自体淋巴细胞宫腔灌注对反复种植失败患者妊娠结局的影响
2016-01-11曹芳,黄晓阳,于春梅等
·论著·
自体淋巴细胞宫腔灌注对反复种植失败患者妊娠结局的影响
曹芳,黄晓阳,于春梅,杨海燕,王宇峰,周伟,吴娟芬,陈莉
作者单位:213000江苏省常州市,南京医科大学附属常州市妇幼保健院
通信作者:陈莉,213000江苏省常州市,南京医科大学附属常州市妇幼保健院;E-mail:shaoshan686@sina.com
【摘要】目的探讨自体外周血淋巴细胞宫腔内灌注治疗对胚胎反复着床失败(RIF)患者的冷冻胚胎移植(FET)周期妊娠结局的影响。方法选取2014年1月—2015年1月在南京医科大学附属常州市妇幼保健院生殖中心行FET的RIF患者74例,将再次FET周期移植前3 d要求接受淋巴细胞宫腔灌注治疗的患者作为治疗组(38例),未接受淋巴细胞宫腔灌注治疗的患者作为对照组(36例)。治疗组患者在行FET前3 d,抽取静脉血20 ml,应用淋巴细胞分离液制备自体淋巴细胞后行宫腔灌注治疗。观察两组内膜准备方案、FET周期移植胚胎数以及妊娠结局。结果两组年龄、不孕年限、基础卵泡刺激素(FSH)、体质指数(BMI)、移植优质胚胎数、不孕类型比较,差异均无统计学意义(P>0.05)。治疗组人工周期33例(86.8%),自然周期5例(13.2%),平均内膜厚度(9.5±1.2)mm,平均移植胚胎数(2.11±0.45)枚,平均移植优质胚胎数(1.51±0.50)枚;对照组人工周期30例(83.3%),自然周期6例(16.7%),平均内膜厚度(9.0±1.7)mm,平均移植胚胎数(2.17±0.44)枚,平均移植优质胚胎数(1.64±0.76)枚。两组内膜准备方案(χ2=0.18,P=0.67)、平均内膜厚度(t=1.35,P=0.18)、平均移植胚胎数(t=-0.58,P=0.55)、平均移植优质胚胎数(t=-0.83,P=0.41)比较,差异均无统计学意义。治疗组胚胎种植率为22.5%(18/80),临床妊娠率为34.2%(13/38);对照组胚胎种植率为7.0%(5/71),临床妊娠率为8.3%(3/36)。治疗组胚胎种植率和临床妊娠率均高于对照组,差异有统计学意义(χ2=7.388,P=0.007;χ2=7.791,P=0.010)。结论通过自体淋巴细胞宫腔内灌注免疫治疗,可以有效改善RIF患者FET周期的妊娠结局。
【关键词】体外受精;胚胎移植;反复种植失败;淋巴细胞;宫腔灌注
基金项目:南京医科大学科技发展基金重点项目(2013NJMU213)
【中图分类号】R 321.3
收稿日期:(2015-01-28;修回日期:2015-10-19)
曹芳,黄晓阳,于春梅,等.自体淋巴细胞宫腔灌注对反复种植失败患者妊娠结局的影响[J].中国全科医学,2015,18(35):4317-4319,4329.[www.chinagp.net]
Cao F,Huang XY,Yu CM,et al.Effect of intrauterine infusion of lymphocytes on the outcome of pregnancy in patients with recurrent implantation failure[J].Chinese General Practice,2015,18(35):4317-4319,4329.
