Mechanistic basis and clinical relevance of the role of transforming growth factor-β in cancer
2015-12-29RunLongLinLuJunZhao
Run-Long Lin, Lu-Jun Zhao
Department of Radiation Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin 300060, China
Mechanistic basis and clinical relevance of the role of transforming growth factor-β in cancer
Run-Long Lin, Lu-Jun Zhao
Department of Radiation Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin 300060, China
Transforming growth factor-β (TGF-β) is a key factor in cancer development and progression. TGF-β can suppress tumorigenesis by inhibiting cell cycle progression and stimulating apoptosis in early stages of cancer progression. However, TGF-β can modulate cancer-related processes, such as cell invasion, distant metastasis, and microenvironment modification that may be used by cancer cells to their advantage in late stages. Corresponding mechanisms include angiogenesis promotion, anti-tumor immunity suppression, and epithelial-to-mesenchymal transition (EMT) induction. The correlation between TGF-β expression and cancer prognosis has also been extensively investigated. Results suggest that TGF-β pathway can be targeted to treat cancer; as such, the feasibility of this treatment is investigated in clinical trials.
Transforming growth factor-β (TGF-β); neoplasms; prognosis; therapeutics
Introduction
According to GLOBOCAN estimates, approximately 14.1 million new cancer cases and 8.2 million deaths occurred in 2012 worldwide1. Transforming growth factor-β (TGF-β) plays an important role in epithelial and neural tissue development, immune system regulation, and wound repair2. TGF-β also participates in cancer development and progression.
TGF-β can suppress tumorigenesis by inhibiting cell cycle progression and inducing apoptosis. Specific mechanisms that mediate the cytostatic effect of TGF-β have been mainly investigated in epithelial cell types. TGF-β inhibits the progression of G1phase via two events shared by skin, lung, and mammary epithelial cells: (I) mobilization of cyclin-dependent kinase (CDK) inhibitors; and (II) suppression of protooncogene c-MYC and ID proteins. Furthermore, the ability of TGF-β to induce apoptosis varies greatly depending on cell type. However, our knowledge of this area remains limited.
Malignant cells can evade suppressive effects of TGF-β and use TGF-β regulatory functions to their advantage. Thus, these cells acquire invasive and metastatic capabilities via angiopoiesis, immune surveillance suppression, epithelial-to-mesenchymal transition (EMT) promotion, and extracellular matrix (ECM) degradation.
TGF-β expression is correlated with poor tissue differentiation, advanced TNM stages, short overall survival, and locally advanced or distant metastasis in various malignancies. However, controversial findings should be considered. TGF-β inhibition can elicit anti-tumor effect, especially when this mechanism is combined with radiotherapy; TGF-β levels increase. Preliminary clinical trials have been performed to evaluate the feasibility of TGF-β inhibitors as anti-tumor modalities. This review summarizes recent advances in this area and discusses potentially relevant mechanisms. Figure 1 shows a schematic of the role of TGF-β in malignancy development.
TGF-β pathway
The TGF-β superfamily includes TGF-β ligands (TGF-β1, -2, and -3), bone morphogenic proteins (BMPs), and activins/ inhibins. TGF-β factors are synthesized as dimeric prohormones and secreted into ECM. Canonical TGF-β/Smad signaling cascade is initiated when a TGF-β/BMP ligand binds to typeII serine/threonine kinase receptors; type II serine/threonine kinase receptors in turn recruit and phosphorylate type I receptors3. Phosphorylated type I receptors then propagate a signal by phosphorylating RSmad, which forms a complex with Smad4. TGF-β ligands transmit the signal through Smad2 and Smad3, whereas BMPs employ Smad1, Smad5, and Smad84,5. Activated Smad complexes are transported into the nucleus, where these complexes, together with co-activators or co-repressors, regulate target gene transcription2. In non-canonical TGF-β signaling, ligand-bound TGF-β receptors activate other signaling pathways, such as p38 and Jun N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK) pathways, phosphoinositide 3-kinase-Akt-mTOR pathway, small GTPase RhoA and Rac/Cdc42 pathways, and Ras-Erk pathway; the activation of these pathways likely enhances tumor growth after canonical TGF-β-Smad signaling is disrupted6,7.
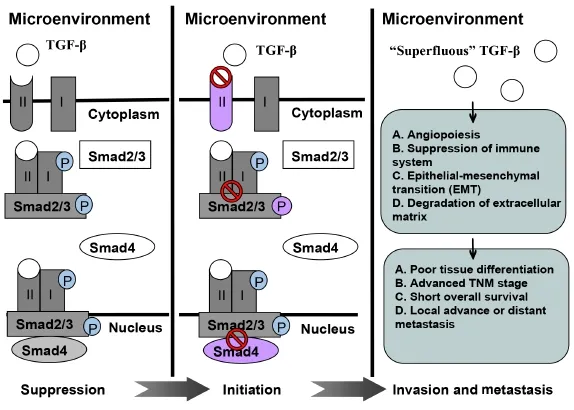
Figure 1 A schematic illustrating role of TGF-β in tumor suppression, initiation, invasion, and metastasis.
