A Study on the Ultrasonic Extraction and Antioxidant Activity of Lychee Pericarp Polysaccharides
2016-01-12,,,,,
, , , , ,
Key Laboratory of Ecological Environmental Analysis and Pollution Control of Universities in Guangxi, Baise University, Baise 533000, China
1 Introduction
Lychee, as one of the characteristic fruits, is mainly grown in tropical and subtropical regions. Lychee fruit is tasty and sweet, and contains a variety of active ingredients[1]. People tend to retain only the pulp and discard pericarp when using the lychee. This will form a large lychee pericarp waste, and it will further pollute the environment if not handled properly. Lychee pericarp has a variety of functional components, and a variety of health benefits. Studies have shown that the lychee pericarp contains polysaccharides with antioxidant activity[2], and it has auxiliary therapeutic effect on hypertension, cancer,etc.[3], so it has great potential value. The development and utilization of lychee pericarp has become the trend of deep processing of lychee, and the extraction and utilization of polysaccharides has also become one of the hotspots. Currently, the extraction of lychee pericarp polysaccharides has been reported, for example, the traditional water extraction method is used to extract lychee pericarp polysaccharides[4], but this method has low extraction rate and flawed extraction process. Research also shows that ultrasonic extraction has high efficiency in extracting lychee pulp polysaccharides[5], but it is rarely reported in the lychee pericarp extraction. Therefore, this study uses ultrasonic extraction method to extract lychee pericarp, and this method has characteristics of quickness and high efficiency, and applying the ultrasonic technology to the extraction of lychee pericarp polysaccharides helps improve the extraction process of lychee pericarp polysaccharides. The study on oxidation activity of polysaccharides obtained from lychee pericarp by ultrasonic extraction contributes to the further use and extraction of active polysaccharides, and provides an important scientific basis for the resource-based reuse of lychee pericarp.
2 Materials and methods
2.1Materials
2.1.1Main instruments. KQ5200 ultrasonic cleaning instrument, DHG-907A blast electric oven, TGL-16M Xiangyi Desk Centrifuge, UV-2700 Shimadzu UV-visible spectrophotometer, BSA124S Sartorius analytical balance.
2.1.2Materials and reagents. Lychee pericarp was removed from fresh black leaf lychee bought from Golden Triangle Market in Youjiang District of Baise City, and after being cleaned and naturally dried, it was crushed, sifted by 60-mesh sieve, dried and stored; phenol was freshly distilled; glucose, concentrated sulfuric acid, absolute ethyl alcohol, hydrogen peroxide, 1, 1-diphenyl-2-trinitrobenzene hydrazine (DPPH), as analytical reagents, were purchased from Sinopharm Chemical Reagent Co., Ltd.; the experimental water was distilled water.
2.2Methods
2.2.1Preparation of standard solution.0.0162 g of anhydrous glucose, dried to constant weight, was precisely weighed, and placed in 100 mL volumetric flask, to get 162 μg/mL glucose solution.
2.2.2Determination method of lychee pericarp polysaccharides. It is based on the sulfuric acid-phenol colorimetry with glucose as standard substance[5].
2.2.3Standard curve drawing. 0.20 mL, 0.40 mL, 0.60 mL, 0.80 mL and 1.00 mL glucose solutions were precisely siphoned and placed in 5 stoppered test tubes, respectively, and then distilled water was added to 2.0 mL, and 1.0 mL of 6% phenol was added, respectively. After being shaken, 10.0 mL of concentrated sulfuric acid was added, respectively. With the same reagent without glucose as control, it was placed in boiling water for 5 min after being kept still for 5 min, and then transferred to cold water to cool. After 30 min, the absorbance was measured at a wavelength of 490 nm[6]. With mass concentration (C) as abscissa and absorbance (A) as vertical axis, the linear regression was done to obtain a linear equation:A=0.0059C+0.0055,r=0.9995.
