Recent Advance in Division of Carbohydrate and Protein Fractions of Ruminant Feed and Their Metabolism in Digestive Tract
2016-01-12,,,*
, , , *
1. State Key Laboratory of Animal Nutrition, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193, China; 2. University of Liège, Gembloux Agro-Bio Tech, Animal Science Unit, Passage des Déportés 2. B-5030 Gembloux, Belgium
1 Introduction
In recent twenty years, the assessment of the ruminant feed nutritional values mainly focused on carbohydrate and protein content, ruminal degradation, outflow and intestinal digestion. The widely used systems for ruminant feed nutritional values include Weende system[16], Van Soest fiber analysis, NRC (2001) and CNCPS systems (The latest version is 6.1). The first two methods are the foundation for evaluation, and have been used for more than 100 years. However, both of them assess feed nutrition statically and couldn’t reveal feedstuff’ digestion properties in digestive tract. NRC (2001) and CNCPS 6.1 are mechanistic and dynamic mathematical models that developed from basic principles of rumen function, microbial growth, feed degradation, passage and animal physiology, also some connections and differences exist in two systems. The objectives of this study were to systematically review the subdivision and degradation of carbohydrate and protein in different CNCPS versions, also compare the different systems for ruminant feed nutritional values evaluation and the application of CNCPS in ruminant nutrition research.
2 Characterization of feed fractions in CNCPS system
2.1CarbohydratefractionThe changes of carbohydrate fractions in CNCPS from the first version to the version 6.1 were demonstrated in Table 1. In CNCPS versions prior to 6.1, carbohydrate fractions were divided into CA, CB1, CB2 and CC[12, 39], sugars, organic acids, and oligosaccharides were included in the CA, starch and soluble fiber compounds in the CB1. Some limitations of the previous schemes have become apparent: (i) the CHO fractions and degradation rates were not precisely defined and generally measured; (ii) Besides, it does not account for the various processing treatments’ effects on NFC digestibility[29]; (iii) In addition, the description and ruminal digestibility of the fraction containing starch and soluble fiber were highlighted[31]; (iv) CHO fractions in NFC differ in rate and extent of fermentation, products of fermentation, and contribution to animal performance[15]. For example, organic acids, which present high concentration in forages, are used less efficiently for microbial growth compared to sugars. Silages are rich in lactate, and contrary to VFA, lactate could produce microbial protein[10, 26]. So in CNCPS v6.1, the carbohydrate pools have been expanded to eight fractions: CA1 (acetic, propionic and butyric acid), CA2 (lactic acid), CA3 (organic acids), CA4 (sugars), CB1 (starch), CB2 (soluble fiber), CB3 (available NDF) and CC (unavailable NDF). The expanded CHO scheme provides a more biologically correct and appropriate feed description that closely related to rumen fermentation characteristics to account for variation in changes in silage quality and diet NFC composition. However, to fully account for differences in feed CHO utilization, further improvements in the methodology used to estimate the fractions and their corresponding degradation rates, inclusion of dietary factors in dry matter intake predictions, and prediction of ruminal VFA production and pH are necessary.
Table1ComparisonofthecarbohydratefractionsindifferentversionsofCNCPS

CNCPSpriorto6.1CNCPS6.1CASugars,organicacidsandshortoligosaccha-ridesCA1Acetate,Propionate,ButyrateCA2lactateCA3OrganicacidsCA4SugarsCB1Starchandsolublefi-berCB1StarchCB2AvailableNDFCB2SolublefiberCB3AvailableNDFCCUnavailableNDFCCUnavailableNDF
2.2ProteinfractionTable 2 demonstrated the evolution of protein fractions in different CNCPS versions. The original CNCPS fractionates CP into 5 fractions (PA, PB1, PB2, PB3 and PC) based on solubility in protein precipitant agents, buffers, and detergent solutions[12, 30, 39]. However, recent researches found some limitations of the previous protein division scheme. Firstly, the assumption that the N insoluble in neutral detergent and in acid detergent represents slowly degradable and unavailable protein fractions, respectively, is not valid for all feeds[7]; Secondly, the assumption that all of the NPN fraction enters the ammonia pool completely and does not provide amino N that can stimulate microbial growth has underpredicted the microbial protein production[2]; Thirdly, the assumption that fraction A is completely degraded does not account for the contributions of free amino acids and peptides to the RUP flows[2, 37]; However, some small peptides and free AA may escape rumen degradation and flow through to the small intestine[14], and Choietal. (2002) suggested 10% of the AA flowing through to the small intestine originated from dietary NPN sources[6], also Velleetal. (1997)[36]infused free AA into the rumen at various rates and showed that up to 20% AA could escape degradation and flow to the small intestine. Based on the limitation mentioned above, an evolution of the protein division was made in the latest versions by Van Amburghetal. (2010)[32], Higgsetal. (2012)[17]and Van Amburgh (2013)[33], who re-divide protein into PA1 (ammonia), PA2 (soluble non-ammonia crude protein), PB1, PB2 and PC, this new configuration shifted a considerable amount of protein from PA1 to PA2. As PA2 fraction contributes MP to the animal, the new scheme enhances the accuracy of the predicted MP supply.
