混合相二氧化钛石墨烯复合物的制备及光催化性能
2015-12-29于建华范闽光董丽辉张飞跃
于建华 范闽光,2,* 李 斌 董丽辉 张飞跃
(1广西大学化学化工学院,南宁530004;2广西石化资源加工及过程强化技术重点实验室,南宁530004)
混合相二氧化钛石墨烯复合物的制备及光催化性能
于建华1范闽光1,2,*李 斌1董丽辉1张飞跃1
(1广西大学化学化工学院,南宁530004;2广西石化资源加工及过程强化技术重点实验室,南宁530004)
采用水热法制备了一系列混合相二氧化钛-石墨烯(TrG)的复合物,并考察了石墨烯的用量对降解污染物甲基蓝的影响.采用X射线衍射(XRD),傅里叶变换红外(FTIR)光谱,高分辨透射电镜(HRTEM),拉曼光谱,紫外-可见漫反射吸收光谱(UV-Vis DRS),X射线光电子能谱(XPS)和比表面积(BET)等测试手段对复合材料进行表征.结果表明,复合材料中TiO2为棒状的混合相,且均匀分散在石墨烯表面.由于石墨烯良好的吸光性能,混合相中的异质结和复合物的良好光电子传递能力以及高比表面积,复合材料具有较高的光催化活性.所制备的TrG复合材料在紫外光下降解甲基蓝的催化活性均高于纯TiO2,且当氧化石墨烯负载量为0.8%(质量分数, w)时,复合材料TrG具有较好的光催化效果.
混合相TiO2;异质结;石墨烯;光电子传递;水热法;光催化
©Editorial office ofActa Physico-Chimica Sinica
1 Introduction
In recent years,semiconductor photocatalytic degradation of organic pollutants has attracted more and more attention in the field of environmental pollution control,because it is one of the most effective technologies for dealing with global environmental pollution and the energy crisis.1-4Among various semiconductor photocatalysts,TiO2-based materials have been the most promising candidates because of their strong oxidizing powers,low cost,desirable electronic,photo-stabilities,and relative nontoxicities.2,5-7TiO2has four natural crystalline polymorphs:anatase (space group:I41/amd),rutile(space group:P42/mnm)and brookite (space group:Pbca),and TiO2(bronze,C2/m),which can be modeled as an edge and corner-linked structure with Ti cations coordinated octahedrally by oxygen anions.Rutile is the most thermodynamically stable form,while the other two are of metastable structure.1,8-10The brookite is rarely investigated due to the difficulty encountered in obtaining its pure phase.11,12Some papers reported that the anatase polymorph generally exhibited better efficiency for photocatalysis and solar energy conversion because of the low recombination rate of its photogenerated electrons and holes,8,13while the photocatalytic performance of rutile is still indistinct.12Generally,rutile TiO2has relatively low photocatalytic activity.1,14In the field of rutile phase photocatalytic degradation has not caused enough attention.Recently,many papers report that the photocatalytic ability of rutile TiO2is excellent in the degradation of organic pollutants.14,15
Among the works which involve the photocatalytic performance of TiO2with its crystal structure,the role of the mixed crystal phases has also been concerned.12,16Mixed crystal phases, such as anatase/rutile,exhibit better photocatalytic performance than pure single crystal phase due to better separation of photogenerated electrons and holes,16-19allowing higher levels of radicals to be generated.20Such as Degussa P-25,which naturally consists of about 80%anatase and 20%rutile with a surface area of 50 m2·g-1,was extensively studied as a standard titania catalyst partially because of its high photocatalytic activity which profits from the mixed phase of anatase and rutile.21
Graphene,as a“rising star”material,has many exceptional properties,such as high electronmobility,theoretically high surface area,and good mechanical properties.14,22-24Recently,graphene oxide(GO)-or graphene-semiconductor nanoparticle composites were synthesized by various methods and their photocatalystic properties were studied.For example,Marcano et al.25used a new method to improve the efficiency of the oxidation process,and provided a greater amount of hydrophilic oxidized graphene material as compared toHummers′method orHummers′method with additional KMnO4.Single-layer GO has been prepared successfully by Cote et al.26using the method of Langmuir Blodgett assembly.Williams et al.27firstly demonstrated that graphene facilitates the charge separation and functions as the electron transfer medium in graphene-TiO2composite photocatalysts.Subsequently,Zhang et al.22reported that P25 TiO2-graphene composites showed enhanced photocatalytic activity for the degradation of methylene blue in aqueous solutions.Many papers have been reported about the composite materials that prepared by TiO2and graphene,but the main phase of TiO2is anatase in these composites.Thus,it is very important to develop a kind of synthetic method in which the crystalline phase of titania can be controlled.Then what kind of results appear when the rutile and anatase phase coexistence of TiO2loaded on graphene,this paper will explore it.
So far,few researches on mixed phase TiO2-graphene composite material in the photocatalytic field were reported.In this work,mixed phase TiO2-graphene composite was prepared by a simple hydrothermal method.The novel TiO2-graphene composites consisting of high quality graphene and high reactive mixed phase TiO2with exposed{110}and{101}facets.The synergistic effect of high reactive facets between anatase and rutile TiO2and graphene may significantly enhance the photocatalytic activity of composites.Thus,we expect that our detail studies on novel TiO2-graphene can help to gain deeper insights into the electron transfer process of semiconductor-carbon for further improving their catalytic activity.