2.2.4Preparation of test solution. 5.00 g of lychee pericarp powder sample was weighed and placed in 250 mL stoppered conical beaker. The distilled water was added according to proportion, and the extraction was carried out based on certain ultrasonic power, temperature and time. The leaching was conducted after extraction, and the filtrate was concentrated in a 100 mL volumetric flask. 10.0 mL of the above solution was taken and mixed with absolute ethyl alcohol for ethanol precipitation in 4℃ refrigerator for 12 h; it was then centrifugalized for 10 min at 8000 r/min to get the ethanol sediments; the water was used to dissolve the ethanol sediments in 25 mL flask to get the test solution.
2.2.5Determination of the test solution. In accordance with the standard solution determination method in "2.2.3", the content was determined from the standard curve by measuring the absorbance of the sample solution, and the polysaccharides extraction rate was calculated. polysaccharides extraction rate = (the amount of polysaccharide extracted/the weight of the lychee pericarp extracted) × 100%.
2.2.6Study of polysaccharides antioxidant activity. With the ethanol sediments obtained in "2.2.4" treatment as lychee pericarp polysaccharides, the dry polysaccharides were prepared into different concentrations for the study of antioxidant activity. According to the literature method, the scavenging effect of lychee pericarp polysaccharides on superoxide anion radical[7-8], hydroxyl radical[9-10]and DPPH radical[11]was examined.
3 Results and analysis
3.1Single-factortest
3.1.1Effect of ultrasonic power and extraction temperature on polysaccharides extraction rateUnder extraction temperature of 70℃, extraction time of 0.5 h and extraction solid-liquid ratio of 1:20, the effect of ultrasonic power of 120 W, 140 W, 160 W, 180 W and 200 W on the polysaccharides extraction rate of lychee pericarp was examined, respectively, as shown in Fig. 1. As can be seen from Fig. 1, when the ultrasonic power increased from 120 W to 200 W, the extraction rate increased first and then decreased, and the extraction rate was highest at 180 W, so the ultrasonic power of 180 W was chosen for the extraction. Under ultrasonic power of 180 W, extraction time of 0.5 h and extraction solid-liquid ratio of 1:20, the effect of extraction temperature of 40℃, 50℃, 60℃, 70℃ and 80℃ on the polysaccharides extraction rate of lychee pericarp was examined, respectively, as shown in Fig. 2. As can be seen from Fig. 2, the polysaccharides extraction rate increased with the rising temperature, because when the temperature was high, the polysaccharides would spread fast from lychee pericarp. However, when the temperature was over 80℃, the water would evaporate quickly, so 80℃ was chosen as the extraction temperature.
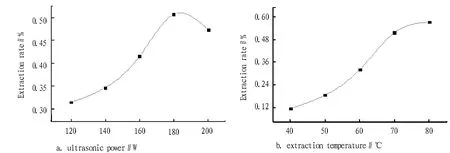
Fig.1 Effect of ultrasonic power and extraction temperature on polysaccharides extraction rate
3.1.2Effect of extraction time and solid-liquid ratio on polysaccharides extraction rate. Under ultrasonic power of 180 W, extraction temperature of 80℃ and extraction solid-liquid ratio of 1:20, the effect of extraction time of 0.5 h, 1.0 h, 1.5 h, 2.0 h and 2.5 h on the polysaccharides extraction rate of lychee pericarp was examined, respectively, as shown in Fig. 3a. As can be seen from Fig. 3a, at 2.0 h, the polysaccharides extraction rate peaked, so the extraction time was chosen as 2.0 h. Under ultrasonic power of 180 W, extraction temperature of 80℃ and extraction time of 2.0 h, the effect of solid-liquid ratio of 1:15, 1:20, 1:25, 1:30 and 1:35 on the polysaccharides extraction rate of lychee pericarp was examined, respectively, as shown in Fig. 3b. As can be seen from Fig. 2b, when the solid-liquid ratio changed from 1:15 to 1:25, the extraction rate increased significantly, and when the solid-liquid ratio changed from 1:25 to 1:35, the extraction rate showed no obvious increasing trend, because at 1:25, a large portion of polysaccharide could be extracted, and if the solvent continued to increase, the dissolution amount of polysaccharides would be very limited. From the perspective of saving energy, the solid-liquid ratio of 1:25 was chosen.