2.3AnalysisofCHOandproteinfractionsofcommonfeedstuffTable 3 lists the CHO and protein fractions for common feedstuff and these contents were calculated by the equations according to Tylutkietal. (2008)[42]. For the new CA expanded scheme, CA1 mainly remains in wet feeds because VFAs are partly volatilized during oven drying. CA2 is the predominant organic acid in ensiled feeds, which can reach up to 50-150 g kg-1DM[25], CA2 also presents in molasses and corn as the degradation product of invert sugar[1], CA3 is almost undetectable in silages[25], but in fresh forages, citric, malic, and aconitic acids can comprise more than 10% of the DM[9], the possible reason is that organic acid degraded in the process of silage. From Table 3, we can conclude that the contents of CHO and protein fractions vary in different feedstuff. For example, sugar is the most abundant fraction for molasses beet and accounts for 700 g kg DM, while in corn or wheat, CA4 content doesn’t reach 50 g kg-1DM basis. Feed processing like roast, extraction and ensilage would change the proportion of each fraction, compared with soybean whole raw, PB1 content of roasted soybean whole decreased from 165.7 to 22.6 g kg-1DM, while PB2 increased from 213.9 to 353.1 g kg-1DM, which will reduces the ruminal degradation of soybean protein. Also, sugar or starch would be utilized by bacteria and degraded into VFAs or organic acids during silage, so CA4 content of alfalfa green chop decreased by 69.53% after silage (101.1 g kg-1vs 30.8 g kg-1, DM basis).
Table2ComparisonoftheproteinfractionsindifferentversionsofCNCPS

CNCPSpriortov6.5CNCPS6.5[33]PANPN(ammonia,pep-tidesandAAs)PA1AmmoniaPA2SolubletrueproteinPB1SolubletrueproteinRapidlydegradedpro-teinPB1Moderatelydegradablepro-teinPB2Intermediatelydegrad-edproteinPB2Slowlydegradableprotein,boundinNDFPB3SlowlydegradabletrueproteinPCADIPPCUnavailableCP
3 Degradation and passage rates of CHO and protein fractions
For ruminants, carbohydrate and protein fractions are firstly degraded by rumen microflora for microbial protein synthesis, and the residue of feedstuff not digested in the rumen will pass to the intestine for further digestion or not. However, as the difference in chemical composition and structure, CHO and protein fractions differ in Kd and Kp, and the degraded quantity of fractions were determined by the simple relationship Kd/(Kd+Kp). So it’s important to study the degradation and passage properties of different fractions for the accurate prediction of feedstuff’ effective nutrition.
3.1DegradationrateTable 4 demonstrated the changes in degradation rates of the various fractions. CA is subdivided into four fractions and each has its own degradation rates. Degradation rate value for CA4 was downward from 200-300 % h-1to 40-60 % h-1(rumen retention time of 100 to 150 min) based on in vitro fermentation studies of Molina (2002)[26], who used a mixed sugar fermentation with mixed rumen bacteria by gas production. Further, Kd of PA reduced from 10 000 % h-1(retention time of 0.6 min) to 200 % h-1, for the 10 000 % h-1was generated to represent the rate of solubilization and not necessarily microbial uptake. Besides, the degradation rate variation in some ranges mainly because the composition of the sugar fraction in feeds and their ability to support microbial growth are different. Take CA4 for example, the fermentation rates of 40 % h-1for glucose and 16 % h-1for arabinose when fermented with a fiber source. As five carbon sugars support less microbial growth than hexoses[41], degradation rates for feeds containing mainly sucrose were set at 40 % h-1for the sugar fraction[26], but for milk derived products the assigned degradation rate for sugars is 30 % h-1as lactose supports less microbial growth than sucrose[24]. For silages, with the exception of immature corn silages, the sugar fraction mainly are arabinose and other simple sugars derived from the hydrolysis of the side chains of pectin and hemicelluloses[20], thus a rate of 20 % h-1, closer to the arabinose fermentation rate was assigned to the sugar fraction of silages.