2 Experimental
2.1 Materials
Graphite(≥99.85%,<30 μm)was purchased from Shanghai Huayi Group Huayuan Chemical Co.,Ltd.H2SO4,H3PO4and HCl were purchased from Chengdu Kelong Chemical Reagent Factory. Hexadecyl trimethyl ammonium bromide(CTAB)and KMnO4was purchased from Tianjin Guangfu Fine Chemical Research Institute and Xi Long Chemical Co.,Ltd.,respectively.Ethylene glycol(EG),NaNO3and H2O2(30%)were purchased from Guangdong Guanghua Science and Technology Co.,Ltd.Isopropyl titanate(TTIP,Aladdin,95%)was purchased from Aaladdin. Methylene blue(MB)was purchased from Shanghai Model Plant Specimens.All chemicals involved were of analytical-grade,and used as received.
2.2 Preparation of samples
2.2.1 Preparation of anatase TiO2
TiO2was obtained by a modified hydrothermal method reported with reference to Sun et al.28In a typical synthesis,0.1 g CTAB was thoroughly dissolved into the mixed solvents composed of 11 mLof double-distilled water and 2 mLof EG by sonication for 30 min.After 3 mLTTIPadded into the solution,an extra 30 min was required to achieve homogeneous solution under sonication and stirring.The solution was transferred to a 20 mL Teflon-lined stainless steel autoclave,placed in an electric oven maintained at 160°C for 24 h.The resulting white powders were collected by centrifugation,neutralization,and washing and finally were dried in an oven at 60°C for 12 h.
2.2.2 Preparation of rutile TiO2.
0.1 g CTAB was thoroughly dissolved into the mixed solvents composed of 9 mLof double-distilled water,2 mLof concentrated hydrochloric acid,and 2 mLof EG by sonication for 30 min.After3 mLTTIP added into the solution,an extra 30 min was required to achieve homogeneous solution under sonication and stirring. The solution was transferred to a 20 mL Teflon-lined stainless steel autoclave,placed in an electric oven maintained at 160°C for 24 h.
2.2.3 Preparation of anatase-rutile TiO2
0.1 g CTAB was thoroughly dissolved into the mixed solvents composed of 10 mLof double-distilled water,1 mLof concentrated hydrochloric acid,and 2 mLof EG by sonication for 30 min.After 3 mLTTIP added into the solution,an extra 30 min was required to achieve homogeneous solution under sonication and stirring. The solution was transferred to a 20 mL Teflon-lined stainless steel autoclave,placed in an electric oven maintained at 160°C for 24 h.
2.2.4 Preparation of TiO2-graphene(TrG)
GO was prepared using an improved method from graphite powders.25First,0.2%,0.4%,0.5%,0.6%,0.8%,or 1.0%(w,mass fraction)of GO was sonicated in 20 mLof deionized water and 10 mL of absolute ethanol for 2 h to achieve uniform dispersions of GO.Next,TiO2powder was added slowly to the GO dispersions while stirring.The TiO2/GO mixture was further stirred for 1 h to ensure complete mixing.Then the mixture was transferred into a Teflon-lined stainless steel vessel for hydrothermal treatment at 180°C for 2 h.The resulting gray powders were collected by centrifugation,and washing and finally were dried in an oven at 60°C. The obtained samples are assigned as TrG0(pure TiO2),TrG0.2, TrG0.4,TrG0.5,TrG0.6,TrG0.8,TrG1.0,respectively.
2.2.5 Preparation of reduced graphene oxide(rGO)
Typically,a certain amount of GO ultrasonically was dispersed in 20 mL H2O and 10 mL absolute ethanol,and then transferred to a stainless steel autoclave of polytetrafluoroethylene,was placed in an electric oven at 180°C incubated 2 h.The resultant product was centrifuged and washed with deionized water repeatedly.The solid obtained was vacuum-dried overnight at room temperature.
3 Characterization
The crystal structures of samples were characterized by powder X-ray diffraction(XRD)and patterns were collected from 10°to 70°in 2θ with 0.04 step·s-1using a Rigaku D/max-3B X-ray diffractometer with Cu Kαradiation source(λ=0.15418 nm)at 40 kV and 36 mA.The nanometric structure and the morphology of the catalysts were observed by a high-resolution transmission electron microscope(HRTEM,TECNAI G2 F30,USA).X-ray photoelectron spectroscopy(XPS)measurements were performed on PHI 5000 Versaprobe spectrometer with monochromatic Al Kα(1486.6 eV)X-ray radiation(200µm,15 kV and 40 W)and hemispherical electron energy analyzer.Fourier transform infrared spectroscopy(FTIR,Bruker VERTEX 70,Germany)was also taken to verify the bonds forming and skeletal of the graphene sheets in the hybrid nanostructures.Raman spectra were performed on a JY HR800 dispersive Raman microscope with excitation laser beam wavelength of 632.8 nm.UV-visible diffuse reflectance spectra were obtained for the dry-pressed disk samples with a UV-visible spectrophotometer(UV-2550,Shimadzu,Japan).BaSO4was used as a reflectance standard in the UV-visible diffuse reflectance experiment.The Brunauer-Emmett-Teller (BET)specific surface areas(SBET)and porous structures of the samples were analyzed by a V-Sorb2800P nitrogen adsorption apparatus(Beijing).
