施氮量对矿山生态型粗齿冷水花磷富集特性的影响
2016-01-27余红梅李廷轩张锡洲郑子成余海英
余红梅,李廷轩,张锡洲,郑子成,余海英
(四川农业大学资源学院,四川 成都 611130)
施氮量对矿山生态型粗齿冷水花磷富集特性的影响
余红梅,李廷轩*,张锡洲,郑子成,余海英
(四川农业大学资源学院,四川 成都 611130)
摘要:采用土培试验,以矿山生态型粗齿冷水花为研究对象,非矿山生态型为对照,探讨了高磷(400 mg P/kg)处理下不同施氮量(0,70,140,210,280和350 mg N/kg)对矿山生态型粗齿冷水花磷富集特性的影响,为利用矿山生态型粗齿冷水花提取土壤中过量的磷,防治磷的非点源污染提供理论依据。结果表明,1)矿山生态型粗齿冷水花地上部和地下部的生物量、磷积累量均在140 mg/kg施氮量下达最大值;其中,矿山生态型地上部磷积累量为223.73 mg/株,非矿山生态型为159.79 mg/株。不同施氮量处理下,矿山生态型地上部生物量和磷积累量显著高于非矿山生态型。粗齿冷水花磷富集系数随施氮量增加逐渐升高,迁移率均高于50%,达到71%~88%。2)随施氮量增加,矿山生态型根系酸性磷酸酶活性逐渐升高,而植酸酶活性先升高后降低,在140 mg/kg达最大值。各施氮量下的矿山生态型根系酸性磷酸酶和植酸酶活性均显著高于非矿山生态型,分别为非矿山生态型的1.22~1.67倍和1.02~1.07倍。在70~210 mg/kg范围施氮有助于促进矿山生态型粗齿冷水花生长,增加植株对磷的积累,提高其富磷潜力。本研究条件下,140 mg/kg为最佳施氮量。
关键词:施氮量;磷富集;粗齿冷水花;生态型;植物修复
DOI:10.11686/cyxb2015023http://cyxb.lzu.edu.cn
余红梅,李廷轩,张锡洲,郑子成,余海英. 施氮量对矿山生态型粗齿冷水花磷富集特性的影响. 草业学报, 2015, 24(8): 85-92.
Yu H M, Li T X, Zhang X Z, Zheng Z C, Yu H Y. Effect of different levels of N supply on P accumulation characteristics of a ‘mining ecotype’ ofPileasinofasciata. Acta Prataculturae Sinica, 2015, 24(8): 85-92.
收稿日期:2015-01-16;改回日期:2015-03-30
基金项目:国家自然科学基金项目(31401377),四川省教育厅重点项目(14ZA0002),四川省科技支撑项目(2013NZ0044)和四川省科技支撑计划(2013NZ0029)资助。
作者简介:余红梅(1989-),女,贵州遵义人,在读硕士。E-mail:18200353350@163.com
通讯作者*Corresponding author. E-mail:litinx@263.net
Effect of different levels of N supply on P accumulation characteristics of a ‘mining ecotype’ ofPileasinofasciata
YU Hong-Mei, LI Ting-Xuan*, ZHANG Xi-Zhou, ZHENG Zi-Cheng, YU Hai-Ying
CollegeofResources,SichuanAgriculturalUniversity,Chengdu611130,China
Abstract:Quantities of P fertilizer and organic fertilizer are supplied in agro-ecosystems to improve the soil available P content and maintain soil fertility, but ultimately resulting in P immobilization and accumulation in the soil. Phytoextraction is a practical method for recovering the excess P after soils have become enriched. In order to provide a theoretical basis for extracting excess P from soil to assist with prevention and control of non-point source pollution, it was necessary to determine the P accumulation characteristics of a ‘mining ecotype’ (ME) of Pilea sinofasciata. This material had previously been screened as showing promise for P extraction from enriched soil. The effects of different levels of nitrogen (N) supply (0, 70, 140, 210, 280, 350 mg N/kg) on plant growth and P accumulation characteristics in the ME of P. sinofasciata were analyzed,with a non-mining ecotype (NME) as contrast. All treatments had the same P supply (400 mg P/kg soil). Pot experiments were carried out in a greenhouse at Sichuan Agricultural University, Sichuan province, China in 2013. Key results were: 1)For both shoot and root biomass, P accumulation of P. sinofasciata significantly increased with increased N supply up to 140 mg/kg, and then decreased with additional N supply. Shoot P accumulation of the ME was maximized at 140 mg/kg N supply, and ME demonstrated greater shoot P accumulation (223.73 mg/plant) than the NME (159.79 mg/plant) under different rates of N supply. The bioaccumulation coefficient of the ME was more than 1, while translation rate was more than 50%, and as high as 71%-88%. 2) The activities of acid phosphatase and phytase in P. sinofasciata peaked at N application rates of 350 mg/kg and 140 mg/kg, respectively, and the activities of these two enzymes in the ME were markedly higher (P<0.05) than those in the NME, being increased by a factor of 1.22-1.67, and 1.02-1.07, respectively. In conclusion, the P. sinofasciata ME showed substantial P accumulation ability under N application rates of 70-210 mg/kg. Thus, P. sinofasciata is a good candidate species for P phytoextraction, with the best results obtained when N was added at 140 mg/kg soil.