Table1Contentofcarbohydrateandproteinfractionsincommonfeedstuff
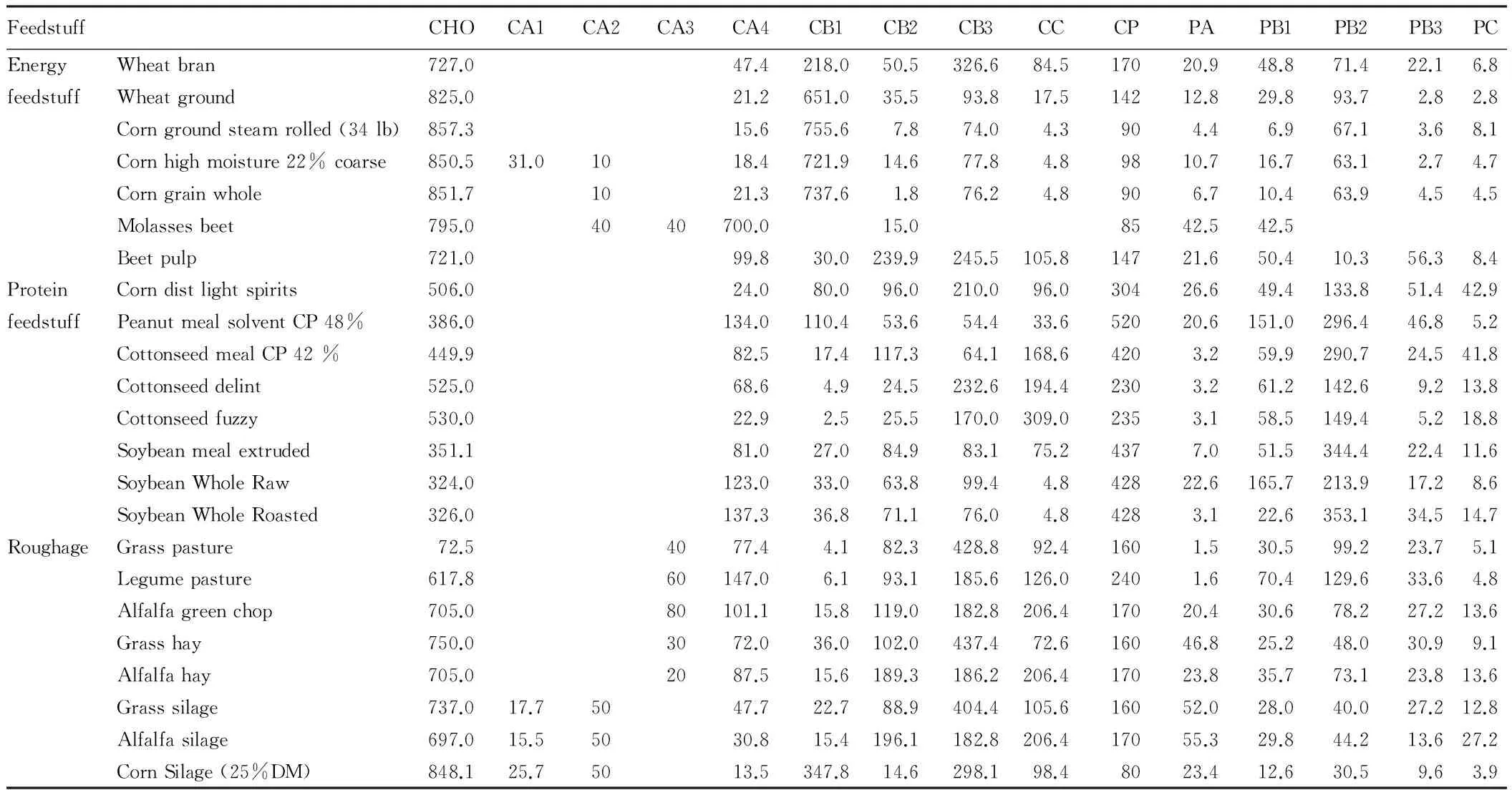
FeedstuffCHOCA1CA2CA3CA4CB1CB2CB3CCCPPAPB1PB2PB3PCEnergyWheatbran727.047.4218.050.5326.684.517020.948.871.422.16.8feedstuffWheatground825.021.2651.035.593.817.514212.829.893.72.82.8Corngroundsteamrolled(34lb)857.315.6755.67.874.04.3904.46.967.13.68.1Cornhighmoisture22%coarse850.531.01018.4721.914.677.84.89810.716.763.12.74.7Corngrainwhole851.71021.3737.61.876.24.8906.710.463.94.54.5Molassesbeet795.04040700.015.08542.542.5Beetpulp721.099.830.0239.9245.5105.814721.650.410.356.38.4ProteinCorndistlightspirits506.024.080.096.0210.096.030426.649.4133.851.442.9feedstuffPeanutmealsolventCP48%386.0134.0110.453.654.433.652020.6151.0296.446.85.2CottonseedmealCP42%449.982.517.4117.364.1168.64203.259.9290.724.541.8Cottonseeddelint525.068.64.924.5232.6194.42303.261.2142.69.213.8Cottonseedfuzzy530.022.92.525.5170.0309.02353.158.5149.45.218.8Soybeanmealextruded351.181.027.084.983.175.24377.051.5344.422.411.6SoybeanWholeRaw324.0123.033.063.899.44.842822.6165.7213.917.28.6SoybeanWholeRoasted326.0137.336.871.176.04.84283.122.6353.134.514.7RoughageGrasspasture72.54077.44.182.3428.892.41601.530.599.223.75.1Legumepasture617.860147.06.193.1185.6126.02401.670.4129.633.64.8Alfalfagreenchop705.080101.115.8119.0182.8206.417020.430.678.227.213.6Grasshay750.03072.036.0102.0437.472.616046.825.248.030.99.1Alfalfahay705.02087.515.6189.3186.2206.417023.835.773.123.813.6Grasssilage737.017.75047.722.788.9404.4105.616052.028.040.027.212.8Alfalfasilage697.015.55030.815.4196.1182.8206.417055.329.844.213.627.2CornSilage(25%DM)848.125.75013.5347.814.6298.198.48023.412.630.59.63.9
Note: Unit of index above is g kg-1DM; " " in the above table means 0 g kg-1DM.
Table4Feeddegradationrates(Kd, %h-1)usedforCHOandproteinpoolsinCNCPSv6.1andpriortoversion6.11

ComponentPriortov6.1v6.1CA1Notmodeled0CA2Notmodeled7CA3Notmodeled5CA4300-50040-60CB120-4020-40CB220-4020-40CB34-94-9CC00PA210000200PB1130-30010-40PB23-203-20PB30.05-2.0Forforages,4-9PC00
Note: 1 This table refers to Van Amburghetal. (2010)[32]; 2For the new protein scheme, the degradation rates for PA1, PA2, PB1, PB2 and PC are 200 % h-1, 10-40 % h-1, 3-20 % h-1, 4-9 % h-1, 0 % h-1, respectively[17].
