新型含2-喹诺酮基的氧杂蒽二酮衍生物的合成*
2016-01-17张珍明王润南张丹丹李树安中国矿业大学化工学院江苏徐州淮海工学院化工学院江苏连云港005
王 璇,张珍明,,王润南,张丹丹,李树安(.中国矿业大学化工学院,江苏徐州 6; .淮海工学院化工学院,江苏连云港 005)
新型含2-喹诺酮基的氧杂蒽二酮衍生物的合成*
王璇1,张珍明1,2,王润南2,张丹丹2,李树安2
(1.中国矿业大学化工学院,江苏徐州221116; 2.淮海工学院化工学院,江苏连云港222005)
摘要:以醋酸为催化剂,DMF为溶剂,取代-2-氯喹啉-3-甲醛和5,5-二甲基-1,3-环己二酮于80℃~100℃反应2.5 h~4.0 h,合成了5个新型的含2-喹诺酮基的氧杂蒽二酮衍生物,收率78%~90%,其结构经1H NMR,IR,HR-ESI-MS和元素分析表征。3,3,6,6-四甲基-9-{ 3-[7-甲基喹啉基-2(1H)酮]}-2,4,5,7,9,10-六氢化氧杂蒽-1,8(2H,5H)二酮(3b)经X-射线单晶衍射表征。3b(CCDC:971 833)属单斜晶系,空间群C12/C1,晶胞参数a=1.411 90(16)nm,b=2.231 4(2)nm,c=1.618 28(18)nm,β=106.904°,V=4.878 1(9)nm3,Dc=1.175 g·cm-3,Z=1,R1=0.044 4,wR2=0.074 6。
关键词:氧杂蒽二酮; 5,5-二甲基-1,3-环己二酮; 2-氯喹啉-3-甲醛;合成;晶体结构
峰资助项目(2009年,2013年);江苏省高校优势学科建设工程资助项目;江苏省海洋资源开发研究院项目(JSIMR 201203);
连云港市科技项目工业攻关(CG1302)
通信联系人:李树安,教授,E-mail:li_shuan@126.com
氧杂蒽含有吡喃环结构,是许多天然药物的重要结构单元,同时也是合成其他杂环化合物的重要中间体,具有广泛的生物活性及药理活性,如抗癌[1]、抗过敏[2]、抗菌[3]、抗高血压[4],治疗过敏性支气管炎[5]、糖尿病[6]和疟疾流行疾病[7],同时还在染料[8]、荧光材料[9]和激光材料[10]等方面也有很好的应用。
合成氧杂蒽衍生物的方法通常由芳香醛衍生物[11-12]、吡啶醛衍生物、呋喃醛衍生物和5,5-二甲基-1,3-环己二酮(2)在相转移催化剂[13]、固体酸[14]、离子液体[15]、微波[16]、超声波[17-18]等催化下合成。本文以醋酸为催化剂,DMF为溶剂,取代-2-氯喹啉-3-甲醛(1a~1e)和2于80℃~100℃反应2.5 h~4.0 h,合成了5个新型的含2-喹诺酮基的氧杂蒽二酮衍生物——3,3,6,6-四甲基-9-{ 3-[喹啉基-2(1H)酮]}-2,4,5,7,9,10-六氢化氧杂蒽-1,8(2H,5H)二酮衍生物(3a~3e,Scheme 1),收率78%~90%,其结构经1H NMR,IR,HR-ESI-MS和元素分析表征。并对3,3,6,6-四甲基-9-{ 3-[7-甲基喹啉基-2(1H)酮]}-2,4,5,7,9,10-六氢化氧杂蒽-1,8(2H,5H)二酮(3b)进行了X-射线单晶衍射分析。