Tumor-suppressive effect of TGF-β
Cytostatic mechanisms
Cell division cycle proceeds by the action of CDKs. TGF-β action inhibits CDKs, which stimulate the G1phase of the cell cycle. In epithelial cells, TGF-β induces the expression of p15Ink4b, which inhibits cyclinD-CDK4/6 complexes; TGF-β also causes the expression of p21Cip1, which inhibits cyclinE/ A-CDK2 complexes8,9. In addition, TGF-β/Smad signaling stimulates p15Ink4bexpression, thereby promoting the release of inactive p27Kip1from cyclin D-CDK4 complexes to allow the p27Kip1-dependent inhibition of cyclin E/A-CDK2 complexes10. TGF-β also induces p16ink4aand p19ARFexpression; this induction contributes to growth arrest and senescence response11. Furthermore, TGF-β reduces CDK activity; as a result, cyclin/CDK complex-induced phosphorylation of the tumor suppressor Rb protein is prevented. Thus, hypophosphorylated Rb can bind to transcription factors in the E2F family and impede the ability of these factors to promote G1/S phase cell cycle progression12.
Another important event in the TGF-β anti-proliferative program is the inhibition of c-MYC expression. c-MYC can bind to an initiator element of p15Ink4bpromoter; as a consequence, p15Ink4bexpression is inhibited13. mRNA and protein levels of c-MYC rapidly decrease in response to TGF-β because the corresponding mRNA and protein are shortlived; thus, c-MYC-mediated p15Ink4brepression is relieved14. Comparing the genome-wide profile of TGF-β gene responses in non-tumorigenic and tumor-derived human mammary cells,Chen et al.15noted that the loss of TGF-β growth inhibitory effect in tumor-derived human mammary cells occurs with a selective loss of the corresponding c-MYC down-regulation.
TGF-β can inhibit ID1 and ID2 expression, in addition to c-MYC down-regulation. ID proteins function as negative regulators implicated in cell differentiation. In ID1 down-regulation, TGF-β stimulation rapidly induces the expression of activating transcription factor-3 (ATF3) in HaCaT skin keratinocytes, HPL1 lung epithelial cells, and MCF-10A mammary epithelial cells via a Smad3-containing transcriptional complex. ATF3 then coordinates with Smad3 and Smad4 to target ID1 and repress transcription16. A link between c-MYC and ID2 expression has been established by showing that the binding of c-MYC to E-box motifs in ID2 promoter supports ID2 expression. Thus, TGF-β-induced c-MYC down-regulation may also inhibit ID2 in specific cell types17.
Pro-apoptotic mechanisms
p38 MAPK activator DAXX is considered as a mediator of TGF-β apoptotic signals because this molecule physically interacts with TGF-β receptor and requires TGF-β-induced apoptosis. The carboxyl terminal of DAXX functions as a dominant negative inhibitor of TGF-β-induced apoptosis in B cell lymphomas; antisense oligonucleotides against DAXX also inhibit TGF-β-induced apoptosis in mouse hepatocytes18. The expression of the death-associated protein kinase (DAP-kinase) is increased during TGF-β-induced apoptosis in hepatoma cells. Overexpression of DAP-kinase triggers apoptosis in the absence of TGF-β, whereas inhibition of DAP-kinase activity protects cells from TGF-β-induced apoptosis. And this process relies on Smad function and sensitizes cells to undergo TGF-β/Smad apoptosis19. Apoptosis-related protein in the TGF-β signaling (ARTS) pathway is an unusual mitochondrial septin-like protein that functions as a tumor suppressor. In an embryonic kidney cell line, ARTS potentiates apoptosis in cells normally resistant to TGF-β-induced cell death; by contrast, reduced ARTS expression impairs TGF-β-induced apoptosis20.
Tumorigenic effects of TGF-β on premalignant cells
TGF-β receptor mutation
The loss of canonical TGF-β/Smad signaling pathway initiates tumors; this phenomenon occurs because some tumor cells may escape the inhibitory effects of the canonical pathway through mutations that can provide a growth advantage over benign tumors21. TGF-β receptors (TGF-β R-I and TGF-β R-II) are required for the proper transduction of TGF-β signaling pathway. Tumor cells may reduce the expression or function of either TGF-β R-I or TGF-β R-II to escape growth-inhibiting effects of TGF-β canonical pathway. This TGF-β receptor-induced loss of sensitivity to TGF-β canonical pathway can cause compensatory TGF-β overexpression; as a result, aggressive effects are elicited22. The loss of TGF-β R-II on myeloma cell surface can also be attributed to gene silencing through hypermethylation; this loss can promote myeloma cell resistance to anti-cancer effects23. TGF-β R-II mutations have also been described in association with microsatellite instable (MSI+) carcinomas. In MSI+cells, DNA base mismatch repair is compromised; thus, errors in a 10 bp poly-adenine repeat segment from the coding region [Poly(A) 10 tract] of TGF-β R-II are frequently observed. These mutations often lead to frame-shift missense mutations or early termination that prevents proper translation of a functional TGF-β R-II protein24.