4 Photocatalytic experiments
The UV light relative photocatalytic activity of each synthesized material with and without the presence of GO,was comparatively investigated by evaluating the photodegradation rate of MB under the irradiation of a 300 W mercury lamp.In a typical process,aqueous solution of the MB dyes(16 mg·L-1,100 mL) and the photocatalysts(0.05 g)were placed in a 100 mL cylindrical quartz beaker.The mixture of catalyst and dye was kept in the dark under stirring for 30 min to ensure the establishing of an adsorption-desorption equilibrium.Following this,the photocatalytic reaction was started by the exposure of UV light and the temperature of the suspension was kept at about 25°C by an external cooling jacket with recycled water.After a setup exposure time,5 mL suspension was sampled,centrifuged,and the supernatant liquid was taken out for UV-Vis absorption spectrum.The absorbance peak of the dye was recorded every 10 min to enable calculation of the degradation rate.
5 Results and discussion
5.1 Comparable textural properties
5.1.1 XRD results
Fig.1 and Fig.2 show the XRD patterns for the graphite,GO, rGO,and TrG nanocomposites synthesized with different contents of graphene compared to the pure TiO2respectively.
According to the Fig.1a,a clear(002)peak at 2θ=26.4°and (004)peak at 2θ=54.5°of graphite flakes can be observed,it indicates that the spatial arrangement of pure graphite sheet layer is very regular.29The(002)peak has become very small and weak after oxidized,and a sharp(001)peak appeared at 2θ=11.5°,indicating a larger interlayer distance of 0.77 nm(the interplanardistance was also estimated from the XRD spectrum by using the Bragg equation:d=λ/2sinθ)than that of graphite(0.34 nm)because of the oxygen-containing groups in the surface of GO.30The oxygen functional groups attached to both sides of the graphite flakes create atomic defects(sp3bonding)in the graphite structure and tend to exfoliate to a few layers or individual layers of GO in an aqueous medium.31Pure rGO shows a strong(002)graphene reflection at 26.4°,which is a clear indication of the conversion of rGO to graphene,31indicating the reduction of GO after hydrothermal treatment.
From Fig.2,the peaks at 25.3°,37.8°,48.0°,53.9°,and 62.7° can be assigned to the diffractions of the(101),(004),(200), (105),and(211)crystal planes of anatase phase TiO2,respectively (JCPDS,No.21-1272).The peaks located at 27.5°,36.0°,41.2°, 44.1°,54.3°,56.7°,62.6°,64.1°,and 69.0°correspond to the diffractions of the(110),(101),(111),(210),(211),(220),(002), (310),and(301)crystal planes of rutile phase TiO2,respectively (JCPDS,No.21-1276).

Fig.2 XRD patterns of anatase TiO2,TrG0(mixed phase TiO2), rutile TiO2,TrG0.2,TrG0.4,TrG0.5,TrG0.6,TrG0.8,and TrG1.0nanocomposites
The TrG composites with different rGO compositions exhibit the characteristic peaks of anatase phase TiO2and rutile phase TiO2,indicating that as for the composites both anatase and rutile phases are coexistence.It is worth noting that the peak positions of(110)of rutile plane and(101)of anatase plane in TrG composites shift to a larger diffracting angle,which may be attributed to the strong mutual interactions between anatase-and rutile-type phases(the coexistence of two phases).Moreover with the increase of the amount of graphene,the crystallinity of composite material(110)crystal face enhanced slightly,similar results have also been reported in the literature,1,32this may be due to the influence of overlapping diffraction peaks between the graphene (002)characteristic diffraction peak and rutile TiO2(110)crystal face peak(27.5°).1,33
5.1.2 BET results
The SBETwas determined by a multipoint BET method using the adsorption data in the relative pressure(p/p0)range of 0.05-0.20. The nitrogen adsorption volume at the relative pressure p/p0of 0.99 was used to determine the pore volume VP.The estimated value of the BET specific surface area and pore volume(VP)are listed in Table 1.The desorption isotherm was used to determine the pore size distribution via the Barrett-Joyner-Halender(BTH) method,assuming a cylindrical pore model.Fig.3 shows the nitrogen sorption isotherms and the corresponding pore size distribution curves for TrG0.8relative to TrG0.According to the Brunauer-Deming-Deming-Teller(BDDT)classification,the isotherm for TrG0is of type IV with a typical hysteresis loop, associated with the capillary condensation of gases within mesopores(2-50 nm),5indicating that both of them are typical mesoporous materials.Due to the low graphene loading,the TiO2-graphene(TrG0.8)nanocomposites exhibit rather similar N2sorption isotherms relative to the pure TiO2(TrG0),suggesting that they have comparable pore structures.The corresponding pore size distribution curves(Fig.3,inset)indicate that both samples contain relative uniform mesopores with wide mesopore size distribution,5the main mesopore size of TrG nanocomposites at 21.5 nm.As shown in Table 1,TrG nanocomposite samples show the higher BET surface area than pure TiO2nanocrystals.Agreater BET surface area of photocatalysts could be beneficial to achieve better adsorption of dye molecules in aqueous suspension and can supply more surface active sites for the photocatalytic reaction, thus can improve the photocatalytic activity of composite materials.1,24

Table1 Summary of physicochemical properties of as-prepared samples

Fig.3 Nitrogen sorption isotherms and the corresponding pore size distribution curves(inset)for TrG0.8and TrG0
5.2 Microstructures of TrG composites
5.2.1 TEM and HRTEM results
HRTEM(Fig.4(a,b)analysis shows the TiO2bars are anchored onto the rGO sheets after hydrothermal treatment,due to interfacial interactions and preferential heterogeneous nucleation, numerous TiO2nanocrystals are densely deposited and dispersed uniformly on the surface of graphene sheets.The surface deposition of the TiO2nanocrystals can obviously alleviate the crumpling and agglomeration of graphene nanosheets,although crumpled edges are still observed.The lattice fringe spacing of 0.353 nm corresponds to the(101)crystallographic planes of anatase TiO2,while the lattice fringe spacing of 0.326 nm matches the(110)planes of rutile phase,respectively.Furthermore,Fig.4d displays the combination of surface rutile in contact with anatase. HRTEM image(Fig.4(c,d))provides direct evidence for the phase junction between anatase and rutile phases.Namely,heterojunctions were formed.