3.2PassagerateTable 5 showed the development of equations for feed passage rates and their difference compared with NRC (2001). Particle size, forage to concentrate ratio, hydration rate and intake level can affect the passage rates of feeds[4, 43]. Sniffenetal. (1992)[39]incorporated these effects into the equations for Kpf and Kpc, and Kp was adjusted for particle size using effective NDF (eNDF), but lacking equation for Kpl. As Kpl could affect the soluble nutrient digestion[19], outflow of rumen metabolites[23], rumen undegraded protein ratio[12]and microbial growth[11], Foxetal. (2004)[12]adding the Kpl equation to CNCPS v5.0, and Kp rates were adjusted by peNDF. The CNCPS version 6.1 absorbed Seo’s researching results, integrating FpBW (Forage DMI as a proportion of BW), CpBW (Concentrate DMI as a proportion of BW) and FDMI (Forage Dry matter intake) factors into the Kp equations, also the peNDF adjustment factor is abandoned, for the double accounting for the particle sizes. For the soluble pools, they were predicted to flow out of the rumen with the solids passage rate in CNCPS prior to v6.1, thus with the high degradation rates and the slow passage rates, all the soluble fractions were considered to be degraded in the rumen. To be more appropriately reflect the biology of the cow, the CNCPS V6.1 reassigned the soluble pools to the liquid passage rate equation, which increasing the predicted outflow of soluble components, thus reducing microbial yield and estimated ammonia production as well as rumen N balance.
Table5EquationsforfeedpassageratesindifferentCNCPSversionsandNRC(2001)

ReferenceEquationAdjustfactor,AfSniffen(1992)Kpf=0.388+(0.002×DMI/BW0.75)+[0.0002×forage2(%DM)]100/(eNDF+70)Kpc=-0.424+1.45×Kpf100/(eNDF+90)NRC(2001)Kpf,wetforage=3.054+0.614X1NoKpf,dryforage=3.362+0.479X1-0.007X2-0.017X3NoKpc=2.904+1.375X-0.020X2NoCNCPSv5.0Kpf=[0.38+(0.022×DMI×1000/BW0.75)+2.0×forage2]/100100/(NDF×peNDF/100+70)Kpc=[-0.424+(1.45×Kpf×100)]/100100/(NDF×peNDF/100+90)Kpl=(4.413+0.191×DMI×1000/FBW)/100NoCNCPSv6.1Kpf=2.365+(0.214×FpBW)+(0.734×CpBW)+(0.069×FDMI)NoKpc=1.169+(1.375×FpBW)+(1.721×CpBW)NoKpl=4.524+(0.223×FpBW)+(2.046×CpBW)+(0.344×FDMI)No
Note: Kpf Passage rate of forages; Kpc Passage rate of concentrate; Kpl Passage rate of liquids; DMI Dry matter intake; BW Body weight; eNDF Effective NDF; peNDF Physical effective NDF; FBW Full body weight; FpBW Forage DMI as a proportion of BW; CpBW Concentrate DMI as a proportion of BW; FDMI Forage Dry matter intake; X1DMI as a proportion of BW; X2Concentrate as a proportion of DMI; X3NDF as a proportion of DMI.