Disruption of downstream canonical signaling pathway
In addition to the crucial role of the canonical signaling pathway in RSmad (Smad2/Smad3) phosphorylation, the role of the activation of the non-canonical pathway by TGF-β receptors likely create phospho-specific Smad2/Smad3. Non-canonical phosphorylation of the RSmads (Smad2/Smad3) via non-canonical pathways, such as MAPK pathway, can inhibit canonical phosphorylation induced by TGF-β receptor complex and disrupt anti-cancer effects of the canonical pathway25. Smad2/Smad3 phosphorylation via the MAPK pathway is possibly implicated in the transformation of oncogenic pSmad2/3L into tumor suppressor pSmad2/3C26. Frequent Smad4 gene mutation is associated with poorer prognosis of colorectal adenocarcinoma27, pancreatic cancer28, and papillary thyroid carcinoma progression29. With canonical TGF-β/ Smad signaling, Smad7 uses a negative feedback mechanism to disrupt Smad2/3 phosphorylation through the competitive inhibition of TGF-β receptor complex; Smad7 also disrupts Smad2/3/4 complex formation and nuclear translocation by recruiting ubiquitin ligases that induce proteasomal degradation30.
Promotive effect of TGF-β on tumor invasion and metastasis
Genetic mutations and downstream alterations in TGF-β/Smad signaling components often inactivate growth inhibitory activities of TGF-β; thus, TGF-β can contribute to cancer progression31,32.Related mechanisms include angiogenesis promotion, anti-tumor immunity suppression, and EMT induction.
Angiopoiesis
New blood vessel formation in tumor tissues (tumor angiogenesis) is necessary to promote tumor cell growth and metastasis. The role of TGF-β in the angiogenesis of cancer cells is highly complex; this function involves the interaction of vascular endothelial growth factor (VEGF) and endothelin.
Soufla et al.33demonstrated that VEGF and TGF-β are involved in endometrial carcinogenesis through transcription activation and down-regulation, respectively; Soufla et al.33also suggested the potential use of VEGF and TGF-β as molecular indicators of disease progression. Tumor-derived VEGF-A triggers enhanced tumor cell proliferation possibly through the paracrine inhibition of TGF-β signaling within a tumor34.
Endoglin is a transmembrane glycoprotein involved in several processes and disorders associated with human circulatory system35. Endoglin acts as a type I transmembrane protein that functions as a co-receptor of TGF-β to modulate signaling by binding to TGF-β receptors and impairing downstream signaling activity36. TGF-β expression is also associated with tumor progression, as in the case of endoglin. TGF-β predicts poorer survival in endoglin-enriched tumors; this phenomenon indicates that TGF-β enhances disease progression in later stages of angiogenesis36,37. Previous studies with experimental models showed that highly expressed endoglin antagonizes the inhibitory effects of TGF-β and contributes to proliferation, migration, and capillary formation of endothelial cells, which are the three key events in angiogenesis. TGF-β also plays an important role in angiogenesis by promoting endothelial cell proliferation and migration at low concentrations and by causing vessel maturation at high concentrations38.
Immune system suppression
TGF-β-induced immunosuppression occurs when tumor cells escape attack by the immune system through various mechanisms, including interaction with CD4+CD25+Foxp3+regulatory T cells (Treg cells), tumor-associated macrophages, tumor-associated neutrophils (TAN), and T helper 17 (TH17). CD8+CTLs (cytotoxic T lymphocytes) are necessary to control tumor progression. Treg cells are a specialized T cell subpopulation, which suppresses immune system activation39. In various tumor types, natural and adaptive Treg cell concentrations are increased in tumor sites and contribute to tumor-induced immunosuppression by suppressing CTL proliferation and function40. In vitro experiments have revealed that the TGF-β secreted by a renal carcinoma cell line and a prostate cancer cell line can induce the transformation of CD4+CD25−T cells in mouse spleen into Treg cells41. The extent of Treg cell infiltration is higher in high-TGF-β-expression group than in low-TGF-β-expression group; this result indicates that TGF-β expression in tumor tissues can increase Treg cell infiltration in a local tumor; thus, tumor cells evade immune responses42. Lu et al.43showed that gastric cancer-induced infiltration of Treg cells predicts the poor prognosis of patients with gastric adenocarcinoma; some of these Treg cells are converted by tumor-produced TGF-β.
Macrophages are also important immune cells in peripheral blood. Macrophages are necessary to prevent metastasis of cancer cells. Classically activated M1 macrophages can phagocytose tumor cells. Therefore, these macrophages are involved in immune function against infection and tumor cell invasion. M1 macrophages also play a critical role in cellular immunity against cancer. Alternatively activated M2 macrophages perform a distinct function from M1 macrophages. M2 macrophages can facilitate tumor cell proliferation, angiogenesis, and tissue remodeling. These effects are mainly achieved through TGF-β secretion44. Other mechanisms, such as TAN and Th17 pathways, associated with the anti-tumor immune effect of the TGF-β pathway have been reported45,46.