该方法具有合成条件温和,后处理方便,产率较高等优点。3a~3e结构中同时含有具有生物活性的喹诺酮和氧杂蒽结构,期望能有较好的应用前景,其抗菌等生物活性正在研究之中。
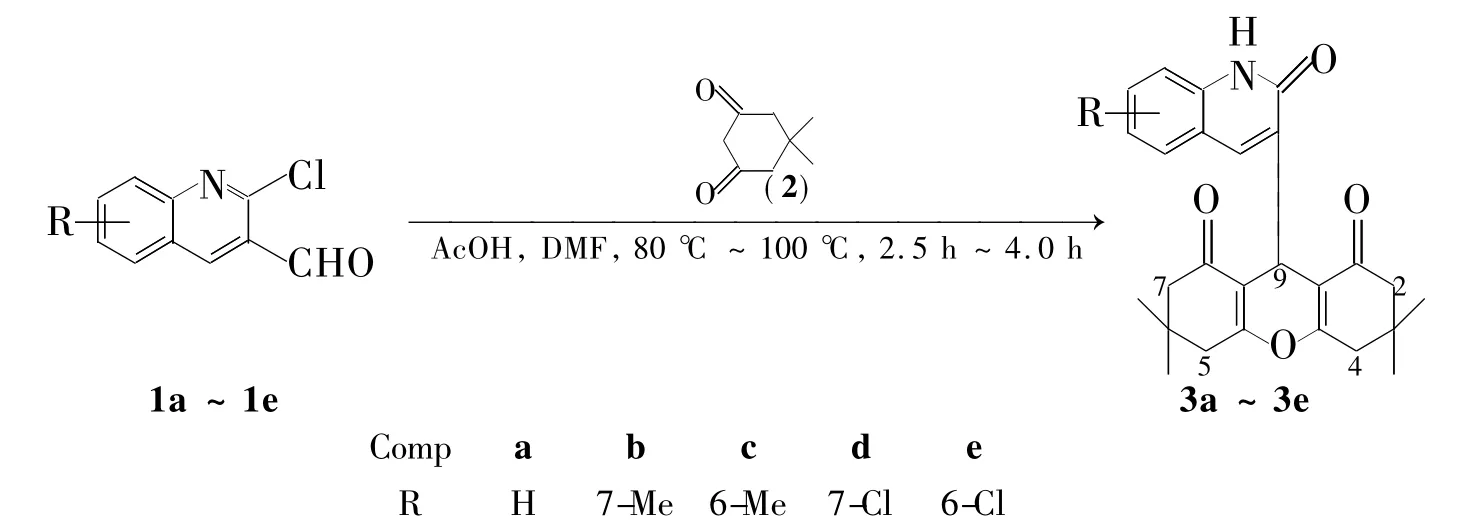
Scheme 1
1 实验部分
1.1仪器与试剂
SGWX-4型熔点仪(温度未校正); Brucker ARX 400 MHz型核磁共振仪(CDCl3为溶剂,TMS为内标); Brucker TENSOR 37型傅里叶红外光谱仪(KBr压片); Agilent Technologies 6230 TOF型质谱仪(Dual AJSESI检测器,EI离子源); PE 2400-Ⅱ型元素分析仪; Brucker SMART 1000型单晶衍射仪。
1a~1e参考文献[19]方法合成;其余所用试剂均为化学纯或分析纯。
1.23a~3e的合成通法[20-22]
在单口烧瓶中加入1 1 mmol,2 2 mmol,DMF 10 mL和两滴醋酸,搅拌下于80℃~100℃反应2.5~4.0 h。倒入50 mL冰水中,静置析晶。过滤,滤饼真空干燥后用无水乙醇重结晶得淡黄色固体3a~3e。
在烧杯中加入3b,乙醇和水,加热使其溶解;冷却至室温,用保鲜膜封口,并扎若干小孔,静置数天析晶。过滤,滤饼干燥得黄色晶体3b。
3a:产率88%;1H NMR δ:8.05(s,1H,NH),7.73~7.71(d,J=8.0 Hz,1H,ArH),7.53~7.49(t,J=8.0 Hz,8.0 Hz,1H,ArH),7.28~7.26(d,J=8.0 Hz,1H,ArH),7.21~7.17(t,J=8.0 Hz,8.0 Hz,1H,ArH),4.85(s,1H,9-H),2.53~2.42(dd,J=17.6 Hz,17.6 Hz,4H,2,7-H),2.26~2.15(dd,J=16.0 Hz,16.0 Hz,4H,4,5-H),1.09(s,6H,3,6-CH3),0.98(s,6H,3,6-CH3); IR ν:3 432(NH),2 919(CH3),1 666(C=O),1 569(Ph)cm-1; HRESI-MS m/z:Calcd for C26H27NO4{[M + H]+} 418.201 8,found 418.202 0; Anal.calcd for C26H27NO4:C 74.80,H 6.52,N 3.35; found C 74.87,H 6.47,N 3.45。