Fig.4 TEM images(a,b)of TrG0.8composites,HRTEM images (c,d)showing the corresponding interplanar space of TiO2
5.2.2 FTIR results
An absorption peak at 1645 cm-1attributed to stretching vibration of C=C sp2hybridized carbons in graphite.After the oxidation of graphite,a series of new infrared absorption peak appeared.The FTIR spectrum(Fig.5)of GO shows strong peaks corresponding to oxygen functional groups such as,carboxylates C—O(1057 cm-1),epoxide C—O—C(1122 cm-1),stretching vibration C—OH(1230 cm-1),out-plane vibrations O—H(1396 cm-1),vibrations of aromatic C=C(1520 cm-1),skeletal ring vibrations C—C(1625-1650 cm-1),and carbonyl moieties C=O (1740 cm-1).While,after GO is reduced,i.e.,rGO,the adsorption bands of oxygen functionalities(e.g.,C—O at 1057 cm-1,O—H at 1396 cm-1,and C=O at 1740 cm-1)disappear,only the peak of C—C at 1645 cm-1remains,indicating the successful reduction of GO via self-assembly by the hydrothermal process,34consistent with the XRD results.According to the Hooke Law,the absorption peak around 1460 cm-1is contributed by the C—Ti skeletal vibration of the TrG0.8.And the absorption below 1000 cm-1could be considered as a combination of Ti—O—Ti and Ti—O—C vibrations,indicating the bonding between TiO2and GO in synthesized hybrids.24

Fig.5 FTIR spectra of graphite,GO,rGO,TrG0,and TrG0.8
5.2.3 Raman results
Raman spectroscopy is generally used to characterize the crystal quality of carbonaceous materials.The Raman spectrum (Fig.6)of as-prepared mixed phase TiO2had four main peaks at 152.3,233.0,431.3,and 610.7 cm-1,35which are in consistency with the fundamental modes observed in the spectra of anataserutile phase.The D peak(about 1350 cm-1)is corresponding to the κ-point phonons of A1gsymmetry,which is a common feature of sp3defects in carbon and usually can be associated with the structural defects,amorphous carbon,or edges that break the symmetry and selection rule,while the G peak(about 1580 cm-1) is common to all sp2carbon,which can provide information on the in-plane vibration of sp2-bonded carbon domains.14,36In the spectrum of pristine graphite,the sharp G peak(1579.7 cm-1)and weak shoulder peak(1357.6 cm-1)indicate that the graphite structure is very regular.30Compared with graphite,the G peak(1585.6 cm-1) of GO becomes wider,and the D peak becomes sharp and strong. The ratio of the intensities(ID/IG)for GO was markedly increased, indicating the formation of some sp3carbon by functionalization.14,37Significantly,in comparison with graphene oxide,there is a notable increase in the Raman intensity ratio of the D and G bands (ID/IG,Fig.6(ii)),with ID/IG=0.99 for the rGO and ID/IG=0.98 for the TrG0.8nanocomposite versus ID/IG=0.90 for graphene oxide.Thischange suggests the effectiveness of the photocatalytic reduction of graphene oxide and the existence of graphene sheets in the TrG nanocomposite.5In addition,the D peak for the TrG nanocomposites is broadened and blue shifted to 1345.7 cm-1after the reduction of graphene oxide(1357.6 cm-1),implying a close interaction between TiO2and graphene.5Moreover,the 2D peaks of TrG0.8(Fig.6(i(a)))at 2692.4 and 2934.7 cm-1imply that rGO exists as single layer and multilayer graphene sheets in the hybrid.37

Fig.6 (i)Enlarged Raman spectra for TrG0.8(a),GO(b),and rGO(c)in the range of 2200-3200 cm-1;(ii)Raman spectra of graphite,GO,rGO,TrG0,and TrG0.8
5.2.4 XPS results
The survey XPS spectra(Fig.7)clearly indicate the existence and binding energies of C 1s,O 1s,and O 2s core electrons for GO.The TrG0.8composite contains Ti,O,and C with binding energies corresponding to Ti 2p3/2,Ti 2p1/2,O 1s,and C 1s.The XPS peaks at 458.6 and 464.4 eV are attributed to the binding energies of the Ti 2p3/2and 2p1/2electrons.