Effect of Intrauterine Infusion of Lymphocytes on the Outcome of Pregnancy in Patients With Recurrent Implantation FailureCAOFang,HUANGXiao-yang,YUChun-mei,etal.ChangzhouWomenandChildrenHealthHospitalAffiliatedtoNanjingMedicalUniversity,Changzhou213000,China
Abstract【】ObjectiveTo investigate the effect of intrauterine infusion of lymphocytes on the outcome of pregnancy of frozen embryo transfer(FET)in patients with recurrent implantation failure(RIF).MethodsWe enrolled 74 RIF patients who received FET in the reproductive center in Changzhou Women and Children Health Hospital Affiliated to Nanjing Medical University from January 2014 to January 2015.Patients who required to have intrauterine infusion of lymphocytes three days before the next FET cycle were assigned into treatment group(n=38),and patients who didn′t have intrauterine infusion of lymphocytes were assigned into control group(n=36).After autologous lymphocytes were prepared using lymphocyte separation medium,and intrauterine infusion treatment was conducted.Intima preparation plan,number of transferred embryo during FET cycle and pregnancy outcomes of the two groups were observed.ResultsThe two groups were not significantly different in age,years of infertility,FSH,BMI,number of high-quality transferred embryos and type of infertility(P>0.05 for all).In treatment group,there were 33(86.8%)cases of artificial cycle and 5(13.2%)cases of natural cycle;the average thickness of intima was(9.5±1.2)mm,the average number of transferred embryo was(2.11±0.45),and the average number of high quality transferred embryo was(1.51±0.50).In control group,there were 30(83.3%)cases of artificial cycle and 6(16.7%)cases of natural cycle;the average thickness of intima was(9.0±1.7)mm,the average number of transferred embryo was(2.17±0.44),and the average number of high quality transferred embryo was(1.64±0.76).The two groups were not significantly different in intima preparation plan(χ2=0.18,P=0.67),the average thickness of intima(t=1.35,P=0.18),the average number of transferred embryo(t=-0.58,P=0.55),and the average number of high quality transferred embryo(t=-0.83,P=0.41).For treatment group,the embryo implantation rate of the treatment group was 22.5%(18/80),and the clinical pregnancy rate was 34.2%(13/38);for control group,the embryo implantation rate for control group was 7.0%(5/71),and the clinical pregnancy rate was 8.3%(3/36).Treatment group was higher than control group in the embryo implantation rate and clinical pregnancy rate(χ2=7.388,P=0.007;χ2=7.791,P=0.010).ConclusionAutologous lymphocyte infusion in intrauterine in FET cycle may be an effective approach to improve the embryo implantation rate and clinical pregnancy rate in patients with RIF.