3.3PossibleproblemsforCHOandproteinfractionsKdandKpvaluesAvornyo (2012)[3]compare three methods (gravimetric, Curve peeling technique, and Cornell values) to estimate protein B2 and B3 degradation rates in the rumen. The results showed that no statistical difference founded among three methods for the degradation rates of protein B2, whereas for protein B3, the degradation rate estimated with the gravimetric method was highest followed by the curve peeling method and then the Cornell values (P<0.01). So the degradation rates assigned to protein B3 in the Cornell databank needs re-examination. Generally, prediction equations of Kp in CNCPS have been developed separately for forage, concentrate and liquid feed, and all Kp equations are based on DMI. However, there some questions found for CNCPS Kp prediction: 1) the equations in CNCPS have been developed based on large sets of empirical data using data of Cr-mordanted fiber as a Kp marker (CNCPS). However, marker type could influences Kp[18]estimated values and Kp equations of forages and concentrates in CNCPS were not corrected for the effect of marker[38]; 2) In CNCPS, Kp for concentrate and forage were calculated separately, and one Kp equation for all forages no matter dry and met forages[38]. However, it is’t possible to separate Kp of forages and concentrate particles, and there are interaction effects between concentrate and forages, as Coluccietal. (1990)[8]observed that Kp of both forage and concentrate particles decreased when the proportion of concentrate in the diet to dairy cows increased as well as Stensigetal. (1998)[40]reported that increased starch supplementation in the diet to dairy cows decreased ruminal particle passage rate; 3) Kp equations in CNCPS don’t containing forage type factors. Forage type affect Kp as Krizsanetal. (2010)[21]indicated that the fastest Kp of iNDF was reported for corn silage diets (2.66 % h-1and 2.87 % h-1); the alfalfa hay diet was in between (1.65 % h-1and 2.17 % h-1), and Kp was lowest for grass hay (1.27 and 1.34 % h-1) when fed to dairy cows supplemented with concentrate or without any supplementation. Krizsanetal. (2010)[21]conducted a meta-analysis of studies using the flux/compartmental pool method with indigestible neutral detergent fiber (iNDF) as internal marker to evaluate Kp equations in CNCPS. He established two models for feeds based on NDF intake: Kp (% h-1) = 1.19 + 0.0879 × NDF intake (g kg-1 of body weight) + 0.792 × proportion of concentrate NDF of total NDF + 1.21 × diet iNDF: NDF ratio when forage type was not included, and Kp(% h-1) = F + 1.54 + 0.0866 × NDF intake for forage type. The models combined the feed type (concentrate, forage and forage type) and forage maturity factors, and by meta-analysis, he reported that prediction of Kp in CNCPS may overestimated and intake of NDF performed better as a predictor of Kp than DMI. So more research is needed to confirm the importance of relative forage differences in a rumen model and to separate animal effects from feed factors in predictions of ruminal particulate matter Kp.
4 Comparison of Weende, Van Soest, NRC (2001) and CNCPD in CHO and protein fraction and digestive metabolism
4.1WeendeandVanSoestfiberanalysis-staticfeednutrientevaluationmethodsFor feed chemical composition division, there are mainly Weende feed proximate analysis, Van Soest fiber analysis, CNCPS and NRC systems. Weende analysis system, also called ’Feed Proxinate Analysis’, was established by Henneberg and Stohmann (1860)[16], which divided feed nutrient into six fractions: moisture, crude protein(CP), ether extract(EE), crude fiber(CF), ash and nitrogen-free extracts(NFE). This concept has been used in feed evaluation systems for more than 150 years and still widely used in China’s feed quality evaluation. However, there has been much dissatisfaction with this system, for example, the crude protein or crude fiber weren’t subdivided to predict their nutritive availability and the NFE content was overestimated because most of the lignin and hemicellulose were extracted into the NFE. Based on Weende analysis system, Van Soest (1967)[34]corrected the CF and NFE and established detergent fiber analysis method, CF was subdivided into cellulose, hemicellulose and lignin according to their solubility in neutral detergent and acidic detergent. This analysis method laid the foundation for the carbohydrate and protein fractions division.