Key words:nitrogen (N) supply; phosphorus enrichment; Pilea sinofasciata; ecotype; phytoremediation
在农业生产中,过量施用含磷肥料以及畜禽粪便任意排放等易造成土壤磷过剩,加剧水体富营养化等环境问题[1-5]。因此,寻找合适的方法减少土壤环境中过量磷已受到广泛关注。植物提取能通过植物收获等方式带走磷,是一种较为有效的治理措施[6-8]。相关研究指出,Marshall和Gulf黑麦草[9-10]、Duo grass[11-12]、黄瓜(Cucumissativus)和黄南瓜(Cucurbitapepovar.melopepo)[13]等均能用于磷过剩环境修复,其地上部磷含量高达10 g/kg以上,然而这些富磷植物多数为一年生植物,且存在生物量小、磷积累量低、对环境适应力较弱等不足,导致磷提取效果不佳。因此,提高富磷植物磷富集潜力已成为当下研究的热点。采用多次收获和暖冷季混种等方式可在一定程度上提高牧草磷富集潜力[14-15],但其应用范围有限,效果不甚理想。Zheng等[16]研究表明,矿山生态型水蓼(Polygonumhydropiper)对畜禽废水中氮、磷去除率随培养时间延长均升高;据Silveira等[17]报道,百喜草(Paspalumnotatum)和象草(Pennisetumpurpureum)等多年生牧草种植在富磷土壤后植株体内氮磷含量均较高;表明植物对氮、磷的吸收存在协同性,适宜施氮可改善植物对土壤磷的吸收积累。Newman等[14]研究表明,适宜施氮可提高多年生暖季牧草百喜草和扁穗牛鞭草(Hemarthriaaltissima)的产量和磷去除率;适宜施氮亦可促进多年生黑麦草(Loliummultiflorum)和狗牙根(Cynodondactylon)吸收土壤中的磷以提高植株生物量和磷含量,从而有效降低表层土壤磷含量[15];表明增施氮肥有利于植物提取土壤中的磷。Xiao等[18-19]调查发现,粗齿冷水花(Pileasinofasciata)是一种多年生草本植物,具有地上部生物量大、磷含量高、对环境适应能力强和易于种植等特点,克服了普通磷富集植物的不足;且矿山生态型粗齿冷水花地上部磷含量高达16.23 g/kg,非矿山生态型仅为6.09 g/kg,具有磷富集植物的优势;前期研究得出,高磷条件下,矿山生态型粗齿冷水花磷富集能力显著高于非矿山生态型,是一种典型的磷富集植物[20-22]。本研究在前期研究基础上,进行土培试验,探讨高磷处理下不同施氮量对矿山生态型粗齿冷水花磷富集特性的影响,明确最佳施氮量,以期为合理利用其提取土壤中过剩的磷提供理论依据。
1材料与方法
1.1 供试材料
供试植物:矿山生态型粗齿冷水花采自四川省什邡市磷矿区(104°50′ E, 30°25′ N),非矿山生态型粗齿冷水花采自四川省雅安市雨城区(102°59′ E, 29°58′ N)。
供试土壤:采自四川省都江堰市白沙镇的灰潮土,其基本理化性质为:pH 6.53、速效磷(P)6.80 mg/kg、碱解氮69.04 mg/kg、速效钾(K)24.90 mg/kg、有机质18.15 g/kg。
供试肥料:尿素(N 46.60%)、磷酸二氢钾(P2O552.16%、K2O 34.61%),均为分析纯。
1.2 试验设计与处理
试验设置0,70,140,210,280和350 mg N/kg土,共6个施氮量处理。施磷量为400 mg/kg,每处理重复5次,共60盆,完全随机排列。采用土培盆栽,土壤风干后,过2 mm筛混匀;每盆(14.5 L)装土15 kg。磷配成溶液一次性施入土壤,充分混匀,陈化5周后,将尿素配成溶液加入土壤,混匀待用。陈化5周后土壤速效磷(P):185.25 mg/kg。