3b:产率90%;1H NMR δ:8.10(s,1H,NH),7.56~7.54(d,J=8.0 Hz,1H,ArH),7.26(s,1H,ArH),7.12(s,1H,ArH),7.04~7.02(d,J=8.0 Hz,1H,ArH),4.80(s,1H,9-H),2.48~2.46(d,J=7.2 Hz,7H,2,7-H,7'-CH3),2.25~2.13(dd,J=16.4 Hz,16.4 Hz,4H,4,5-H),1.06(s,6H,3,6-CH3),0.92(s,6H,3,6-CH3); IR ν:3 312(NH),2 956,2 938(CH3),1 666(C=O),1 566(Ph),1 222(C-O)cm-1; HR-ESI-MS m/z:Calcd for C27H29NO4{[M + H]+} 432.217 5,found 432.221 3; Anal.calcd for C27H29NO4:C 75.15,H 6.77,N 3.25; found C 75.25,H 6.65,N 3.18。
3c:产率85%;1H NMR δ:8.11(s,1H,NH),7.70~7.68(d,J=8.8 Hz,1H,ArH),7.42(s,1H,ArH),7.42~7.40(d,J=8.8 Hz,1H,ArH),4.81(s,1H,9-H),2.44~2.32(dd,J=16.0 Hz,16.0 Hz,4H,2,7-H),2.29(s,3H,6'-CH3),2.14~2.02(dd,J=16.0 Hz,16.0 Hz,4H,4,5-H),1.01(s,6H,3,6-CH3),0.97(s,6H,3,6-CH3); IR ν:3 427(NH),2 960,2 939(CH3),1 663(C=O),1 599(Ph),1 223(C-O)cm-1; HR-ESI-MS m/z:Calcd for C27H29NO4{[M + H]+} 432.217 5,found 432.221 3; Anal.calcd for C27H29NO4:C 75.15,H 6.77,N 3.25; found C 75.23,H 6.63,N 3.29。
3d:产率78%;1H NMR δ:8.25(s,1H,NH),7.73~7.71(d,J=8.0 Hz,1H,ArH),7.53~7.49(t,J=8.0 Hz,8.0 Hz,1H,ArH),7.28~7.26(d,J=8.0 Hz,1H,ArH),4.85(s,1H,9-H),2.53~2.42(dd,J=17.6 Hz,17.6 Hz,4H,2,7-H),2.26~2.15(dd,J=16.0 Hz,16.0 Hz,4H,4,5-H),1.09(s,6H,3,6-CH3),0.98(s,6H,3,6-CH3); IR ν:3 426(NH),2 955,2 924(CH3),1 660(C=O),1 563(Ph),1 216(C-O)cm-1; HR-ESI-MS m/z:Calcd for C26H26NO4Cl{[M + H]+} 452.162 9,found 452.164 6; Anal.calcd for C26H26NO4Cl:C 69.10,H 5.80,N 3.10; found C 69.07,H 5.91,N 3.17。