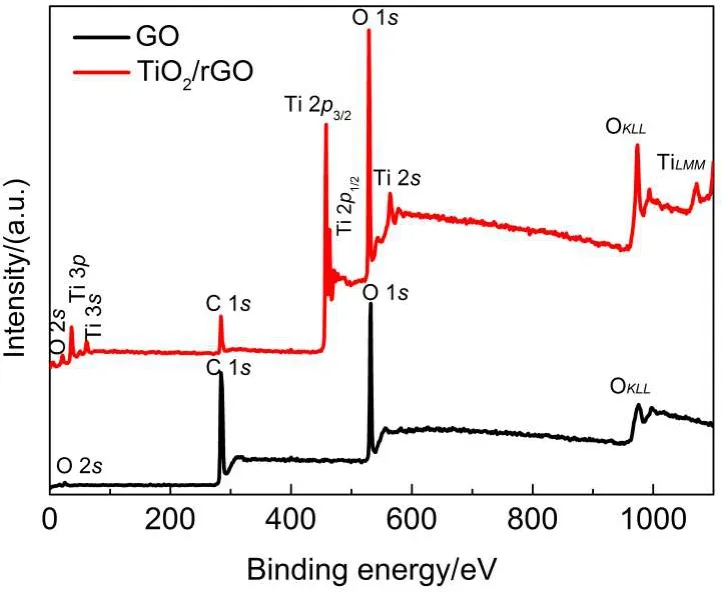
Fig.7 Survey XPS spectra of GO and TrG0.8
Three typical peaks are observed in GO(Fig.8),which represent chemically different species are located at 284.8,286.9,and 288.8 eV,respectively.These peaks correspond to elemental carbon/sp2-hybridized carbon from GO,oxygen-containing alcohol, or ether linkages(C—OH and C—O)and carboxylate(O=C—O),respectively.38TrG0.8displays a predominant peak associated with C=C/C—C(284.8 eV),but the peak attributed to C—O (286.4 eV)becomes relative weak compared to the carbon bond of C—O(286.9 eV)in original GO sample,this change indicates the effectiveness of the photocatalytic reduction of graphene oxide and the existence of graphene sheets in the TrG nanocomposite, which is consistent with the results of Raman.In addition,an additional shoulder peak located at 282.7 eV was found,which was usually assigned to the formation of a chemical bond between a carbon atom and a titanium atom in the lattice of TiO2,which further confirm the formation of C—Ti bonds,39this is consistent with the FTIR results well.The significant decreases of the C—O signals in TrG0.8demonstrate a high degree of deoxygenating and successful reduction from GO sample to TrG0.8during the hydrothermal process.

Fig.8 Deconvoluted peaks of XPS core levels of C 1s of GO and TrG0.8electrons
5.2.5 UV-Vis results
The UV-Vis DRS is measured to evaluate the optical absorption properties of the catalysts,as shown in Fig.9.The presence of different amounts of rGO affects the optical property of light absorption for the TrG nanocomposites significantly.The addition of rGO induces the increased light absorption intensity in the visible region,as observed in all of the TrG nanocomposites with different addition ratios of rGO.A plot of the transformed Kubelka-Munk function as a function of energy of light is shown in Table 1,by which the roughly estimated band gaps are 3.08, 2.92,2.95,2.88,2.85,2.83,2.82,2.78,and 2.76 eV corresponding to anatase TiO2,rutile TiO2,TrG0,TrG0.2,TrG0.4,TrG0.5,TrG0.6, TrG0.8,and TrG1.0,respectively.This phenomenon can be explained as follows:during heat treatment of the GO,the functional groups on the surface of the GO(i.e.,—OH,—COOH and so on)disappeared.Upon this situation,the p electron of the carbon atom did not entirely bond with others to form the delocalized large p bond,and some unpaired electrons bonded with the free electrons on the surface of TiO2to form a Ti—O—C structure,which then shifted up the valence band edge and reduced the band gap.The stronger absorption intensity of light for the TrG nanocomposites than bare TiO2suggests that they could have higher photocatalytic activity for a given reaction.

Fig.9 UV-Vis diffuse reflectance spectra of anatase TiO2, rutile TiO2,TrG0,and TrG composites
5.3 Dye photodecomposition
The photocatalytic degradation efficiency of MB under UV light follows the order:rutile TiO2<anatase TiO2<TrG0<TrG0.2<TrG0.4<TrG0.5<TrG0.6<TrG1.0<TrG0.8,as shown in Fig.10.Clearly,the activity of mixed phase TiO2is higher than rutile TiO2and anatase TiO2,when the weight addition ratio of rGO is increased to 1.0%, the activity of TrG1.0will be lower than TrG0.8,although it is higher than bare TiO2.According to the above results,the phenomenon can be ascribed to the following reasons:(i)with the increase of rGO content light harvesting competition between TiO2and rGO is intensified,which leads to the decrease of the photocatalytic performance;and(ii)the excessive rGO can act as a kind of charge carrier recombination center and promote the recombination of electron-hole(e--h+)pairs in rGO.