两种生态型粗齿冷水花幼苗于2013年5月上旬采集。幼苗的扦插管理采用刘霜等[20]的方法。待幼苗生长30 d后,将长势一致的幼苗移栽至盆中,每盆种2株。采用自然光照,每周浇水4~5次,按田间持水量的70%确定灌水量,并记录植株生长状况,及时除草、防治病虫害等。试验于2013年6月至10月在四川农业大学教学科研园区有防雨设施的网室中进行。
1.3 样品采集与制备
于移栽后9周(花期)采样,采样时将整盆倒出,植株先用自来水冲洗,再用蒸馏水润洗,洗净后用吸水纸擦干,任取一株,将其分为地上部和地下部,装袋后在105℃杀青30 min,75℃烘干至恒重,称重测定生物量,粉碎后过1 mm筛用于磷含量测定;另一株取根系经液氮迅速固定后,保存于-80℃(Thermo Freezer 700, USA)冰箱,用于酸性磷酸酶和植酸酶活性的测定。
1.4 测定项目及方法
土壤基本理化性质采用常规分析方法[23];植株全磷测定采用H2SO4-H2O2消煮-钒钼黄比色法[23];酸性磷酸酶活性测定采用对硝基苯磷酸二钠法[24];植酸酶活性测定采用Starnes等[25]的方法。
1.5 数据处理
富集系数=植株磷含量/陈化后土壤有效磷含量[26];迁移率=地上部磷积累量/植株磷积累量×100%[26];采用Excel 2007、DPS 11.0进行统计分析,Origin Pro 8.0进行图表制作。
2结果与分析
2.1 施氮量对两种生态型粗齿冷水花生物量和磷积累量的影响
从表1可知,随施氮量增加,粗齿冷水花地上部和地下部生物量均呈现先升高后降低趋势,在140 mg/kg达最大值。矿山生态型地上部生物量在70,140和210 mg/kg施氮量下显著高于对照,分别比对照增加60%,81%和46%;非矿山生态型地上部生物量在70,140和210 mg/kg显著高于对照,分别比对照增加26%,63%和49%;各施氮量下矿山生态型地上部生物量均高于非矿山生态型,为非矿山生态型的1.36~3.47倍。矿山生态型地下部生物量仅在140 mg/kg显著高于对照,非矿山生态型地下部生物量在70~280 mg/kg均高于对照;矿山生态型地下部生物量随施氮量变化小于非矿山生态型。其中,矿山生态型地下部生物量仅在70和140 mg/kg低于非矿山生态型,在其余施氮量下均高于非矿山生态型。表明140 mg/kg施氮量能使矿山生态型粗齿冷水花地上部生物量增幅最大。
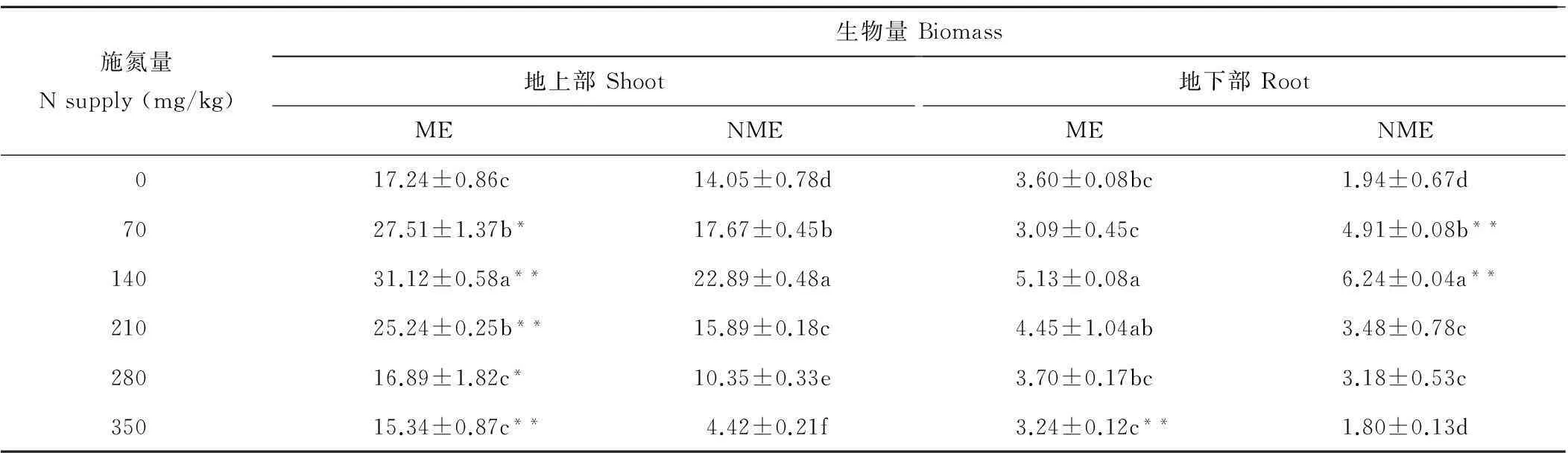
表1 施氮量对两种生态型粗齿冷水花生物量的影响
注:ME表示矿山生态型,NME表示非矿山生态型;同列不同字母表示不同施氮量间差异显著(P<0.05),*表示不同生态型间差异显著(P<0.05),** 表示不同生态型间差异极显著(P<0.01)。下同。
Note: ME means mining ecotypes, NME means non-mining ecotypes; Mean values labeled with different letters in the same column are significantly different (P<0.05) at different N supply. * means significantly different (P<0.05) at different ecotypes at the same N supply. ** means significantly different (P<0.01) at different ecotypes at the same N supply. The same below.