3.2OrthogonaltestOn the basis of the above single-factor test, extraction time, ultrasonic power, extraction temperature and solid-liquid ratio were selected as the influencing factors, and with polysaccharide extraction rate as indicator, 4-factor and 3-level L9(34) orthogonal test was performed. The factor level and test results are shown in Table 1 and Table 2, respectively. From the range, it was found thatRC>RB>RA>RD, so the influencing factors were in the order of extraction temperature> ultrasonic power> extraction time> solid-liquid ratio. By the comparison of the mean, it was found that the optimum process combination wasA3B2C3D2, that is, the optimum extraction process parameters were extraction time of 2.5 h, ultrasonic power of 180 W, extraction temperature of 80 ℃ and solid-liquid ratio of 1:25.
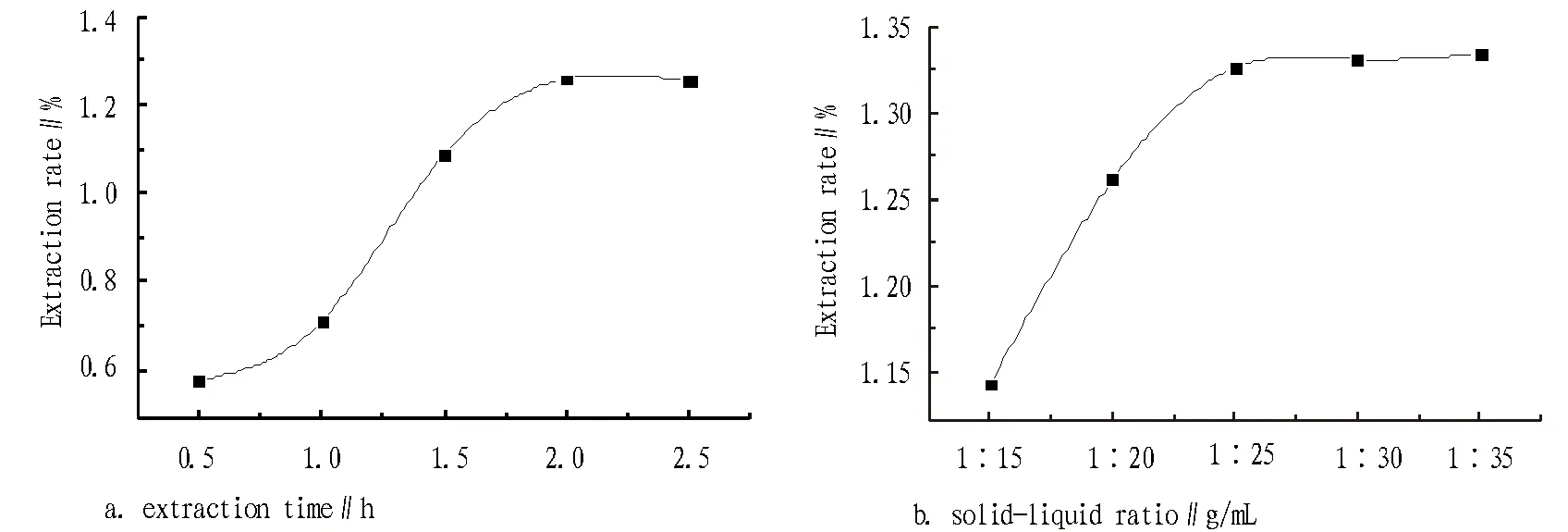
Fig.2 Effect of extraction temperature on the extraction time and solid-liquid ratio of polysaccharides extraction rate
Table1Factorleveldesign
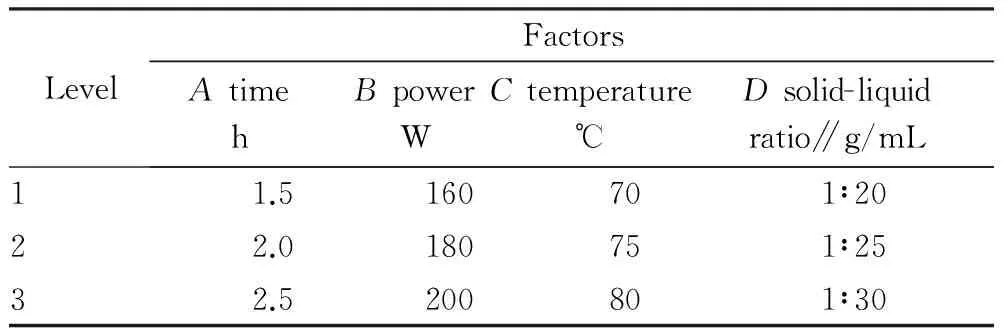
LevelFactorsAtimehBpowerWCtemperature℃Dsolid-liquidratio∥g/mL11.5160701∶2022.0180751∶2532.5200801∶30
Table2L9(34)orthogonaltestresults

No.FactorsABCDExtractionrate∥%111110.601212220.976313331.176421230.930522311.412623120.721731321.306832130.824933210.935K10.9180.9460.7150.983K21.0211.0710.9471.001K31.0220.9441.2980.977R0.1040.1270.5830.024
3.3TheoptimumprocessverificationThe lychee pericarp was extracted under optimum process conditions, and its content was determined. It was repeated 5 times and it was calculated that the average extraction rate of lychee pericarp polysaccharides was 1.45% andRSDwas 1.92%, so it was an optimum process.