4.2NRC(2001)andCNCPSsystems-dynamicfeednutrientevaluationmethodsThe feed proximate analysis and Van Soest fiber analysis method evaluate feed nutrition statically, not considering factors like animal body condition, feed particle size, digestion. Both NRC (2001) and CNCPS system represent a large step to the dairy industry in that feed composition is described by carbohydrate and protein fractions and their degradation rates, as well as rumen fermentation and animal factors were integrated in two systems. However, there are still some difference and relationship between NRC (2001) and CNCPS for carbohydrate and protein fractions, degradation and passage rates, mainly including: (i) For carbohydrate and protein fractions, NRC (2001) absorbed the theoretical achievements of CNCPS before 2001, and divided carbohydrate simply into structural carbohydrate (SC) and non-structural carbohydrate (NSC), protein was subdivided into three fractions according to in situ method: Protein A (NPN, solubilized protein, and protein in particles smaller than the porosity of the nylon bag), protein B (potentially degradable protein) and protein C(unavailable protein, the remaining nitrogen at the end of predetermined incubation time). Whereas the CNCPS adopted the chemical partitioning method (solubility) to partition carbohydrate into eight fractions as described above, and protein was subdivided into five fractions (PA, PB1, PB2, PB3 and PC). (ii) Using different index to describe feed nutrient. NRC (2001) uses DM, CP(%DM), NDIP(%CP), ADIP(%CP), EE(%DM), NDF, ADF, Lignin(%DM), Ash (%DM), while CNCPS6.1 uses DM, OM(%DM), CP(%DM), SP(%CP), NPN(%CP), ADIP(%CP), NDIP(%CP), NFC(%DM), Sugar(%DM), Starch (%DM), SF(%DM), ADF(%DM), NDF(%DM), peNDF(%NDF), Lignin(%NDF), Ash(%DM). (iii) As showed in Table 5, the NRC (2001) and CNCPS models use different equations for predicting passage rate of undigested feed. NRC (2001) developed separate equations for wet forages and dry forages and found that the Kp of wet forages was higher (P<0.01) than that of dry forages (0.0432 h-1versus 0.0377 h-1), and Kp equation for liquid was not developed, though the liquid Kp may affect digestion of soluble nutrients[19], outflow of end products of fermentation[23], peptide escape[12]and microbial growth[11]. Because of the lack of data for both development and evaluation, CNCPS system developed one equation for forages Kp prediction, wet forage and dry forage are not calculated separately, and Kp equation for liquid was also established. (iv) NRC (2001) uses RDP=A+B (Kd/Kd+Kp) and RUP= B(Kp/Kd+Kp)+C to calculate RUP and RDP, respectively, which is similar to CNCPS system.
5 Conclusions
CNCPS is a dynamic ruminant nutrition model that integrate animal, environment, physiological functions and metabolic processes, also carbohydrate and protein fractions and their degradation and passage rates continuously update. This update has allowed us to predict feed availability with more accuracy. Also the feed library and programs like CPM Dairy, AMTS.Cattle, NDS, DinaMilk of CNCPS help predict feed nutritional values and optimize ruminant diets more accurately and efficiently. However, the assessment of feed nutritional values mainly based on Weende system in China, and CNCPS was not commonly used for its meticulous division of carbohydrate, protein fractions, and complicated index, which is difficult to be determined for producer. So for the utilization of CNCPS in China’s ruminant production, feedstuff database should be built and the integration of CNCPS models with computer technology should be further strengthened to realize China’s precision farming.
[1] AMIN A. Gas-chromatographic separation and identification of organic-acids in beet molasses and date syrup[J]. Nahrung, 1980(24): 705-711.
[2] AQUINO DL, TEDESCHI LO, LANZAS CS,etal. Evaluation of CNCPS predictions of milk production of dairy cows fed alfalfa silage[C]. In Cornell Nut. Conference of Feed Manufacture, Cornell University, Syracuse, NY, 2003.
[3] AVRONYO FK. Comparison of three approaches of estimating protein B2 and B3 degradation rates in the rumen of sheep[J]. Online Journal of Animal and Feed Research (OJAFR), 2012, 2(2):166-173.
[4] BHATTI SA, BOWMAN JGP, FIRKINS JL,etal. Effect of intake level and alfalfa substitution for grass hay on ruminal kinetics of fiber digestion and particle passage in beef cattle[J].Journal of Animal Science, 2008, 86(1): 134-145.
