelo S, et al.Regulation of serum paraoxonase activity by genetic,nutritional,and lifestyle factors in the general population.Clin Chem2003;49:1491–7.
30.Mackness MI,Arrol S,Mackness B,Durrington PN.Alloenzymes of paraoxonase and effectiveness of high density lipoproteins in protecting low density lipoprotein against lipid peroxidation.Lancet1997;349:851–2. 31.Kontush A,Chantepie S,Chapman MJ.Small,dense HDL particles exert potent protection of atherogenic LDL against oxidative stress.Arterioscler Thromb Vasc Biol2003;23:1881–8.
Received 14 July 2014;revised 15 November 2014;accepted 26 January 2015
Available online 28 May 2015
Peer review under responsibility of Shanghai University of Sport.
*Corresponding author.
E-mail address:gulbinrn@gmail.com(G.R.Nalcakan).
http://dx.doi.org/10.1016/j.jshs.2015.01.010
2095-2546/©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Conclusion:These results showed that a regular aerobic exercise program can improve PON1 activity depending on PON1-192 phenotype,but not on lipid and lipoprotein levels,in middle-aged Turkish women.
©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Journal of Sport and Health Science的其它文章
- Biomarker-guided classificatio scheme of neurodegenerative diseases
- Mechanism of neurodegeneration through tau and therapy for Alzheimer’s disease
- Examining the relationship between sport and health among USA women: An analysis of the Behavioral Risk Factor Surveillance System
- The microbiome,microbial-generated proinflammato y neurotoxins, and Alzheimer’s disease
- Longitudinal trajectories of physical activity in women using latent class growth analysis:The WIN Study
- Evidence of a conservative gait strategy in athletes with a history of concussions