3e:产率80%;1H NMR δ:8.08(s,1H,NH),7.55~7.53(d,J=8.0 Hz,1H,ArH),7.26(s,1H,ArH),7.03~7.01(d,J=8.0 Hz,1H,ArH),4.79(s,1H,9-H),2.51~2.46(dd,J=10.8 Hz,10.8 Hz,4H,2,7-H),2.25~2.13(dd,J=16.0 Hz,16.0 Hz,4H,4,5-H),1.06(s,6H,3,6-CH3),0.91(s,6H,3,6-CH3); IR ν:3 425(NH),2 960,2 939(CH3),1 663(C=O),1 599(Ph),1 221(C-O)cm-1; HR-ESI-MS m/z:Calcd for C26H26NO4Cl{[M + H]+} 452.162 9,found 452.163 3; Anal.calcd for C26H26NO4Cl:C 69.10,H 5.80,N 3.10; found C 69.17,H 5.91,N 3.01。
1.3晶体结构测定
将3b(0.12 mm×0.15 mm×0.20 mm)置衍射仪上,采用石墨单色化的Mo Kα射线(λ=0.710 73Å),于296(2)K下以ω/2θ的方式在1.09°≤θ≤25.25°内收集12 755个强反射数据,其中独立衍射点4 520个[R(int)=0.027 7]。收集的数据经APEX2软件还原和晶胞参数修正,用SADAB作吸收校正,采用SHELXL-97程序完成结构分析和计算[23],所得数据经Lp因子和经验吸收校正。
2 结果与讨论
2.1表征
由于3a~3e的熔点都大于300℃,且熔点仪并未校正,所以本文并未列出其熔点。以3b为例分析进行分析。1H NMR分析表明:δ 0.92和δ 1.06处出现了两个单峰,归属氧杂蒽环上的4个甲基上的H,由于空间位阻的原因导致碳原子上连接的两个甲基的化学位移不相同;δ 2.1~2.4间有多重峰出现,为4个亚甲基上的H; δ 4.79左右处出现的单峰,归属氧杂蒽环9-位上的H;δ 7.0~8.1间出现的化学位移为喹诺酮环上氢的化学位移。IR分析表明:3 312 cm-1处有较强的N-H对称伸缩振动吸收峰,1 666 cm-1处有强的羰基的伸缩振动吸收峰。MS分析表明:m/z=432.22,因为采用阳离子的轰击模式,所以物质的分子量比分子离子峰少1,为431.21,与3b构式的分子量相吻合。
2.2晶体结构