TGF-β pathway and EMT
Tumor invasion and metastasis are initiated by decreased cell-to-cell adhesion, increased motility, and invasive properties that allow carcinoma cells to detach from primary tumor and invade surrounding tissues through collective or individual cell migration. TGF-β functions as a potent stimulator of cancer progression by inducing EMT; in this process, epithelial cells acquire a mesenchymal phenotype and exhibit enhanced motility and invasion47. Cells undergoing EMT down-regulate the expression of E-cadherin epithelial marker and increase the expression of N-cadherin, a mesenchymal marker48. Cells can respond to TGF-β through growth inhibition and EMT. Pino et al.49reported that TGF-β induces EMT in colon cancer cell lines with a wild-type TGF-β R-II. However, no changes in cell morphological characteristics, differentiation marker expression, motility, and invasion have been observed in cells with homozygous TGF-β R-II mutations. This finding reveals that growth inhibition and EMT may share canonical TGF-β/Smad pathway as a common signaling pathway.
TGF-β levels are positively associated with tumor resistance to radiotherapy or chemotherapy; this positive associationmay attribute to treatment-initiated EMT of tumor cells. Zhao et al.50observed that increased TGF-β levels during radiation therapy are strongly correlated with poor prognosis among patients with non-small cell lung cancer. In addition, poor prognosis of glioblastoma (GBM) routinely treated with ionizing radiation has been attributed to the relative radioresistance of glioma-initiating cells (GICs). GICs are sensitive to treatment, but response is mediated by undefined factors in a microenvironment. GIC resistance to radiation, which is mediated by a tumor microenvironment, can be abolished by inhibiting TGF-β/Smad signaling pathway51. Tas et al.52showed that patients with chemotherapy-unresponsive epithelial ovarian cancer present higher serum TGF-β levels than responsive patients (P=0.02). These studies support the current hypothesis that a subtle relationship exists among TGF-β, EMT phenotype, and therapy resistance. TGF-β may be a new molecular subtype that can cause resistance to therapy.
ECM degradation
Tumor ECM degradation is a critical step in tumor invasion and metastasis. TGF-β plays an important role in ECM degradation. ECM is mainly degraded by proteolytic enzymes, such as matrix metalloproteinases (MMPs). MMPs are a group of proteolytic enzymes that can degrade tumor ECM and are up-regulated in several tumor tissues. Yang et al.53found that TGF-β expression levels exhibit a significantly positive correlation with MMP2 expression in renal clear cell carcinoma. A similar finding has been observed in melanoma54. TGF-β is also significantly correlated with MMP9 expression; MMP9 can facilitate tumor cell infiltration in lymphatic or blood systems by degrading basement membrane components55. This effect is another mechanism in the tumor-promoting effect of TGF-β.
Clinical significance of TGF-β
TGF-β plays an important role in cancer development and progression. TGF-β expression may predict the prognosis of patients with malignancy. Studies have investigated the prognostic role of TGF-β protein/mRNA expression in cancer. Some of the related studies are summarized in Table 1. These studies have indicated that a high TGF-β expression may predict poor prognosis, including poor tissue differentiation, advanced TNM stage, short overall survival, and locally advanced or distant metastasis. However, other studies have revealed controversial findings. Discrepancies may be attributed to several factors, such as differentiation status of analyzed tumors and disease stage. TGF-β pathway regulation occurring post-transcription may differ among samples. Discrepancies may also be caused by different methods used to detect TGF-β expression.
Opportunities and challenges in therapeutically targeting TGF-β
Targeting TGF-β may elicit a significant anti-tumor effect because TGF-β is implicated in cancer development and progression. TGF-β inhibitors have been preclinically evaluated; some of these inhibitors are in early stage clinical studies. Preliminary studies are summarized in Table 2. TGF-β inhibition among cancer patients has also been evaluated through clinical trials by using an antibody (GC1008) or an oligonucleotide (AP12009). These trials suggest that TGF-β inhibition exhibits promising efficacy and safety. However, large clinical trials should be conducted to clarify the feasibility and safety of treatments. Other challenges related to this approach include targeting a tumor microenvironment by using TGF-β inhibitors without affecting TGF-β function in hosts to maintain systemic homeostatic processes.
Conclusion
TGF-β functions as a trigger of a canonical suppression pathway, inducer of tumor angiogenesis, tumor-derived immunosuppressor, promoter of carcinoma invasion and metastasis, and influencing factor of chemotherapy and radiotherapy; thus, the dual role of TGF-β has been extensively investigated. TGF-β levels determined during diagnosis and treatment may also be a reliable marker; this method may be used to predict the prognosis of patients with cancer. High TGF-β1 levels are also associated with poor tissue differentiation, advanced TNM stage, and decreased overall survival. TGF-β inhibitors, especially those used in late cancer stage, may elicit anti-tumor effects via novel mechanisms, such as tumor angiogenesis suppression, immune system promotion, and EMT reversal. However, the optimal timing of TGF-β blockade and the ideal combination of this approach with other therapies, such as radiotherapy and chemotherapy, remain unknown. These questions are currently addressed in ongoing preclinical studies and will be resolved in future clinical trials.