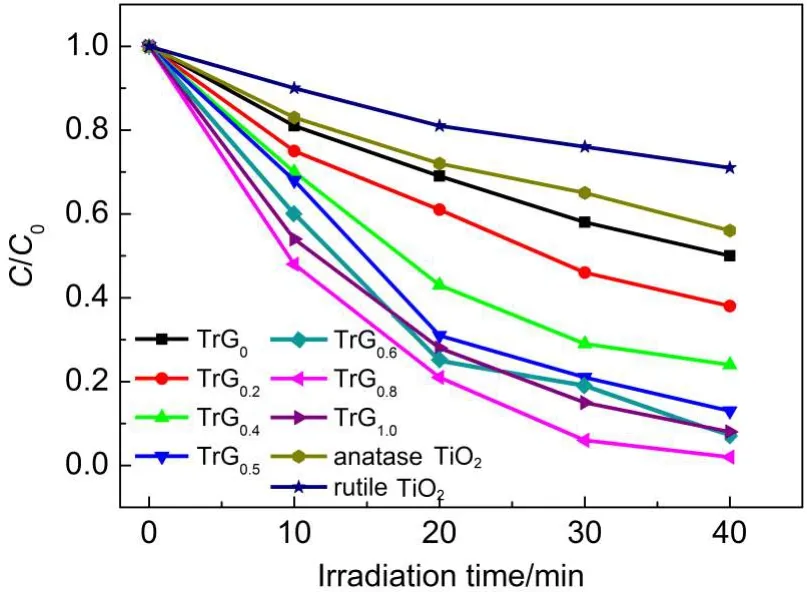
Fig.10 Liquid-phase photocatalytic degradation of methylene blue(MB)under the irradiation of UV light over the anatase TiO2,rutile TiO2,and TrG nanocomposites
The TrG nanocomposites have higher catalytic activity results from the mixed phase of TiO2similar as P25,and the photocarrier nanofilm graphene.(1)Under the UV irradiation,either rutile or anatase TiO2is excited to produce photo-generated electrons and holes,which then carry out interfacial reactions to initiate the photocatalytic procedure.In a hetero-phase catalyst of rutile and anatase,charge transfer between the two phases should be also concerned.When anatase interweaves with rutile,the establishment of a heterojunction by the coupling of two kinds of TiO2polymorphs at their interface should allow an easier transfer of photogenerated electrons from one phase to another,electron paramagnetic resonance experiments focusing on mixed anatase/ rutile samples have demonstrated that electrons flow from rutile into anatase,with holes moving in the opposite direction (Fig.11).40This charge transfer is favored by the close contact between small anatase particles and larger rutile particles,and stabilizes the e--h+separation in the presence of rutile.On the other hand,the rutile phase with a narrower band gap could extend the useful range of the photoresponse and harvest more light. (2)The strong mutual interactions between anatase-and rutiletype phases may be attributed to the stress within molecules of multiphase particles.This factor may result in increasing surface free energy,in order to decrease the surface free energy,one or a few atomic layers occur reconstruction,relaxation phenomenon, and anisotropic growth phenomenon in the interface,consequently a order nanostructure formed that beneficial to the separation of photogenerated electron hole pairs.These effects of interface may be beneficial to the absorbance of O2and OH-yielding as a result of an increase in the O-2•and•OH.The generation of O-2•and·OH are key factors in the reaction of the photo-oxidation of the target pollutant in aqueous media because of its high reactivity.41(3) Graphene possesses a remarkable electrical transport property.In the TrG composite,the portion of graphene plays a role for conducting electrons,which makes the photocarrier transferring to the surface of graphene(Fig.11),and improves the separation of the electron-hole pairs and also the photocatalytic efficiency.(4) Graphene is of great specific surface area and strong capacity of absorption.Therefore,graphene attributes to increase specific surface area of the composite.During degradation,at first,MB molecules were absorbed on the graphene surface,and reacted with the photocarrier transferred from TiO2.

Fig.11 Schematic of a proposed model for photocatalytic activity for degradation of TiO2-graphene
6 Conclusions
The TiO2-graphene(TrG)composite materials with high photocatalytic activity were prepared by hydrothermal method.The influence of the amount of graphene oxide on the photocatalytic activity of the TrG composites was studied.The results show that: (1)The as-formed TiO2was formed in the anatase and rutile phase with a bar structure,and dispersed uniformly on the surface of graphene sheets.(2)The composites possess higher specific areas and strong capacity of absorption results in photocatalytic degradation performance.(3)The higher photocatalytic performance of the TiO2-graphene composite material,which may be due to the synergistic effect of graphene and mixed phase TiO2,can effectively separate the photogenerated electron hole pairs,improve the utilizationrate rate of the photogenerated electrons and holes,and provide more reactive points,thereby significantly improve the photocatalytic activity for degradation of pollutants.