3b(CCDC:971 833)的晶体学数据见表1,部分键长和键角数据见表2,晶体结构见图1。从图1可见,在3b的晶体结构有4个平面{平面1[C(11),C(12),C(15),C(16)],平面2[C(17),C(18),C(21),C(22)],平面3[C(11),C(16),C(17),C(22)]和平面4(喹诺酮环)}。平面3与平面1和平面2之间的二面角为7.4°和6.5°;平面3与喹诺酮环之间的二面角为92.5°。从键长[C(21)-O(2)(0.122 2 nm),C(12)-O(3)(0.121 7 nm)和C(6)-O(4)(0.124 64 nm)]可以看出,C(21)-O(2),C(12)-O(3)和C(6)-O(4)均为双键。C(6)-O(4)键长为0.124 64 nm,在C-O键范围内,而不是C-Cl(0.176 nm)键。从C(3)-C(4)(0.142 7 nm)和C(5)-C(6)(0.146 0 nm)的键长可以看出,C(3)-C(4)和C(5)-C(6)均为单键。C(4)-C(5)(0.134 7 nm)的键长在C=C键范围内; C(3)-C(7)(0.140 4 nm)的键长介于C-C和C=C的键长间,可以看出其并不是单纯的碳碳单键或者双键,即为苯环中的碳碳键。C(6)-N(1)和C(7)-N(1)的键长由于受到羰基和苯环的作用比一般的C-N单键键长要短,证明3b中含有喹诺酮结构单元,而不是喹啉结构单元。这与3b的元素分析中C含量(75.25%),H含量(6.65%)和N含量(3.18%)相吻合。如果是C-Cl键,按C27H28NO3Cl理论计算C含量是72.07%,H的含量为6.27%,N的含量为3.11%,与实际测量值相差太大。
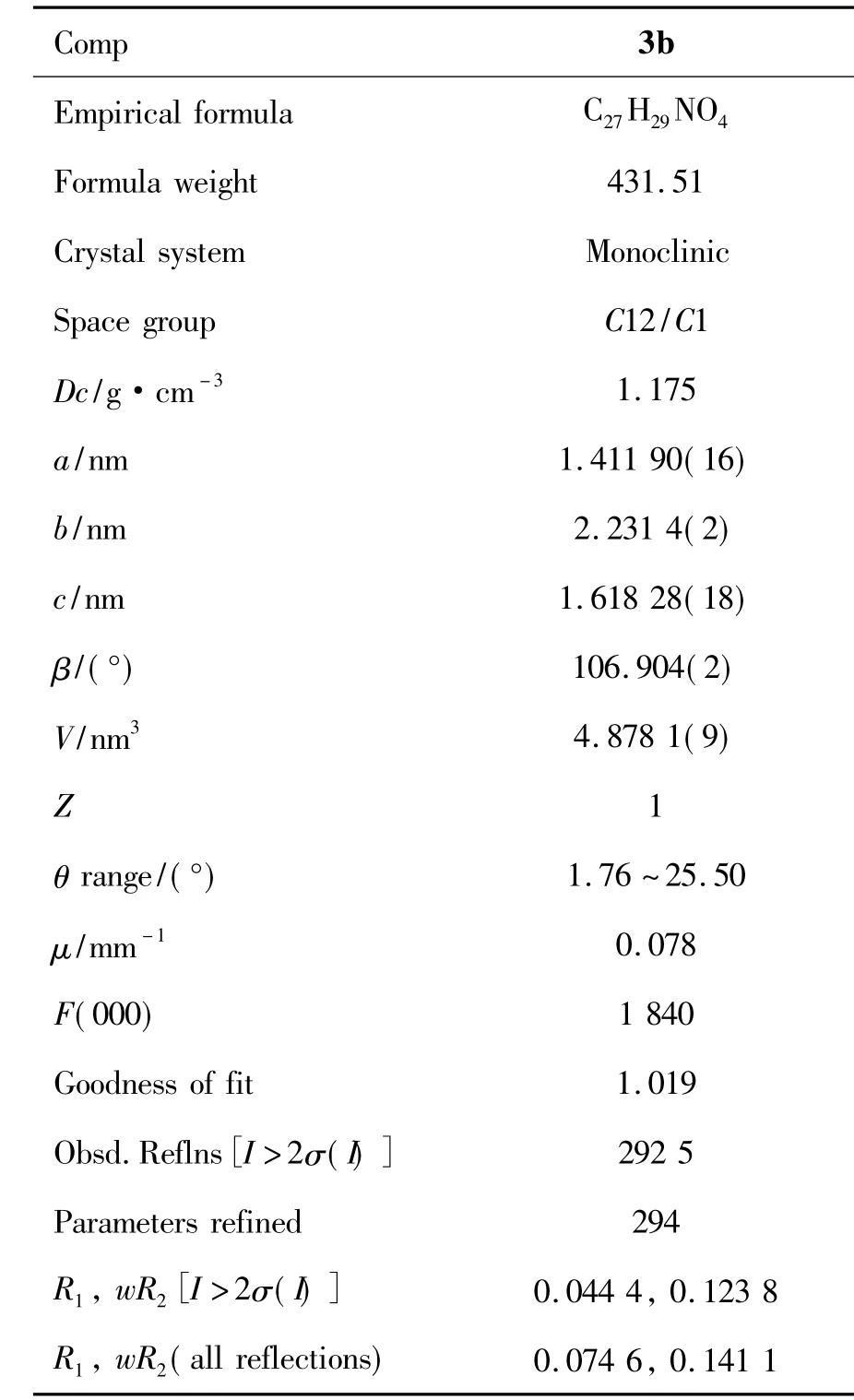
表1 1的晶体学数据*Table 1 Crystal data and refinement details of 1

图1 1的晶体结构Figure 1 The crystal structure of 1

表2 1的选择性键长和键角Table 2 Selected bond distances and bond angles of 1
2.3反应机理
1和2在醋酸的催化下并没有得到预期的3,3,6,6-四甲基-9-[3-(2-氯喹啉基)]-2,4,5,7,9,10-六氢化氧杂蒽-1,8-(2H,5H)二酮及其衍生物,而是得到了3,3,6,6-四甲基-9-{ 3-[喹啉基-2(1H)酮]-2,4,5,7,9,10-六氢化氧杂蒽-1,8-(2H,5H)二酮及其衍生物。可能反应机理是在醋酸的条件下,氢离子优先和喹啉环中的N结合,使其季铵化,另外3-位上连接吸电子基团的醛基,因此2-位C-Cl(本身电负性大的Cl的-I效应)上的碳原子电子云密度进一步降低,有利于亲核试剂H2O对2-位碳原子的进攻,发生水解反应,形成C-OH后,异构化成2-喹啉酮。
参考文献
[1]Tchamo D N,Silvere N,Etienne T.Xanthones as therapeutic agents:Chemistry and pharmacology[J].Advances in Phytomedicine,2006,2:273-298.