从表2可知,随施氮量增加,粗齿冷水花地上部和地下部磷积累量先升高后降低,在140 mg/kg达最大值。其中,矿山生态型地上部磷积累量在70,140和210 mg/kg显著高于对照,分别比对照增加29%,73%和41%;非矿山生态型在70,140和210 mg/kg显著高于对照,分别比对照增加46%,76%和31%。各施氮量下矿山生态型地上部磷积累量均高于非矿山生态型,为非矿山生态型的1.34~2.59倍。矿山生态型地下部磷积累量仅在140和210 mg/kg显著高于对照,非矿山生态型在70,140和210 mg/kg显著高于对照。除70和140 mg/kg施氮量外,矿山生态型地下部磷积累量均高于非矿山生态型。表明矿山生态型粗齿冷水花地上部和地下部磷积累量受施氮量影响较大,在140 mg/kg施氮量下其磷积累量最大。
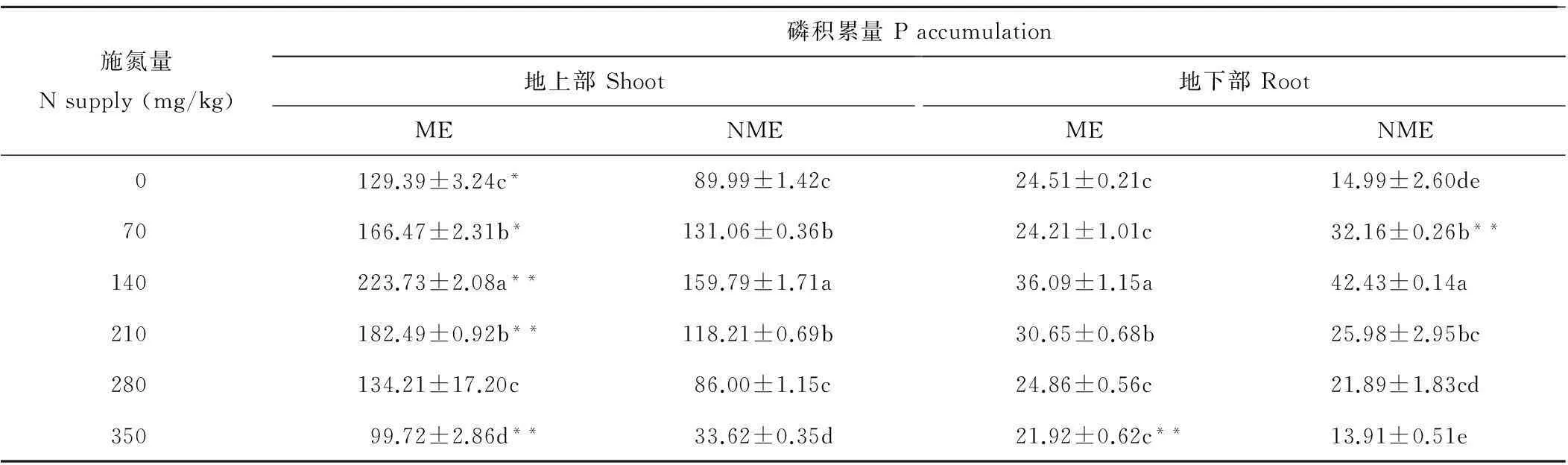
表2 施氮量对两种生态型粗齿冷水花磷积累量的影响
2.2 施氮量对两种生态型粗齿冷水花磷富集系数和迁移率的影响
由表3可知,矿山生态型粗齿冷水花磷富集系数随施氮量增加而升高,在350 mg/kg施氮量达最大值。各施氮量下矿山生态型磷富集系数均大于1,且高于对照。两种生态型粗齿冷水花磷富集系数在不同施氮量下差异较小,表明适宜施氮可促进矿山生态型粗齿冷水花对磷的吸收,增加植株生物量,而对其体内磷含量有一定稀释作用,导致富集系数差异较小。
迁移率能很好地反映粗齿冷水花向地上部转移磷的能力。由表3可知,在不同施氮量下,矿山生态型粗齿冷水花磷迁移率均大于50%。除对照外,矿山生态型粗齿冷水花迁移率在不同施氮量下均高于非矿山生态型,在70和140 mg/kg处理下表现最为明显。表明适宜增施氮肥能提高矿山生态型向地上部迁移磷的能力,增加植株对磷的积累。
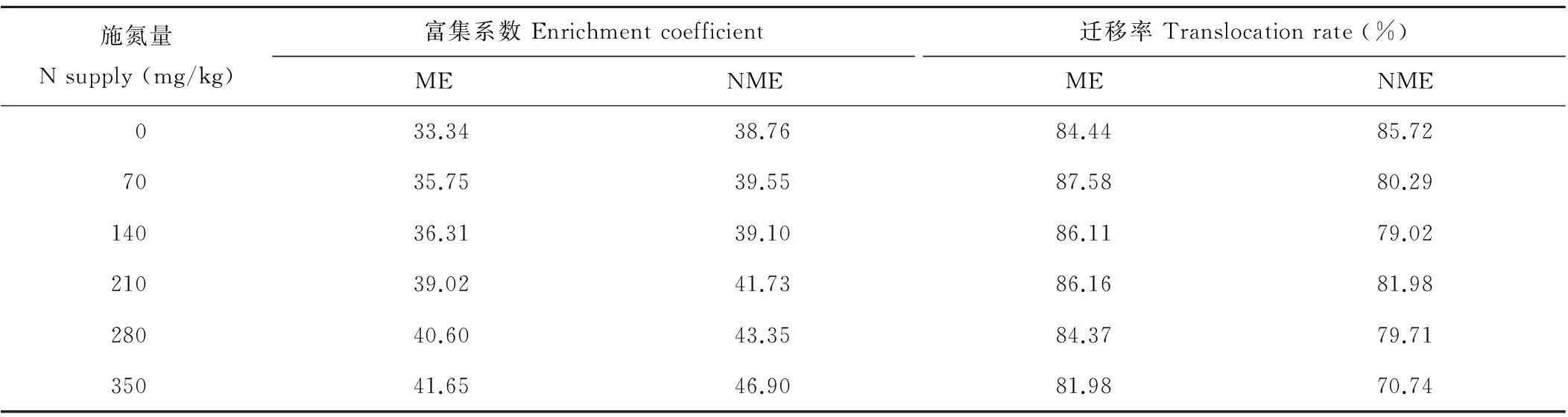
表3 施氮量对两种生态型粗齿冷水花磷富集系数和迁移率的影响
2.3 施氮量对两种生态型粗齿冷水花根系酸性磷酸酶和植酸酶活性的影响