3.4AntioxidationoflycheepericarppolysaccharidesThe antioxidant activity results of lychee pericarp polysaccharides can be shown in Table 3. From Table 3, it was found that the lychee pericarp polysaccharides had a certain scavenging effect on superoxide anion radical O2-·, hydroxyl radical OH· and DPPH radical. When the amount of polysaccharides increased from 25 g to 100 g, the scavenging ability would also increase. This shows that lychee pericarp polysaccharides have antioxidant properties, consistent with the literature[2, 12].
Table3Radicalscavengingabilityofpolysaccharides
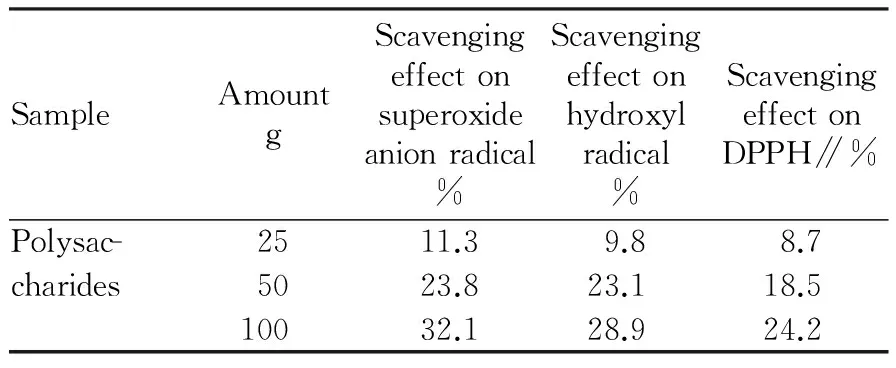
SampleAmountgScavengingeffectonsuperoxideanionradical%Scavengingeffectonhydroxylradical%ScavengingeffectonDPPH∥%Polysac-2511.39.88.7charides5023.823.118.510032.128.924.2
4 Conclusions and discussions
In this study, the optimum extraction process was extraction time of 2.5 h, ultrasonic power of 180 W, extraction temperature of 80℃, and solid-liquid ratio of 1:25. Under the optimum process, the extraction rate of lychee pericarp polysaccharides reached 1.45%. In the concentration of 25 g to 100 g, lychee pericarp polysaccharides showed different scavenging effect on superoxide anion radical, hydroxyl radical and DPPH radical, and had good antioxidant activity. The extraction rate of lychee pericarp polysaccharides under ultrasonic extraction was higher than under traditional water extraction[4], mainly because ultrasound could speed up the dissolution of polysaccharide molecules from the lychee pericarp. The dissolution of active ingredients of lychee pericarp was affected by solid-liquid ratio, ultrasonic power, extraction time and extraction temperature, and the dissolution mechanism among the factors was yet to be studied. The extraction process in this study has high polysaccharides extraction rate, so it can be used for extraction of lychee pericarp polysaccharides, the potentially huge value of lychee pericarp will be further developed, and it will create greater economic value for industry and agriculture.