[2]Chand N,Diamantis W,Sofia R D.Modulation of in vitro anaphylaxis of guinea-pig isolated tracheal segments by azelastine,inhibitors of arachidonic acid metabolism and selected antiallergic drugs[J].British Journal of Pharmacology,1986,87(2):443-448.
[3]Jenekhe S A,Lu L,Alam M M.New conjugated polymers with donor-acceptor architectures:Synthesis and photophysics of carbazole-quinoline and phenothiazine-quinoline copolymers and oligomers exhibiting large intramolecular charge transfer[J].Macromolecules,2001,34(21):7315-7324.
[4]Greenblatt M S,Bennett W P,Hollstein M,et al.Mutations in the p53 tumor suppressor gene:Clues to cancer etiology and molecular pathogenesis[J].Cancer Research,1994,54(18):4855-4878.
[5]Limsuwan S,Trip E N,Kouwen T R H M,et al.Rhodomyrtone:A new candidate as natural antibacterial drug from Rhodomyrtus tomentosa[J].Phytomedicine,2009,16(6):645-651.
[6]Itoh T,Ohguchi K,Iinuma M,et al.Inhibitory effect of xanthones isolated from the pericarp of Garcinia mangostana L.on rat basophilic leukemia RBL-2H3 cell degranulation[J].Bioorganic&Medicinal Chemistry,2008,16(8):4500-4508.
[7]Niederman M S.Principles of appropriate antibiotic use[J].Int J Antimicrob Agents 26 Suppl,2005,3:S170-S175.
[8]Alizadeh N,Babaei M,Aghamohammadi M,et al.Electrosynthesis of dixanthylene photochromic dye,characterization and ab initio calculations[J].Dyes and Pigments,2008,76(3):596-603.
[9]Musiol R,Jampilek J,Buchta V,et al.Antifungal properties of new series of quinoline derivatives[J].Bioorganic&Medicinal Chemistry,2006,14(10):3592-3598.
[10]Ahmad M,King T A,Ko D K,et al.Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases[J].Journal of Physics D:Applied Physics,2002,35(13):1473.
[11]Nagarajan K,Shenoy S J.Chemistry of dimedone:Structures of aldehyde-dimedone adducts[J].Indian Journal of Chemistry,1992,31B:73-87.
[12]Shi D Q,Mu L L,Lu Z S,et al.Synthesis and crystal structure of 3,3,6,6-tetramethyl-9-orthochlorophenyl-1,8-dioxo-3,4,5,6,7,9-hexahydroxanthene [J].Chinese Journal of Structural Chemistry,1997,(6):484-487.
[13]史达清,庄启亚,陈景,等.水溶剂中芳醛与5,5-二甲基-1,3-环己二酮的反应[J].有机化学,2003,23(7):694-696.
[14]Jin T S,Zhang J S,Wang A Q,et al.Solid-state condensation reactions between aldehydes and 5,5-dimethyl-1,3-cyclohexanedione by grinding at room temperature[J].Synthetic Communications,2005,35(17):2339-2345.
[15]Cao S T,Fang D,Gong K,et al.Reaction of aromatic aldehydes with 5,5-dimethyl-1,3-cyclohexandione in water catalyzed by functionalized ionic liquid [J].Chinese Journal of Applied Chemistry,2009,26(9):1123-1125.