【Key words】Fertilization in vitro;Embryo transfer;Recurrent implantation failure;Lymphocytes;Intrauterine administration
目前,体外受精-胚胎移植(in vitro fertilization-embryo transfer,IVF-ET)技术是治疗不孕不育的有效手段,但反复着床失败(repeated implantation failure,RIF)是IVF-ET亟待解决的难题之一。胚胎着床发生在很短的“植入窗”,成功的植入不仅需要优质的胚胎,还需要良好的子宫内膜容受性,以及胚胎与子宫内膜的同步性发育[1-2]。子宫内膜容受性是指子宫内膜处于允许胚胎黏附、穿透并植入的一种状态,与子宫内膜厚度、血流情况、移植窗胞饮突的数量、内膜微环境等相关,此时内膜除受内分泌系统调控外,免疫系统也参与了胚胎种植过程的调节[3-4]。近年来,改善子宫内膜容受性的免疫学机制越来越受到重视。为减少刺激周期促排卵等药物对子宫内膜的影响,本研究对拟行冷冻胚胎移植(FET)周期的RIF患者进行宫腔内自体淋巴细胞灌注治疗,探讨宫腔内灌注免疫治疗对RIF患者妊娠结局的影响。
1对象与方法
1.1研究对象选取2014年1月—2015年1月在南京医科大学附属常州市妇幼保健院生殖中心行FET的RIF患者74例,纳入标准:(1)已移植卵裂期优质胚胎3次或以上;既往周期胚胎移植(ET)前日子宫内膜Salle评分≥13分;(2)基础血清卵泡刺激素(FSH)<15 U/L;(3)夫妇双方染色体核型正常,患者无子宫畸形、子宫内膜异位症等盆腔器质性病变。告知入选患者淋巴细胞宫腔内灌注治疗的意义及潜在风险,将再次FET周期移植前3 d要求接受淋巴细胞宫腔灌注治疗的患者作为治疗组(38例),未接受淋巴细胞宫腔灌注治疗的患者作为对照组(36例)。患者均签署知情同意书。
1.2方法
1.2.1自体淋巴细胞制备治疗组患者在行FET前3 d,抽取静脉血20 ml,采用肝素抗凝,37 ℃水浴30 min,取上清液置于3 ml磷酸盐缓冲液(PBS)中,混匀后缓慢加入含3 ml淋巴分离液的离心管中,以1 500 r/min离心15 min,离心半径为7 cm,收集乳白色淋巴细胞层,置于3 ml PBS中混匀,以1 200 r/min离心6 min,离心半径为7 cm,去上清液,沉淀中加入PBS 3 ml再次混匀,以1 200 r/min离心6 min,离心半径为7 cm,去上清液,将淋巴细胞团重悬于0.3 ml 0.9%氯化钠溶液中混匀备用。
1.2.2宫腔灌注操作方法患者取膀胱截石位,以0.1%碘伏冲洗外阴及阴道后,腹部B超监测下观察到内膜,用人工授精管连接1 ml注射器,抽取已制备好的淋巴细胞,将人工授精管缓慢推注入宫腔内,停留15 s,取出人工授精管,嘱患者卧床10~20 min。
1.2.3观察指标(1)胚胎复苏及评价:利用玻璃化解冻液(Vitrification Media VT101,Thawing Media VT102,Japan)进行卵裂期胚胎解冻,复苏胚胎在体外培养3 h后进行移植。卵裂期胚胎评分标准:1级胚胎:卵裂球数目≥7个,卵裂球规则,球形,折光适度,透明带完整,没有碎片或碎片少于10%;2级胚胎:卵裂球数目≥7个,卵裂球略不规则,轻度折光,碎片含量为10%~19%。1级胚胎和2级胚胎均为优质胚胎。(2)常规FET周期内膜准备:根据患者各自的临床特点选择子宫内膜准备方案:月经规律、正常排卵或内膜厚度≥8 mm者,行自然周期内膜准备方案;月经不规律、排卵异常或内膜厚度<8 mm者,行人工周期内膜准备方案(雌孕激素替代疗法)。(3)移植后第14天检测血清人绒毛膜促性腺激素(HCG)判断是否妊娠。HCG阳性确诊为生化妊娠,继续动态监测血清HCG,胚胎移植后4周行超声检查,见孕囊或胎心搏动可确定为临床妊娠。胚胎种植率=(着床胚胎数/移植胚胎总数)×100%;临床妊娠率=(临床妊娠数/移植周期数)×100%。
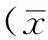
2结果
2.1两组一般资料比较两组年龄、不孕年限、基础FSH、体质指数(BMI)、移植优质胚胎数、不孕类型比较,差异均无统计学意义(P>0.05,见表1)。
2.2两组内膜准备方案及FET周期移植胚胎比较治疗组人工周期33例(86.8%),自然周期5例(13.2%),平均内膜厚度(9.5±1.2)mm,平均移植胚胎数(2.11±0.45)枚,平均移植优质胚胎数(1.51±0.50)枚;对照组人工周期30例(83.3%),自然周期6例(16.7%),平均内膜厚度(9.0±1.7)mm,平均移植胚胎数(2.17±0.44)枚,平均移植优质胚胎数(1.64±0.76)枚。两组内膜准备方案(χ2=0.18,P=0.67)、平均内膜厚度(t=1.35,P=0.18)、平均移植胚胎数(t=-0.58,P=0.55)、平均移植优质胚胎数(t=-0.83,P=0.41)比较,差异均无统计学意义。
2.3两组妊娠结局比较治疗组胚胎种植率为22.5%(18/80),临床妊娠率为34.2%(13/38);对照组胚胎种植率为7.0%(5/71),临床妊娠率为8.3%(3/36)。治疗组胚胎种植率和临床妊娠率均高于对照组,差异有统计学意义(χ2=7.388,P=0.007;χ2=7.791,P=0.010)。
3讨论
胚胎着床是一个复杂的过程并且受诸多因素的精细调控,RIF是指不孕患者移植优质胚胎3次以上或多次移植优质胚胎数10枚或10枚以上仍未获得临床妊娠[5],有研究报道其发生率约为10%[6]。近年来,辅助生殖技术控制性超促排卵方案不断优化,实验室胚胎培养、移植技术也不断发展,但是RIF对临床医生和胚胎学专家仍是一个难题。研究发现约有2/3的RIF是由子宫内膜容受性异常所致[7]。目前通过各种药物治疗、宫腹腔镜手术和内膜搔刮机械刺激等方法改善子宫内膜容受性,一定程度上改善了RIF患者的妊娠结局[8],但存在药物不良反应大、机械刺激创伤性疼痛等缺陷。