[1] DONG ZY. Study on extraction, purification and antioxidative activity of polysaccharide from litchi flesh[D]. Xianyang: North West Agriculture and Forestry University, 2006-06. (in Chinese).
[2] YANG B. Study on preparation of bio-active compounds from litchi (Litchi Chinensis Sonn.) pericarp tissues and their biological activities[D]. Guangzhou: South China University of Technology, 2006-06.(in Chinese).
[3] JAVIER RL, CESAR OF, PEDRO WE. Changes in anthocyanin concentration in lychee (litchi chinensis sonn) pericarp during maturation[J].Food Chemistry,1999,65: 195-200.
[4] HUI C, LU MY, LIN DZ. Study on the optimized handicraft of traditional water formulation of Litchi pericarp polysaccharide[J]. China Medical Herald, 2011, 8(28):29-31. (in Chinese).
[5] WU YJ, ZHANG MW, SUN YM. Investigation on the ultrasonic wave-assisted extraction technology for litchi polysaccharides [J]. Journal of South China Normal University(Natural Science Edition),2007,5(2):119-124.(in Chinese).
[6] DONG Q, ZHENG LY, FANG JN. Modified phenol-sulfuric acid method for determination of the content of oligo- and polysaccharides[J].Chinese Pharmaceutical Journal, 1996,31(9):550-552.(in Chinese).
[7] TIAN LM, ZHAO Y, GUO C,etal. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata[J].Carbohydrate Polymers, 2011,83(2):537-544.
[8] LV XR, GUO L, CHANG MC,etal. Antioxidant activity of polysaccharide from Agaricus blazei Murill[J].Acta Edulis Fungi,2010, 17(1):69-71.(in Chinese).
[9] CHEN LY, MENG XJ. The study on the antitumor activity and scavenging free radical and immune effect of the water-soluble polysaccharides from A. Persica. L. var. seleropersica[J]. Food Science, 2004, 25(2): 167-170. (in Chinese).
[10] WANG CC, CHANG SC, INBARAJ S,etal. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L.and evaluation of antioxidant activity[J]. Food Chemistry, 2010, 120(1):184-192.
[11] HE NW, YANG XB, TIAN LM,etal. In vitro antioxidant activity of cucumber polysaccharides[J].Food Science,2011,32(19):70-74. (in Chinese).
[12] SUN J. Analysis of composition and antioxidant activities of litchi pericarp polysaccharide[J].Science and Technology of Cereals,Oils and Foods,2006,14(5):44-53.(in Chinese).
杂志排行
Asian Agricultural Research的其它文章
- Economic Analysis on the Rational Allocation of Agricultural Production Factors in Henan Province
- Special Space and Plant Landscape Design of Urban Ecological Buildings
- Main Methods Applied in Fertigation Technology
- The Fishery Industrial Structure in China Based on the Application of Shift-Share Analysis
- Spatial Structure of Tourism Resources in Liaoning Province
- Analysis on Technical Efficiency and Influencing Factors of Rapeseed Production