[16]唐然肖,何红岩,杨旭哲,等.微波辐射下酸性离子液体促进氧杂蒽二酮类衍生物的合成[J].化学试剂,2007,29(3):173-174.
[17]Jin T S,Zhang J S,Wang A Q,et al.Ultrasoundassisted synthesis of 1,8-dioxo-octahydroxanthene derivatives catalyzed by p-dodecylbenzenesulfonic acid in aqueous media[J].Ultrason.Sonochem,2006,13:220-224.
[18]Venkatesan K,Pujari S S,Lahoti R J,et al.An efficient synthesis of 1,8-dioxo-octahydro-xanthene derivatives promoted by a room temperature ionic liquid at ambient conditions under ultrasound irradiation [J].Ultrason Sonochem,2008,15:548-553.
[19]Meth-Cohn O,Taylor D L.The reverse vilsmeier approach to the synthesis of quinolines,quinolinium salts and quinolones[J].Tetrahedron,1995,51(47):12869-12882.
[20]冯友健,章晓镜,缪春宝,等.对苯二甲醛或间苯二甲醛与5,5-二甲基-1,3-环己二酮的反应:含有双4(H)-吡喃,1,4-二氢吡啶结构单元的杂环化合物的合成[J].有机化学,2004,24(8):950-952.
[21]王甦惠,史达清,屠树江,等.无外加催化剂条件下邻取代芳香醛与5,5-二甲基-1,3-环己二酮的反应研究[J].有机化学,1999,19:483-488.
[22]Krishnakumar V,Mandal B K,Khan F R N,et al.Water mediated catalyst-free efficient domino synthesis of 9-(quinolin-2(1H)-one)-xanthene-1,8(5H, 9H)-diones using parallel synthesizer[J].Tetrahedron Letters,2014,55(27):3717-3720.
[23]Sheldrick G M.SHELX-97,Program for the solution and refinement of crystal structures[K].University of Göttingen,Germany,1997.
·研究简报·
·快递论文·
Synthesis of Novel Xanthene-dione Derivatives Containing2-Quinolone Moiety
WANG Xuan1,ZHANG Zhen-ming1,2,
WANG Run-nan2,ZHANG Dan-dan2,LI Shu-an2
(1.School of Chemical Technology,China University of Mining and Technology,Xuzhou 221116,China; 2.School of Chemical Technology,Huaihai Institute of Technology,Lianyungang 222005,China)
Abstract:Five novel xanthene-dione derivatives containing 2-quinolone moiety were synthesized in yields of 78%~90% by reaction of substituted 2-chloroquinoline-3-carbaldehyde with 5,5-dimethyl-1,3-cyclohexanedione,using acetic acid as catalyst and DMF as solvent at 80℃~100℃for 2.5 h~4.0 h.The structures were characterized by1H NMR,IR,HR-ESI-MS and elemental analysis.X-ray single crystal diffractometer of 3,3,6,6-tetramethyl-9-{ 3-[7-methylquinolyl-2(1H)-one]}-2,4,5,7,9,10-hexahydro-xantene-1,8(2H,5H)-dione(3b)tests indicated that 3b(CCDC:971 833)belongs to monoclinic crystal system,space group C12/C1 with a=1.411 90(16)nm,b=2.231 4(2)nm,c=1.618 28(18)nm,β=106.904°,Z=1,Dc=1.175 g·cm-3,V=4.878 1(9)nm3,R1=0.044 4,ωR2=0.074 6.
Keywords:xanthene-dione; 5,5-dimethyl-1,3-cyclohexanedione; 2-chloroquinoline-3-carbaldehyde; synthesis; crystal structure
作者简介:王璇(1991-),男,汉族,安徽砀山人,硕士研究生,主要从事药物中间体的合成研究。E-mail:wangx_518@163.com
基金项目:国家海洋公益性行业科研专项项目(201305007);江苏省高校产业化推进项目(JHB2011-60);江苏省六大人才高
收稿日期:2015-07-24
DOI:10.15952/j.cnki.cjsc.1005-1511.2015.12.1135 *
文献标识码:A
中图分类号:O625.15; O626.3