(1)Gan,Y.P.;Qin,H.P.;Huang,H.;Tao,X.Y.;Fang,J.W.; Zhang,W.K.Acta Phys.-Chim.Sin.2013,29,403.[甘永平,秦怀鹏,黄 辉,陶新永,方俊武,张文魁.物理化学学报,2013,29,403.]doi:10.3866/PKU.WHXB201211022
(2)Liu,S.W.;Yu,J.G.;Jaroniec,J.J.Am.Chem.Soc.2010,132, 11914.doi:10.1021/ja105283s
(3)Kong,M.;Li,Y.Z.;Chen,X.;Tian,T.T.;Fang,P.F.;Zheng,F.; Zhao,X.J.J.Am.Chem.Soc.2011,133,16414.doi:10.1021/ ja207826q
(4)Yu,X.M.;Kim,B.;Kim,Y.K.ACS Catal.2013,3,2479.doi: 10.1021/cs4005776
(5)Liu,S.W.;Liu,C.;Wang,W.G.;Cheng,B.;Yu,J.G. Nanoscale2012,4,3193.doi:10.1039/c2nr30427a
(6)Long,R.;English,N.J.;Prezhdo,O.V.J.Am.Chem.Soc.2012,134,14238.
(7)Zhou,J.;Song,B.;Zhao,G.L.;Han,G.R.Nanoscale Res.Lett.2012,7,217.doi:10.1186/1556-276X-7-217
(8)Zhao,B.;Lin,L.;He,D.N.J.Mater.Chem.A2013,1,1659. doi:10.1039/c2ta00755j
(9)Zhao,M.L.;Li,L.P.;Lin,H.F.;Yang,L.S;Li,G.S.Chem. Commun.2013,49,7046.doi:10.1039/c3cc43416h
(10)Jiao,Y.C.;Zhao,B.;Chen,F;Zhang,J.L.CrystEngComm2011,13,4167.doi:10.1039/c0ce00932f
(11)Li,K.;Xu,J.L.;Shi,W.Y.;Wang,Y.B.;Peng,T.Y.J.Mater. Chem.A2014,2,1886.doi:10.1039/c3ta13597g
(12)Zhao,B.;Chen,F.;Jiao,Y.C.;Yang,H.Y.;Zhang,J.L.Journal of Molecular Catalysis A:Chemical2011,348,114.doi: 10.1016/j.molcata.2011.08.015
(13)Yanagisawa,K.;Ovenstone,J.J.Phys.Chem.B1999,103, 7781.doi:10.1021/jp990521c
(14)Zhang,J.;Yan,S.;Fu,L.;Wang,F.;Yuan,M.Q.;Luo,G.X.; Xu,Q.;Wang,X.;Li,C.C.J.Catal.2011,32,983.
(15)Kandiel,T.A.;Dillert,R.;Feldhoff,A.;Bahnemann,D.W. J.Phys.Chem.C2010,114,4909.
(16)Zhang,J.H.;Xiao,X.;Nan,J.M.J.Hazard.Mater.2010,176, 617.doi:10.1016/j.jhazmat.2009.11.074
(17)Romero-Gomez,P.;Borras,A.;Barranco.A.;Espinos,J.P.; Gonzalez-Elipe,A.R.Chem.Phys.Chem.2011,12,191.
(18)Zhang,X.R.;Lin,Y.H.;He,D.Q.;Zhang,J.F.;Fan,Z.Y.;Xie, T.F.Chem.Phys.Lett.2011,504,71.doi:10.1016/j. cplett.2011.01.060
(19)Deak,P.;Aradi,B.;Frauenheim,T.J.Phys.Chem.C2011,115, 3443.doi:10.1021/jp1115492
(20)Mahshid,S.;Askari,M.;Sasani Ghamsari,M.;Afshar,N.; Lahuti,S.J.Alloy.Compd.2009,478,586.doi:10.1016/j. jallcom.2008.11.094
(21)Yan,M.C.;Chen,F.;Zhang,J.L.;Masakazu,A.J.Phys.Chem. B2005,109,8673.doi:10.1021/jp046087i
(22)Zhang,Y.H.;Tang,Z.R.;Fu,X.Z.;Xu,Y.J.ACS Nano2010,4,7303.doi:10.1021/nn1024219
(23)Huang,Q.W.;Tian,S.Q.;Zeng,D.W.;Wang,X.X.;Song,W. L.;Li,Y.Y.;Xiao,W.;Xie,C.S.ACS Catal.2013,3,1477. doi:10.1021/cs400080w
(24)Zhang,Y.P.;Pan,C.X.J.Mater.Sci.2011,46,2622.doi: 10.1007/s10853-010-5116-x
(25)Marcano,D.C.;Kosynkin,D.V.;Berlin,J.M.;Sinitskii,A.; Sun,Z.Z.;Slesarev,A.;Alemany,L.B.;Lu.W.;Tour,J.M. ACS Nano2010,4,4806.doi:10.1021/nn1006368
(26)Cote,L.J.;Kim,F.;Huang,J.X.J.Am.Chem.Soc.2009,131, 1043.doi:10.1021/ja806262m
(27)Williams,G.;Seger,B.;Kamat,P.V.ACS Nano2008,2,1487. doi:10.1021/nn800251f
(28)Sun,Z.Q.;Kim,J.H.;Zhao,Y.;Bijarbooneh,F.;Malgras,V.; Lee,Y.;Kang,Y.M.;Dou,S.X.J.Am.Chem.Soc.2011,133, 19314.doi:10.1021/ja208468d
(29)Ma,W.S.;Zhou,J.W.;Cheng,S.X.Journal of Chemical Engineering of Chinese Universities2010,24,719.