由图1可知,在高磷(400 mg/kg)条件下,随施氮量增加,矿山生态型粗齿冷水花根系酸性磷酸酶活性逐渐升高,植酸酶活性则先升高后降低,在140 mg/kg达最大值。各施氮量下矿山生态型根系酸性磷酸酶和植酸酶活性均显著高于非矿山生态型(P<0.05),分别为非矿山生态型的1.22~1.67倍和1.02~1.07倍。在70~350 mg/kg施氮量下,矿山生态型根系酸性磷酸酶活性分别比对照增加34%~281%,非矿山生态型根系酸性磷酸酶活性比对照增加23%~178%。矿山生态型根系植酸酶活性在施氮量为70,140和210 mg/kg时分别比对照增加3.80%,13.60%和8.40%,非矿山生态型根系植酸酶活性则比对照增加4.2%,10.0%和5.2%。表明高磷条件下,适宜施氮可显著提高矿山生态型粗齿冷水花根系酸性磷酸酶和植酸酶活性。
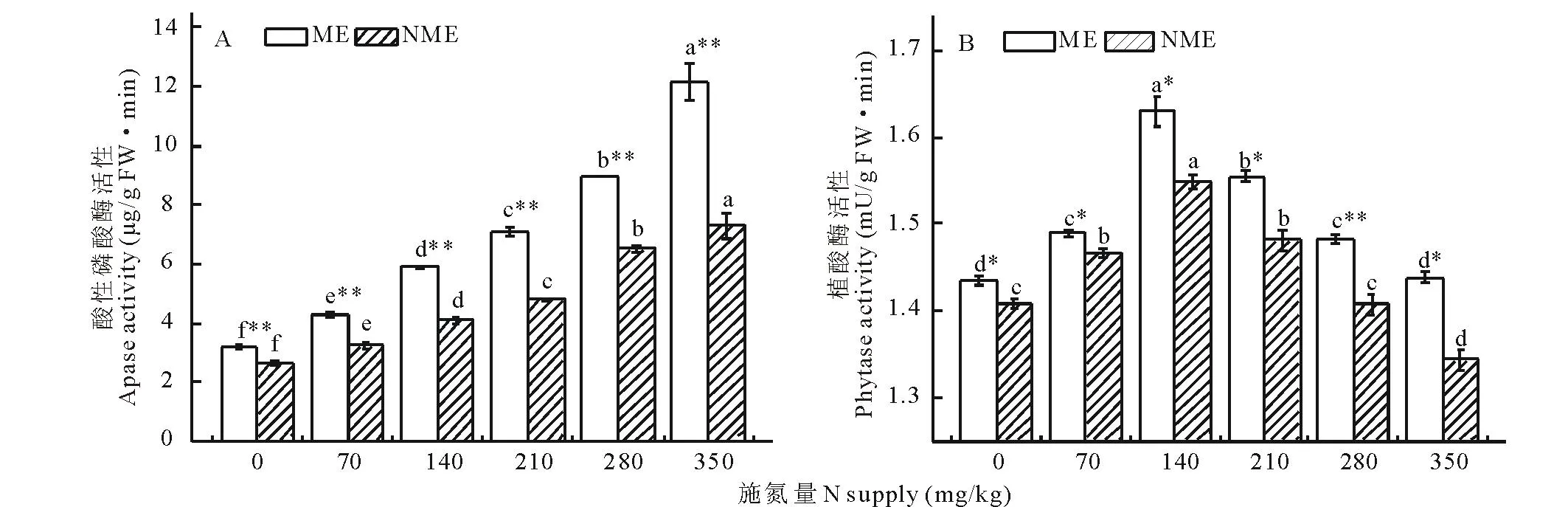
图1 施氮量对两种生态型粗齿冷水花根系酸性磷酸酶(A)和植酸酶(B)活性的影响Fig.1 Effect of different N supply on the activities of apase (A) and phytase (B) in the root of two ecotypes of P. sinofasciata ME表示矿山生态型,NME表示非矿山生态型;不同字母表示同种生态型不同施氮量间差异显著(P<0.05),* 表示同一处理不同生态型间差异显著(P<0.05),** 表示同一处理不同生态型间差异极显著(P<0.01)。ME means mining ecotypes, NME means non-mining ecotypes; Mean values labeled with different letters in the same ecotypes are significantly different (P<0.05) at different N supply. * means significantly different (P<0.05) at different ecotypes at the same N supply. ** means significantly different (P<0.01) at different ecotypes at the same N supply.