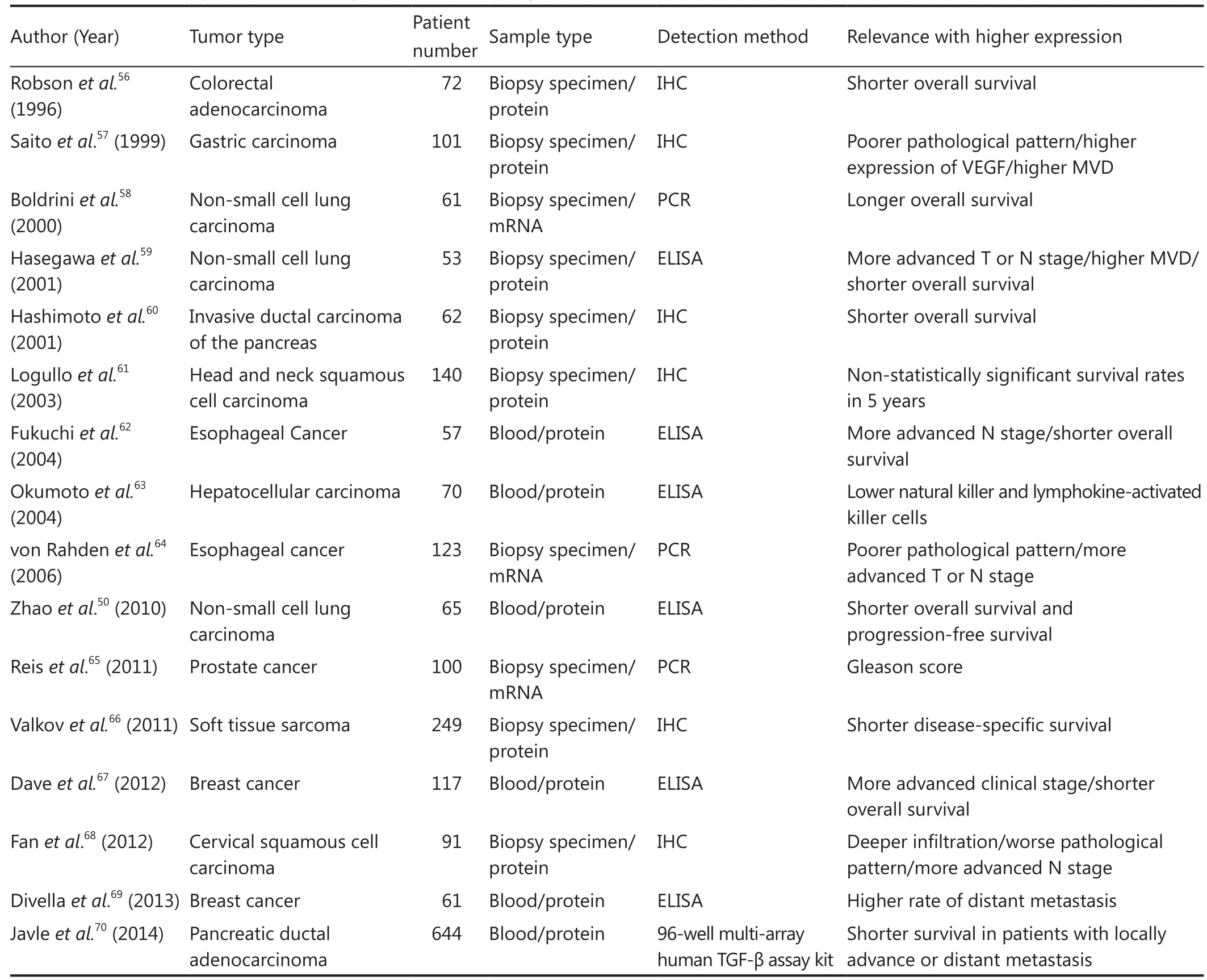
Table 1 Relevance analysis between TGF-β expression and prognosis
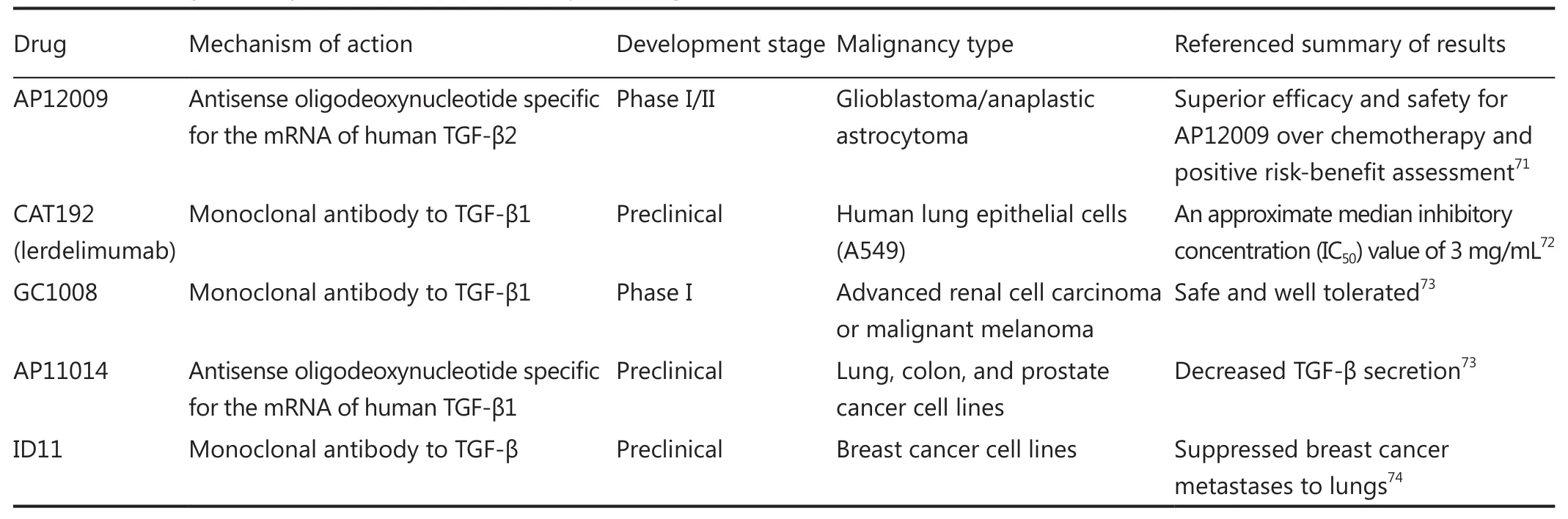
Table 2 Summary of TGF-β inhibitors as novel therapeutic targets
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81372429).