(30)Tong,X.C.;Wang,J.;Zhang,J.Guangzhou Chemical Industry2012,40,4.[童小翠,王 静,张 瑾.广州化工,2012,40,4.]
(31)Perera,S.D.;Mariano,R.G.;Vu,K.;Nour,N.;Seitz,O.; Chabal,Y.;Balkus,K.J.ACS Catal.2012,2,949.doi:10.1021/ cs200621c
(32)Tao,H.C.;Fan,L.Z.;Yan,X.Q.;Qu,X.H.Electrochim.Acta2012,69,328.doi:10.1016/j.electacta.2012.03.022
(33)Li,N.;Liu,G.;Zhen,C.;Li,F.;Zhang,L.L.;Cheng,H.M.Adv. Funct.Mater.2011,21,1717.doi:10.1002/adfm.201002295
(34)Zhang,Z.Y.;Xiao,F.;Guo,Y.L.;Wang,S.;Liu,Y.Q.ACS Appl.Mater.2013,5,2227.doi:10.1021/am303299r
(35)Wang,J.Q.;Xin,B.F.;Yu,H.T.;Xie,Y.T.;Zhao,B.;Fu,H.G. Chem.J.Chin.Univ.2003,24,1237.[王建强,辛柏福,于海涛,谢玉涛,赵 冰,付宏刚.高等学校化学学报,2003,24, 1237.]
(36)Long,M.C.;Qin,Y.L.;Chen,C.;Guo,X.Y.;Tan,B.H.;Cai, W.M.J.Phys.Chem.C2013,117,16734.doi:10.1021/ jp4058109
(37)Pu,X.P.;Zhang,D.F.;Gao,Y.Y.;Shao,X.;Ding,G.Q.;Li,S. S.;Zhao,S.P.J.Alloy.Compd.2013,551,382.doi:10.1016/j. jallcom.2012.11.028
(38)Etacheri,V.;Yourey,J.E.;Bartlett,B.M.ACS Nano2014,8, 1491.doi:10.1021/nn405534r
(39)Wang,Y.J.;Li,H.X.;Ji,L.;Liu,X.H.;Wu,Y.X.;Lv,Y.H.; Fu,Y.Y.;Zhou,H.D.;Chen,J.M.J.Phys.D:Appl.Phys.2012,45,295301.doi:10.1088/0022-3727/45/29/295301
(40)Scanlon,D.O.;Dunnill,C.W.;Buckeridge,J.Nature Materials2013,12,798.doi:10.1038/nmat3697
(41)Zhang,Y.L.;Gan,H.H.;Zhang,G.K.Chem.Eng.J.2011,172,936.doi:10.1016/j.cej.2011.07.005
Preparation and Photocatalytic Activity of Mixed Phase TiO2-Graphene Composites
YU Jian-Hua1FAN Min-Guang1,2,*LI Bin1DONG Li-Hui1ZHANG Fei-Yue1
(1School of Chemistry and Chemical Engineering,Guangxi University,Nanning 530004,P.R.China;2Guangxi Key Laboratory Petrochemical Rescource Processing and Process Intensification Technology,Nanning 530004,P.R.China)
Aseries of composites consisting of anatase-rutile TiO2and graphene(TrG)were synthesized by a hydrothermal route.The influence of the amount of graphene oxide on the photocatalytic activity during the degradation of methyl blue was studied.The photocatalysts were characterized by X-ray diffraction(XRD), Fourier transform infrared(FTIR)spectroscopy,high-resolution transmission electron microscopy(HRTEM), Raman spectroscopy,ultraviolet-visible diffuse reflectance spectroscopy(UV-Vis DRS),X-ray photoelectron spectroscopy(XPS),and Brunauer-Emmett-Teller(BET)specific surface area measurements.The results show that as-prepared TiO2formed in the anatase and rutile phase with a bar structure and it dispersed uniformly over the surface of the graphene sheets.The composites possess higher catalytic activity because of the strong absorption capacity of graphene,the establishment of heterojunctions between rutile and anatase TiO2,the remarkable electrical transport between TiO2and graphene and the high specific surface areas.The photodegradation performance of methyl blue by the TrG composites under UV light was studied.Our results indicate that the photocatalytic activities of titanium dioxide-graphene composites were higher than those of pure TiO2.We also found that the TrG composites prepared with a loading of 0.8%(mass fraction,w)graphene oxide had the best photocatalytic activity.
Mixed phase TiO2;Heterojunction;Graphene;Photoelectron transfer;Hydrothermal route;Photocatalysis
O643;O645
10.3866/PKU.WHXB201412291www.whxb.pku.edu.cn
Received:October 27,2014;Revised:December 26,2014;Published on Web:December 29,2014.
∗Corresponding author.Email:fanmg@gxu.edu.cn;Tel:+86-771-3233718.
The project was supported by the National Key Basic Research Program of China(973)(2012CB21500203)and Dean Project of Guangxi Key
Laboratory of Petrochemical Resource Processing and Process Intensification Technology,China(2013K009,2013Z001).
国家重点基础研究发展规划项目(973)(2012CB21500203)及广西石化资源加工及过程强化技术重点实验室院长项目(2013K009,2013Z001)资助