研究表明增生期子宫内膜中的自然杀伤(NK)样细胞数量很少,但是在分泌期子宫内膜NK样细胞大量出现,在胚胎种植时数量达到最多,子宫内膜转化为蜕膜时,大颗粒淋巴细胞大量增生。另外,体外实验发现未孕大鼠胸腺淋巴细胞可促进子宫内膜分化,诱导胚胎种植,提示某些免疫细胞可能具有调节子宫内膜分化,开启胚胎种植窗的作用[9]。Ideta等[10]将牛自体单细胞核淋巴细胞在体外培养48 h后注入宫腔后行胚胎移植,可有效提高胚胎种植率和妊娠率。提示淋巴细胞对子宫内膜容受性起正向调节作用,有助于胚胎种植。
为避免促排卵药物对子宫内膜的影响,本研究纳入的RIF患者均行FET。结果显示,治疗组胚胎种植率(22.5%vs.7.0%)和临床妊娠率(34.2%vs.8.3%)均高于对照组,提示自体淋巴细胞宫腔内灌注联合FET治疗可提高RIF患者临床妊娠率。
自体淋巴细胞宫腔内灌注治疗RIF的作用机制有待进一步研究。自体淋巴细胞宫腔内灌注后,子宫内膜表现为非感染性炎性改变,宫腔内分泌蛋白酶,介导内膜细胞与胚胎信号传导,使子宫内膜的分化与胚胎发育同步化[11]。此外,自体淋巴细胞可刺激子宫内膜NK样细胞等分泌大量细胞因子及趋化因子,促使子宫内膜分化,有利于开放子宫内膜种植窗期,促进滋养细胞的侵入,从而利于胚胎的种植[12]。
本研究提示淋巴细胞宫腔内灌注是治疗RIF的有效方法之一,且操作简单,患者依从性好,对生殖调控、不孕治疗和提高妊娠率具有重大意义。但是需要进一步随访活婴出生率及淋巴细胞宫腔内灌注的治疗方法对子代发育是否有影响。
参考文献
[1]Zanatta A,Rocha AM,Carvalho FM,et al.The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility:a review[J].J Assist Reprod Genet,2010,27(12):701-710.
[2]Wu Z,Li R,Qiao J.Research of endometrial receptivity in assisted reproductive technology[J].Reproduction and Contraception,2011,31(8):538-543.(in Chinese)
武泽,李蓉,乔杰.辅助生殖技术治疗中子宫内膜容受性变化的研究进展[J].生殖与避孕,2011,31(8):538-543.

表1 两组患者一般资料比较
注:a为χ2值;FSH=卵泡刺激素,BMI=体质指数
[3]Carlino C,Stabile H,Morrone S,et al.Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy[J].Blood,2008,111(6):3108-3115.
[4]Lash GE,Robson SC,Bulmer JN.Review:Functional role of uterine natural killer(uNK)cells in human early pregnancy decidua[J].Placenta,2010(31 Suppl):S87-92.
[5]Simon A,Laufer N.Repeated implantation failure:clinical approach[J].Fertil Steril,2012,97(5):1039-1043.
[6]Voullaire L,Collins V,Callaghan T.High incidence of complex chromosome abnormality in cleavage embryos from patients with repeated implantation failure[J].Fertil Steril,2007,87(5):1053-1058.
[7]Coughlan C,Ledger W,Wang Q,et al.Recurrent implantation failure:definition and management[J].Reprod Biomed Online,2014,28(1):14-38.
[8]Hitit M,Guzeloglu A,Ozel C,et al.109 expression of genes related to endometrial receptivity in equine endometrium during the estrous cycle and early pregnancy[J].Reprod Fertil Dev,2014,27(1):147.
[9]van Mourik MS,Macklon NS,Heijnen CJ.Embryonic implantation:cytokines,adhesion molecules,and immune cells in establishing an implantation environment[J].J Leukoc Biol,2009,85(1):4-19.
[10]Ideta A,Sakai S,Nakamura Y,et al.Administration of peripheral blood mononuclear cells into the uterine horn to improve pregnancy rate following bovine embryo transfer[J].Anim Reprod Sci,2010,117(1/2):18-23.
[11]Fujiwara H,Ideta A,Araki Y,et al.Immune system cooperatively supports endocrine system-primed embryo implantation[J].Journal of Mammalian Ova Research,2009,26:122-128.
[12]Okitsu O,Kiyokawa M,Oda T,et al.Intrauterine administration of autologous peripheral blood mononuclear cells increases clinical pregnancy rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure[J].J Reprod Immunol,2011,92 (1/2):82-87.
(本文编辑:贾萌萌)