3讨论
目前,有关富磷植物的研究主要集中在不同植物品种和同一品种不同基因型或生态型间富磷能力比较等方面[27-32],对如何提高其磷富集潜力的研究较少;况且报道的富磷植物多为陆生植物,对于既适合陆生又适合湿生的植物研究较为缺乏。粗齿冷水花是一种多年生草本植物,广泛分布于中国大陆南方地区的路边、河边和山坡上,可陆生和湿生,具有生物量大、磷含量高、根系分布深、对环境适应能力强、可连续多年提取等优点。前期土培试验表明,高磷处理下的矿山生态型粗齿冷水花对磷的富集能力较强[20],其磷富集潜力远高于非矿山生态型粗齿冷水花、向日葵(Helianthusannus)、黄南瓜、Marshall和Gulf黑麦草等磷富集植物[9,13]。本研究在前期研究的基础上进一步揭示了施氮量对高磷处理下的两种生态型粗齿冷水花磷富集潜力的影响。相关研究表明,合理施氮可促进甜瓜(Cucumismelo)[33]、饲草玉米(Zeamays)[34]、百喜草和扁穗牛鞭草[14]地上部的生长,增加收获部分生物量;不合理施氮则会引发氮肥增产效益降低,对植物生长产生毒害等问题。本研究中,在140 mg/kg施氮量下,矿山生态型生物量达36.25 g/株,远高于非矿山生态型(29.13 g/株)以及相同磷浓度和同一采样时期下的矿山生态型生物量(4.49 g/株)[21];当施氮量超过140 mg/kg,生物量显著下降,表明适宜施氮可有效提高矿山生态型生物量,不合理施氮则会对植株产生毒害,进而降低其生物量。
施氮不仅会影响植物的生长,也会影响植物对养分的吸收积累。汤明尧等[35]研究表明,施氮可促进加工番茄(Lycopersiconesculentum)植株对磷的吸收,各施氮处理下的磷积累量为对照的1.49~2.63倍;有关甜瓜[33]和杂交棉(Gossypiumhirsutum)[36]等的研究指出,适宜增施氮肥可有效提高植株磷积累量,过量施氮则不利于植株磷的积累。本研究中,在0~140 mg/kg施氮范围内,高磷处理下的矿山生态型粗齿冷水花植株磷积累量逐渐增加;当施氮量高于140 mg/kg,矿山生态型磷积累量显著下降;表明在0~140 mg/kg范围内施氮可显著提高矿山生态型粗齿冷水花磷积累量,过量施氮则会影响植株磷积累。植物修复的高效率表现在通过地上部带走污染物的总量[6],矿山生态型地上部和整株磷积累量在140 mg/kg施氮量下可达223.73和259.82 mg/株,非矿山生态型达159.79和202.22 mg/株,远高于相同磷浓度和同一采样时期下的矿山生态型整株磷积累量(30.25 mg/株)[21];而高磷(猪粪)处理下矿山生态型粗齿冷水花整株磷积累量仅为99.40 mg/株[22];可水陆两生的水蓼(Polygonumhydropiper)在高磷条件下磷积累量也仅为114.88 mg/株[26];用于污水修复的凤眼莲(Eichhorniacrassipes)和粉绿狐尾藻(Myriophyllumaquaticum)体内磷积累量最高也仅为80.13和38.72 mg/株[37]。迄今为止研究最为深入的牧草Ptilotus[38]、Marshall和Gulf黑麦草[9-10],在高磷处理下的地上部磷积累量也仅能达到120,29.33和29.70 mg/盆,远低于本研究中的矿山生态型粗齿冷水花的单株磷积累量。因此,适宜增施氮能显著提高矿山生态型粗齿冷水花磷积累量,强化其富磷潜力。植物根系分泌酸性磷酸酶是提高有机磷在土壤中的生物有效性的重要途径之一[39-40],植酸酶是对植酸盐类具有高度的专一性的磷酸单酯水解酶,为肌醇与磷酸(盐)一类酶的总称[12, 24],对植酸盐类具有高度的专一性。根系分泌酸性磷酸酶被认为是植物适应低磷胁迫的一种机制[41-43]。然而,研究得出,高磷处理下的富磷植物黄瓜、黄南瓜[13]、水蓼[26]、Duofestulolium[10,24]、Marshall和Gulf黑麦草[9-10]等的根系酸性磷酸酶和植酸酶活性均高于对照;叶代桦等[26]认为,富磷植物水蓼体内较高的酸性磷酸酶和植酸酶活性是其对磷富集的机理之一。本研究中,高磷处理下的粗齿冷水花酸性磷酸酶活性随施氮量增加而升高,植酸酶活性则先升高后降低,表明适宜施氮可显著提高高磷处理下的粗齿冷水花根系酸性磷酸酶和植酸酶活性,增加植物对磷的富集;矿山生态型根系酸性磷酸酶和植酸酶活性始终高于非矿山生态型,说明各施氮量下矿山生态型粗齿冷水花对磷的富集作用更强。以上研究表明,矿山生态型粗齿冷水花在适宜施氮量下的生长状况更佳,比非矿山生态型以及普通富磷植物更具磷富集优势。除多次收获和暖冷季混种等方式外,适宜施氮亦可有效提高植物对磷的富集潜力。
4结论
随施氮量的增加,矿山生态型粗齿冷水花生物量和磷积累量先升高后降低,在140 mg/kg施氮量下达最大值。其中,矿山生态型地上部和整株磷积累量达223.73和259.82 mg/株。各施氮量下矿山生态型生物量和磷积累量均高于非矿山生态型。矿山生态型磷富集系数随施氮量增加而增加,磷迁移率均高于50%。与非矿山生态型相比,矿山生态型对磷的富集潜力更大。
矿山生态型根系酸性磷酸酶和植酸酶活性分别在350和140 mg/kg达峰值,且各施氮量下矿山生态型根系酸性磷酸酶活性和植酸酶活性均显著高于非矿山生态型。在140 mg/kg施氮量下,高磷环境中的矿山生态型粗齿冷水花根系较高的酸性磷酸酶和植酸酶活性是其高效积累磷的原因之一。
本研究发现,140 mg/kg为最佳施氮量,该施氮量下矿山生态型粗齿冷水花的长势和磷富集潜力均最优。
References:
[1]Chakraborty D, Nair V D, Chrysostome M,etal. Soil phosphorus storage capacity in manure-impacted Alaquods: Implications for water table management. Agriculture, Ecosystems & Environment, 2011, 142(3): 167-175.