[5] CHOI CW, AHVENJARVI S, VANHATALO A,etal. Quantitation of the flow of soluble non-ammonia nitrogen entering the omasal canal of dairy cows fed grass silage based diets[J]. Animal Feed Science and Technology, 2002, 96(3): 203-220.
[6] CHOI CW, VANHATALO A, AHVENJARVI S,etal. Effects of several protein supplements on flow of soluble nonammonia nitrogen from the forestomach and milk production in dairy cows[J]. Animal Feed Science and Technology, 2002(102):15-33.
[7] COBLENTZ WK, FRITZ JO, FICK WH,etal. In situ disappearance of neutral detergent insoluble nitrogen from alfalfa and eastern gamagrass at three maturities[J]. Journal of Animal Science,1999(77):2803-2809.
[8] COLUCCI PE, MACLEOD GK, GROVUM WL,etal. Digesta kinetics in sheep and cattle fed diets with different forage to concentrate ratios at high and low intakes[J].Journal of Dairy Science, 1990(73):2143-2156.
[9] DIJKSHOORN W. Organic acids and their role in ion uptake. In: Bailey, R.W. (Ed.), Chemistry and Biochemistry of Herbage[M]. Academia Press, NY, USA,1973:163-188.
[10] DOANE PH, PELL AN, SCHOFIELD P. The effect of preservation method on the neutral detergent soluble fraction of forages[J]. Journal of Animal Science, 1997(75): 1140-1148.
[11] EUN JS, FELLNER V, GUMPERTZ ML. Methane production by mixed ruminal cultures incubated in dual-flow fermentors[J]. Journal of Dairy Science, 2004, 87(1): 112-121.
[12] FOX DG, TEDESCHI LO, TYLUTKI TP,etal. The cornell net carbohydrate and protein system model for evaluating herd nutrition and nutrient excretion[J]. Animal Feed Science and Technology, 2004, 112(1): 29-78.
[13] FOX DG, SNIFFEN CJ, OCONNOR JD,etal. A net carbohydrate and protein system for evaluating cattle diets: III. Cattle requirements and diet adequacy[J].Journal of Animal Science, 1992, 70(11): 3578-3596.
[14] GIVENS DI, RULQUIN H. Utilisation by ruminants of nitrogen compounds in silage-based diets[J]. Animal Feed Science and Technology, 2004, 114(1): 1-18.
[15] HALL MB, HEREJK C. Differences in yields of microbial crude protein from in vitro fermentation of carbohydrates[J]. Journal of Dairy Science, 2001(84): 2486-2493.
[16] HENNEBERG W, STROHMANN F. Beitr ge zur Begründung einer rationellen fütterung der Wiederk uer.[Z]. Praktisch-landwirthschaftliche und physiologische Untersuchungen. Heft 1u.2, Braunschweig, 1860: 1860-1864.
[17] HIGGS RJ, CHASE LE, ROSS DA,etal. Evaluating and refining the cncps feed library using commercial laboratory feed databases[O]. In Proceedings Cornell Nutrient Conference, Syracuse, NY, 2012.
[18] HUHTANEN P, KUKKONEN U. Comparison of methods, markers, sampling sites and models for estimating digesta passage kinetics in cattle fed at two levels of intake[J]. Animal Feed Science and Technology, 1995(52):141-158.
[19] ILLIUS AW, GORDON IJ. Prediction of intake and digestion in ruminants by a model of rumen kinetics integrating animal size and plant characteristics[J]. Journal of Agricultural Science, 1991, 116(1), 145-57.
[20] JONES BA, HATFIELD RD, MUCK RE. Effect of fermentation and bacterial inoculation on lucerne cell walls[J]. Journal of the Science of Food and Agriculture, 1992(60): 147-153.
[21] KRIZSAN SJ, AHVENJARVI S, HUHTANEN P. A meta-analysis of passage rate estimated by rumen evacuation with cattle and evaluation of passage rate prediction model[J]. Journal of Dairy Science, 2010, 93(12): 5890-5901.
[22] LANZAS C, SNIFFEN CJ, SEO S,etal. A revised CNCPS feed carbohydrate fractionation scheme for formulating rations for ruminants[J]. Animal Feed Science and Technology, 2007, 136(3): 167-190.
[23] LOPEZ S, HOVELL FDD, DIJKSTRA J,etal. Effects of volatile fatty acid supply on their absorption and on water kinetics in the rumen of sheep sustained by intragastric infusions[J].Journal of Animal Science, 2003, 81(10): 2609-2616.