Conflict of interest statement
No potential conflicts of interest are disclosed.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108.
2. Massagué J. TGFbeta in Cancer. Cell 2008;134:215-230.
3. Attisano L, Cárcamo J, Ventura F, Weis FM, Massagué J, Wrana JL. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 1993;75:671-680.
4. Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol 2000;1:169-178.
5. Massagué J. TGF-beta signal transduction. Annu Rev Biochem 1998;67:753-791.
6. Mu Y, Gudey SK, Landstrom M. Non-Smad signaling pathways. Cell Tissue Res 2012;347:11-20.
7. Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res 2009;19:128-139.
8. Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 1994;371:257-261.
9. Li CY, Suardet L, Little JB. Potential role of WAF1/Cip1/p21 as a mediator of TGF-beta cytoinhibitory effect. J Biol Chem 1995;270:4971-4974.
10. Reynisdóttir I, Massagué J. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev 1997;11:492-503.
11. Vijayachandra K, Higgins W, Lee J, Glick A. Induction of p16ink4a and p19ARF by TGFbeta1 contributes to growth arrest and senescence response in mouse keratinocytes. Mol Carcinog 2009;48:181-186.
12. Bierie B, Moses HL. Gain or loss of TGFbeta signaling in mammary carcinoma cells can promote metastasis. Cell Cycle 2009;8:3319-3327.
13. Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, et al. Repression of p15INK4b expression by Myc through association with Miz-1. Nat Cell Biol 2001;3:392-399.
14. Warner BJ, Blain SW, Seoane J, Massagué J. Myc downregulation by transforming growth factor beta required for activation of the p15(Ink4b) G(1) arrest pathway. Mol Cell Biol 1999;19:5913-5922.
15. Chen CR, Kang Y, Massague J. Defective repression of c-myc in breast cancer cells: A loss at the core of the transforming growth factor beta growth arrest program. Proc Natl Acad Sci U S A 2001;98:992-999.
16. Kang Y, Chen CR, Massague J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell 2003;11:915-926.
17. Lasorella A, Noseda M, Beyna M, Yokota Y, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 2000;407:592-598.
18. Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat Cell Biol 2001;3:708-714.
19. Jang CW, Chen CH, Chen CC, Chen JY, Su YH, Chen RH. TGF-beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol 2002;4:51-58.
20. Xu F, Sun W, Li P, Chen J, Zhu D, Sun X, et al. Specificity protein 1 transcription factor regulates human ARTS promoter activity through multiple binding sites. PLoS One 2015;10:e0120072.
21. Barrack ER. TGF beta in prostate cancer: a growth inhibitor that can enhance tumorigenicity. Prostate 1997;31:61-70.
22. Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Aakre M, et al. Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res 2008;68:1809-1819.
23. de Carvalho F, Colleoni GW, Almeida MS, Carvalho AL, Vettore AL. TGFbetaR2 aberrant methylation is a potential prognostic marker and therapeutic target in multiple myeloma. Int J Cancer 2009;125:1985-1991.
24. Levy L, Hill CS. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 2006;17:41-58.
25. Tarasewicz E, Jeruss JS. Phospho-specific Smad3 signaling: impact on breast oncogenesis. Cell Cycle 2012;11:2443-2451.
26. Boye A, Kan H, Wu C, Jiang Y, Yang X, He S, et al. MAPK inhibitors differently modulate TGF-beta/Smad signaling in HepG2 cells. Tumour Biol 2015. [Epub ahead of print].
27. Handra-Luca A, Olschwang S, Flejou JF. SMAD4 protein expression and cell proliferation in colorectal adenocarcinomas. Virchows Arch 2011;459:511-519.
28. Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang X, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res 2009;15:4674-4679.
29. D’Inzeo S, Nicolussi A, Donini CF, Zani M, Mancini P, Nardi F, et al. A novel human Smad4 mutation is involved in papillary thyroid carcinoma progression. Endocr Relat Cancer 2012;19:39-55.