[2]Waldrip H M, He Z, Erich M S. Effects of poultry manure amendment on phosphorus uptake by ryegrass, soil phosphorus fractions and phosphatase activity. Biology and Fertility of Soils, 2011, 47(4): 407-418.
[3]Schwartz R, Dao T H, Bell J M. Manure and mineral fertilizer effects on seasonal dynamics of bioactive soil phosphorus fractions. Agronomy Journal, 2011, 103(6): 1724-1733.
[4]Chien S H, Prochnow L I, Tu S,etal. Agronomic and environmental aspects of phosphate fertilizers varying in source and solubility: an update review. Nutrient Cycling in Agroecosystems, 2011, 89(2): 229-255.
[5]DeLaune P B, Moore P A, Carman D K,etal. Evaluation of the phosphorus source component in the phosphorus index for pastures. Journal of Environmental Quality, 2004, 33(6): 2192-2200.
[6]Pant H K, Mislevy P, Rechcigl J E. Effects of phosphorus and potassium on forage nutritive value and quantity: environmental implications. Agronomy Journal, 2004, 96(5): 1299-1305.
[7]Xiang W, Xiao E Y, Rengel Z. Phytoremediation facilitates removal of nitrogen and phosphorus from eutrophicated water and release from sediment. Environmental Monitoring and Assessment, 2009, 157(1): 277-285.
[8]Padmanabhan P, Sahi S V. Suppression subtractive hybridization reveals differential gene expression in sunflower grown in high P. Plant Physiology and Biochemistry, 2011, 49(6): 584-591.
[9]Sharma N C, Sahi S V, Jain J C,etal. Enhanced accumulation of phosphate byLoliummultiflorumcultivarsgrown in phosphate enriched medium. Environmental Science & Technology, 2004, 38(8): 2443-2448.
[10]Sharma N C, Sahi S V. Enhanced organic phosphorus assimilation promoting biomass and shoot P hyperaccumulations inLoliummultiflorumgrown under sterile conditions. Environmental Science & Technology, 2011, 45(24): 10531-10537.
[11]Padmanabhan P, Starnes D L, Sahi S V. Differential responses ofDuograss(Lolium×Festuca), a phosphorus hyperaccumulator to high phosphorus and poultry manure treatments. African Journal of Biotechnology, 2013, 12(21): 3191-3195.
[12]Priya P, Sahi S V. Influence of phosphorus nutrition on growth and metabolism ofDuograss(Duofestulolium). Plant Physiology and Biochemistry, 2009, 47(1): 31-36.
[13]Sharma N C, Starnes D L, Sahi S V. Phytoextraction of excess soil phosphorus. Environmental Pollution, 2007, 146(1): 120-127.
[14]Newman Y C, Agyin-Birikorang S, Adjei M B,etal. Enhancing phosphorus phytoremedation potential of two warm-season perennial grasses with nitrogen fertilization. Agronomy Journal, 2009, 101(6): 1345-1351.
[15]Read J J. Spring nitrogen fertilization of ryegrass-bermudagrass for phytoremediation of phosphorus enriched soils. Agronomy Journal, 2012, 104(4): 908-916.
[16]Zheng Z C, Li T X, Zeng F F,etal. Accumulation characteristics of and removal of nitrogen and phosphorus from livestock wastewater byPolygonumhydropiper. Agricultural Water Management, 2013, 117: 19-25.
[17]Silveira M L, Vendramini J M B, Sui X,etal. Screening perennial warm-season bioenergy crops as an alternative for phytoremediation of excess soil P. BioEnergy Research, 2013, 6(2): 469-475.
[18]Xiao G L, Li T X, Zhang X Z,etal. Uptake and accumulation of phosphorus by dominant plant species growing in a phosphorus mining area. Journal of Hazardous Materials, 2009, 171(1-3): 542-550.
[19]Xiao G L, Li T X, Zhang X Z,etal. Effect of different phosphorus treatments on the physiological and biochemical characteristics ofPileasinofasciata. Communications in Soil Science and Plant Analysis, 2010, 41(12): 1433-1444.
[20]Liu S, Li T X, Ji L,etal. Phosphorus accumulation and root morphological difference of two ecotypes ofPileasinofasciatagrown in different phosphorus treatments. Acta Prataculturae Sinica, 2013, 22(3): 211-217.
[21]Zheng Z C, Li T X, Zhang X Z,etal. Phosphorous accumulation and distribution of two ecotypes ofPileasinofasciatagrown in phosphorous-enriched soils. Applied Soil Ecology, 2014, 84: 54-61.
[22]Ye D H, Li T X, Zhang X Z,etal. P uptake characteristics and P removal potentials ofPileasinofasciatagrown under soils amended with swine manure. Ecological Engineering, 2014, 73: 553-559.
[23]Lu R K. Analytical Methods of Soil and Agricultural Chemistry[M]. Beijing: China Agricultural Scientech Press, 2000: 146-315.
[24]Sharma N C, Sahi S V. Characteristics of phosphate accumulation inLoliummultiflorumfor remediation of phosphorus-enriched soils. Environmental Science & Technology, 2005, 39(14): 5475-5480.
[25]Starnes D L, Padmanabhan P, Sahi S V. Effect of P sources on growth, P accumulation and activities of phytase and acid phosphatases in two cultivars of annual ryegrass (LoliummultiflorumL.). Plant Physiology and Biochemistry, 2008, 46(5-6): 580-589.