[24] MCCORMICK ME, REDFEARN DD, WARD JD,etal. Effect of protein source and soluble carbo-hydrate addition on rumen fermentation and lactation performance of holstein cows[J]. Journal of Dairy Science, 2001 (84): 1686-1697.
[25] MCDONALD P, HENDERSON AR, HERON SJE. The biochemistry of silage, 2nd[Z]. Cambrian Printers Ltd., Aberystwyth, UK, 1991.
[26] MOLINA DO. Prediction in intake of lactating cows in the tropics and of the energy value of organic acids[D]. Ph. D. Dissertation, Cornell University, Ithaca, NY, 2002.
[27] MORRISON FB. Feeds and feeding[M]. 22th Clinton: Morrison Publishing Co, 1956.
[28] NRC. Nutrient requirements of dairy cattle, 7thed[M].Washington: National Academy Press, 2001.
[29] OFFNER A, SAUVANT D. Comparative evaluation of the molly cncps, and les rumen models[J]. Animal Feed Science and Technology, 2004(112): 107-130.
[30] PICHARD D. Forage nutritive value: Continuous and batch in vitro rumen fermentations and nitrogen solubility[D]. Cornell University, 1977.
[31] PITT RE, VANKESSEL, JS, FOX DG,etal. Prediction of ruminal volatile fatty acids and ph within the net carbohydrate and protein system[J]. Journal of Animal Science, 1996(74): 226-244.
[32] VAN AMBURGH ME., CHASE LE, OVERTON TR,etal. Updates to the cornell net carbohydrate and protein system v6.1 and implications for ration formulation[D]. Department of Animal Science at the New York State College of Agriculture and Life Sciences (A Statutory College of the State University of New York) Cornell University, 2010:144.
[33] VAN AMBURGH ME, FOSKOLOS A, COLLAO-SAENZ EA,etal. Updating the CNCPS feed library with new feed amino acid profiles and efficiencies of use: evaluation of model predictions-version 6.5.[C].Syracuse NY: Cornell Nutrition Conference, 2013: 59-76.
[34] VAN SOEST PJ. Development of a comprehensive system of feed analyses and its application to forages[J]. Journal of Animal Science, 1967, 26(1): 119-128.
[35] VAN SOEST PJ, ROBERTSON JB, LEWIS, B. A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition[J]. Journal of Dairy Science, 1991, 74(10): 3583-3597.
[36] VELLE W, SJAASTAD OV, AULIE A,etal. Rumen escape and apparent degradation of amino acids after individual intraruminal administration to cows[J]. Journal of Dairy Science, 1997, 80(12): 3325-3332.
[37] REYNAL SM, IPHARRAGUERRE IR, LINEIRO M,etal. Omasal flow of soluble proteins, peptides, and free amino acids in dairy cows fed diets supplemented with proteins of varying ruminal degradabilities[J]. Journal of Dairy Science, 2007(90):1887-1903.
[38] SEO S, TEDESCHI LO, LANZAS C,etal. Development and evaluation of empirical equations to predict feed passage rate in cattle[J]. Animal Feed Science and Technology, 2006, 128(1): 67-83.
[39] SNIFFEN CJ, OCONNOR JD, VAN SOEST PJ,etal. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability[J]. Journal of Animal Science, 1992, 70(11): 3562-3577.
[40] STENSIG T, WEISBJERG MR, HVELPLUND. Digestion and passage kinetics of fibre in dairy cows as affected by the proportion of wheat starch or sucrose in the diet[J]. Acta Agric Scand, 1998, 48:129-140.
[41] STROBEL HJ, RUSSELL JB. Effect of pH and energy spilling on bacterial protein synthesis by carbohydrate-limited cultures of mixed rumen bacteria[J]. Journal of Dairy Science, 1986(69): 2941-2947.
[42] TYLUTKI TP, FOX DG, DURBAL VM,etal. Cornell net carbohydrate and protein system: A model for precision feeding of dairy cattle[J]. Animal Feed Science and Technology, 2008, 143(1-4):174-202.
[43] YANG WZ, BEAUCHEMIN KA, RODE LM. Effects of particle size of alfalfa-based dairy cow diets on site and extent of digestion[J]. Journal of Dairy Science, 2002, 85(8), 1958-1968.
杂志排行
Asian Agricultural Research的其它文章
- Economic Analysis on the Rational Allocation of Agricultural Production Factors in Henan Province
- Special Space and Plant Landscape Design of Urban Ecological Buildings
- Main Methods Applied in Fertigation Technology
- A Study on the Ultrasonic Extraction and Antioxidant Activity of Lychee Pericarp Polysaccharides
- The Fishery Industrial Structure in China Based on the Application of Shift-Share Analysis
- Spatial Structure of Tourism Resources in Liaoning Province