30. Luo L, Li N, Lv N, Huang D. SMAD7: a timer of tumor progression targeting TGF-beta signaling. Tumour Biol 2014;35:8379-8385.
31. White RA, Malkoski SP, Wang XJ. TGFbeta signaling in head andneck squamous cell carcinoma. Oncogene 2010;29:5437-5446.
32. Chatterjee R, Mitra A. An overview of effective therapies and recent advances in biomarkers for chronic liver diseases and associated liver cancer. Int Immunopharmacol 2015;24:335-345.
33. Soufla G, Sifakis S, Porichis F, Spandidos DA. Prognostic value of tgfb1 protein in endometrioid adenocarcinoma. Eur J Clin Invest 2013;43:79-90.
34. Weidenaar AC, ter Elst A, Kampen KR, Meeuwsen-de Boer TG, de Jonge HJ, Scherpen FJ, et al. Stromal interaction essential for vascular endothelial growth factor A-induced tumour growth via transforming growth factor-beta signalling. Br J Cancer 2011;105:1856-1863.
35. Zakrzewski PK, Cygankiewicz AI, Mokrosiński J, Nowacka-Zawisza M, Semczuk A, Rechberger T, et al. Expression of endoglin in primary endometrial cancer. Oncology 2011;81:243-250.
36. Lin H, Huang CC, Ou YC, Huang EY, Changchien CC, Tseng CW, et al. High immunohistochemical expression of TGF-beta1 predicts a poor prognosis in cervical cancer patients who harbor enriched endoglin microvessel density. Int J Gynecol Pathol 2012;31:482-489.
37. Pardali E, van der Schaft DW, Wiercinska E, Gorter A, Hogendoorn PC, Griffioen AW, et al. Critical role of endoglin in tumor cell plasticity of Ewing sarcoma and melanoma. Oncogene 2011;30:334-345.
38. Bellone G, Gramigni C, Vizio B, Mauri FA, Prati A, Solerio D, et al. Abnormal expression of Endoglin and its receptor complex (TGF-beta1 and TGF-beta receptor II) as early angiogenic switch indicator in premalignant lesions of the colon mucosa. Int J Oncol 2010;37:1153-1165.
39. Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 2000;101:455-458.
40. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006;6:295-307.
41. Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol 2007;178:2883-2892.
42. Lin GH, Wang J, Li SH, Wang J, Xu L, Li SP. Relationship and clinical significance of TGF-beta1 expression with Treg cell infiltration in hepatocellular carcinoma. Chin J Cancer 2010;29:403-407.
43. Lu X, Liu J, Li H, Li W, Wang X, Ma J, et al. Conversion of intratumoral regulatory T cells by human gastric cancer cells is dependent on transforming growth factor-beta1. J Surg Oncol 2011;104:571-577.
44. Luo H, Hao Y, Tang B, Zeng D, Shi Y, Yu P. Mouse forestomach carcinoma cells immunosuppress macrophages through transforming growth factor-beta1. Mol Med Rep 2012;5:988-992.
45. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009;16:183-194.
46. Chalmin F, Mignot G, Bruchard M, Chevriaux A, Végran F, Hichami A, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 2012;36:362-373.
47. Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol 2013;25:76-84.
48. Cheng JC, Auersperg N, Leung PC. TGF-beta induces serous borderline ovarian tumor cell invasion by activating EMT but triggers apoptosis in low-grade serous ovarian carcinoma cells. PLoS One 2012;7:e42436.
49. Pino MS, Kikuchi H, Zeng M, Herraiz MT, Sperduti I, Berger D, et al. Epithelial to mesenchymal transition is impaired in colon cancer cells with microsatellite instability. Gastroenterology 2010;138:1406-1417.
50. Zhao L, Ji W, Zhang L, Ou G, Feng Q, Zhou Z, et al. Changes of circulating transforming growth factor-beta1 level during radiation therapy are correlated with the prognosis of locally advanced nonsmall cell lung cancer. J Thorac Oncol 2010;5:521-525.
51. Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res 2012;72:4119-4129.
52. Tas F, Karabulut S, Serilmez M, Ciftci R, Duranyildiz D. Clinical significance of serum transforming growth factor-beta 1 (TGF-beta1) levels in patients with epithelial ovarian cancer. Tumour Biol 2014;35:3611-3616.
53. Yang SD, Sun RC, Mu HJ, Xu ZQ, Zhou ZY. The expression and clinical significance of TGF-beta1 and MMP2 in human renal clear cell carcinoma. Int J Surg Pathol 2010;18:85-93.
54. Malaponte G, Zacchia A, Bevelacqua Y, Marconi A, Perrotta R, Mazzarino MC, et al. Co-regulated expression of matrix metalloproteinase-2 and transforming growth factor-beta in melanoma development and progression. Oncol Rep 2010;24:81-87.
55. Chen Y, Zhang W, Geng N, Tian K, Jack Windsor L. MMPs, TIMP-2, and TGF-beta1 in the cancerization of oral lichen planus. Head Neck 2008;30:1237-1245.
56. Robson H, Anderson E, James RD, Schofield PF. Transforming growth factor beta 1 expression in human colorectal tumours: an independent prognostic marker in a subgroup of poor prognosis patients. Br J Cancer 1996;74:753-758.
57. Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, et al. The expression of transforming growth factor-beta1 is significantly correlated with the expression of vascular endothelial growth factorand poor prognosis of patients with advanced gastric carcinoma. Cancer 1999;86:1455-1462.
58. Boldrini L, Calcinai A, Samaritani E, Pistolesi F, Mussi A, Lucchi M, et al. Tumour necrosis factor-alpha and transforming growth factor-beta are significantly associated with better prognosis in non-small cell lung carcinoma: putative relation with BCL-2-mediated neovascularization. Br J Cancer 2000;83:480-486.
59. Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 2001;91:964-971.
60. Hashimoto K, Nio Y, Sumi S, Toga T, Omori H, Itakura M, et al. Correlation between TGF-beta1 and p21 (WAF1/CIP1) expression and prognosis in resectable invasive ductal carcinoma of the pancreas. Pancreas 2001;22:341-347.
61. Logullo AF, Nonogaki S, Miguel RE, Kowalski LP, Nishimoto IN, Pasini FS, et al. Transforming growth factor beta1 (TGFbeta1) expression in head and neck squamous cell carcinoma patients as related to prognosis. J Oral Pathol Med 2003;32:139-145.
62. Fukuchi M, Miyazaki T, Fukai Y, Nakajima M, Sohda M, Masuda N, et al. Plasma level of transforming growth factor beta1 measured from the azygos vein predicts prognosis in patients with esophageal cancer. Clin Cancer Res 2004;10:2738-2741.
63. Okumoto K, Hattori E, Tamura K, Kiso S, Watanabe H, Saito K, et al. Possible contribution of circulating transforming growth factorbeta1 to immunity and prognosis in unresectable hepatocellular carcinoma. Liver Int 2004;24:21-28.
64. von Rahden BH, Stein HJ, Feith M, Pühringer F, Theisen J, Siewert JR, et al. Overexpression of TGF-beta1 in esophageal (Barrett’s) adenocarcinoma is associated with advanced stage of disease and poor prognosis. Mol Carcinog 2006;45:786-794.
65. Reis ST, Pontes-Júnior J, Antunes AA, Sousa-Canavez JM, Abe DK, Cruz JA, et al. Tgf-beta1 expression as a biomarker of poor prognosis in prostate cancer. Clinics (Sao Paulo) 2011;66:1143-1147.
66. Valkov A, Sorbye SW, Kilvaer TK, Donnem T, Smeland E, Bremnes RM, et al. The prognostic impact of TGF-beta1, fascin, NF-kappaB and PKC-zeta expression in soft tissue sarcomas. PLoS One 2011;6:e17507.
67. Dave H, Shah M, Trivedi S, Shukla S. Prognostic utility of circulating transforming growth factor beta 1 in breast cancer patients. Int J Biol Markers 2012;27:53-59.
68. Fan DM, Wang XJ, He T, Wang Y, Zhou D, Kong GQ, et al. High expression of TGF-beta1 in the vaginal incisional margin predicts poor prognosis in patients with stage Ib-IIa cervical squamous cell carcinoma. Mol Biol Rep 2012;39:3925-3931.
69. Divella R, Daniele A, Savino E, Palma F, Bellizzi A, Giotta F, et al. Circulating levels of transforming growth factor-betaeta (TGF-beta) and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of distant seeding of circulating tumor cells in patients with metastatic breast cancer. Anticancer Res 2013;33:1491-1497.
70. Javle M, Li Y, Tan D, Dong X, Chang P, Kar S, et al. Biomarkers of TGF-beta signaling pathway and prognosis of pancreatic cancer. PLoS One 2014;9:e85942.
71. Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 2011;13:132-142.
72. Benigni A, Zoja C, Corna D, Zatelli C, Conti S, Campana M, et al. Add-on anti-TGF-beta antibody to ACE inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol 2003;14:1816-1824.
73. Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 2010;10:554-567.
74. Nam JS, Suchar AM, Kang MJ, Stuelten CH, Tang B, Michalowska AM, et al. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor beta in a mouse model of breast cancer. Cancer Res 2006;66:6327-6335.
Cite this article as: Lin RL, Zhao LJ. Mechanistic basis and clinical relevance of the role of transforming growth factor-β in cancer. Cancer Biol Med 2015;12:385-393. doi: 10.7497/j.issn.2095-3941.2015.0015
Lu-Jun Zhao
E-mail: zhaolujun@tjmuch.com
Received March 8, 2015; accepted May 12, 2015.
Available at www.cancerbiomed.org
Copyright © 2015 by Cancer Biology & Medicine
杂志排行
Cancer Biology & Medicine的其它文章
- Pathological clavicular fracture as first presentation of renal cell carcinoma: a case report and literature review
- Synergistic suppression of the PI3K inhibitor CAL-101 with bortezomib on mantle cell lymphoma growth
- Prognostic value of body mass index before treatment for laryngeal squamous cell carcinoma
- Dermatofibrosarcoma protuberans: from translocation to targeted therapy
- Upgrading the definition of early gastric cancer: better staging means more appropriate treatment
- Advances in drug delivery system for platinum agents based combination therapy