[26]Ye D H, Li T X, Zhang X Z,etal. Effect of high phosphate supply on P accumulation characteristics of mining ecotype ofPolygonumhydropiper. Plant Nutrition and Fertilizer Science, 2013, 20(1): 196-204.
[27]Missouri A M, Boerma H R, Bouton J H. Genetic variation and heritability of phosphorus uptake in Alamo switchgrass grown in high phosphorus soils. Field Crops Research, 2005, 93(2): 186-198.
[28]Marschner P, Solaiman Z, Rengel Z. Brassica genotypes differ in growth, phosphorus uptake and rhizosphere properties under P-limiting conditions. Soil Biology and Biochemistry, 2007, 39(1): 87-98.
[29]Muir J P, Bow J R. Herbage, phosphorus, and nitrogen yields of winter-season forages on high-phosphorus soil. Agronomy Journal, 2009, 101(4): 764-768.
[30]Huang X, Li T X, Zhang X Z,etal. Growth, P accumulation, and physiological characteristics of two ecotypes ofPolygonumhydropiperas affected by excess P supply. Journal of Plant Nutrition and Soil Science, 2012, 175(2): 290-302.
[31]Ye D H, Li T X, Chen G D,etal. Influence of swine manure on growth, P uptake and activities of acid phosphatase and phytase ofPolygonumhydropiper. Chemosphere, 2014, 105(2014):139-145.
[32]Hammond J P, White P J. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany, 2008, 59(1): 93-109.
[33]Hu G Z, Feng J X, Zhang Y,etal. Effects of nitrogen fertilization on nutrient uptake, assignment, utilization and yield of melon. Plant Nutrition and Fertilizer Science, 2013, 19(3): 760-766.
[34]Cheng Y X, Cheng X, Cheng X P,etal. Effects of different nitrogen additions on the yield, quality and nutrition absorption of forge maize. Acta Prataculturae Sinica, 2014, 23(3): 255-261.
[35]Tang M Y, Zhang Y, Hu W,etal. Effects of different nitrogen rates on nutrition absorption, distribution and yield in tomato. Plant Nutrition and Fertilizer Science, 2010, 16(5): 1238-1245.
[36]Li L L, Fang W P, Ma Z B,etal. Effects of nitrogen fertilization on uptake and utilization of NPK and yield and quality of hybrid cotton. Plant Nutrition and Fertilizer Science, 2010, 16(3): 663-667.
[37]Polomski R F, Taylor M D, Bielenberg D G,etal. Nitrogen and phosphorus remediation by three floating aquatic macrophytes in greenhouse-based laboratory-scale subsurface constructed wetlands. Water, Air, and Soil Pollution, 2009, 197: 223-232.
[38]Ryan M H, Ehrenberg S, Bennett R G,etal. Putting the P inPtilotus: a phosphorus-accumulating herb native to Australia. Annals of Botany, 2009, 103: 901-911.
[39]Richardson A E, Hadobas P A, Hayes J E. Acid phosphomonoesterase and phytase activities of wheat (TriticumaestivumL.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant, Cell & Environment, 2000, 23(4): 397-405.
[40]Ramesh A, Sharma S K, Joshi O P,etal. Phytase, phosphatase activity and P-nutrition of soybean as influenced by inoculation of Bacillus. Indian Journal of Microbiology, 2011, 51(1): 94-99.
[41]George T S, Gregory P J, Hocking P,etal. Variation in root-associated phosphatase activities in wheat contributes to the utilization of organic P substrates in vitro, but does not explain differences in the P-nutrition of plants when grown in soils. Environmental and Experimental Botany, 2008, 64(3): 239-249.
[42]Qiu H, Liu C, Yu T,etal. Identification of QTL for acid phosphatase activity in root and rhizosphere soil of maize under low phosphorus stress. Euphytica, 2014, 197(1): 133-143.
[43]Huang Y, Zhang H W, Xu F S. Research progress on plant acid phosphatase. Journal of Huazhong Agricultural University, 2008, 27(1): 148-154.
参考文献:
[20]刘霜, 李廷轩, 戢林, 等. 不同磷处理下两种生态型粗齿冷水花的富磷特征及根系形态差异. 草业学报, 2013, 22(3): 211-217.
[23]鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000: 146-315.
[26]叶代桦, 李廷轩, 张锡洲, 等. 高磷对矿山生态型水蓼磷富集特性的影响. 植物营养与肥料学报, 2013, 20(1): 196-204.
[33]胡国智, 冯炯鑫, 张炎, 等. 不同施氮量对甜瓜养分吸收、分配、利用及产量的影响. 植物营养与肥料学报, 2013, 19(3): 760-766.
[34]陈远学, 陈曦, 陈新平, 等. 不同施氮对饲草玉米产量品质及养分吸收的影响. 草业学报, 2014, 23(3): 255-261.
[35]汤明尧, 张炎, 胡伟, 等. 不同施氮水平对加工番茄养分吸收、分配及产量的影响. 植物营养与肥料学报, 2010, 16(5): 1238-1245.
[36]李伶俐, 房卫平, 马宗斌, 等. 施氮量对杂交棉氮、磷、钾吸收利用和产量及品质的影响. 植物营养与肥料学报, 2010, 16(3): 663-667.
[43]黄宇, 张海伟, 徐芳森. 植物酸性磷酸酶的研究进展. 华中农业大学学报, 2008, 27(1): 148-154.
