Synergistic suppression of the PI3K inhibitor CAL-101 with bortezomib on mantle cell lymphoma growth
2015-12-29FuLianQuBingXiaSuXiaLiChenTianHongLiangYangQianLiYaFeiWangYongYuYiZhuoZhang
Fu-Lian Qu*, Bing Xia*, Su-Xia Li*, Chen Tian, Hong-Liang Yang, Qian Li, Ya-Fei Wang, Yong Yu, Yi-Zhuo Zhang
1Department of Hematology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin 300060, China;2Department of Medical Oncology, Kaifeng Central Hospital, Kaifeng 475000, China;3Department of Geriatric Hematology, Chinese PLA General Hospital, Beijing 100853, China
Synergistic suppression of the PI3K inhibitor CAL-101 with bortezomib on mantle cell lymphoma growth
Fu-Lian Qu1,2*, Bing Xia1*, Su-Xia Li3*, Chen Tian1, Hong-Liang Yang1, Qian Li1, Ya-Fei Wang1, Yong Yu1, Yi-Zhuo Zhang1
1Department of Hematology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin 300060, China;2Department of Medical Oncology, Kaifeng Central Hospital, Kaifeng 475000, China;3Department of Geriatric Hematology, Chinese PLA General Hospital, Beijing 100853, China
Objective: To investigate the effects of CAL-101, particularly when combined with bortezomib (BTZ) on mantle cell lymphoma (MCL) cells, and to explore its relative mechanisms.
Methods: MTT assay was applied to detect the inhibitory effects of different concentrations of CAL-101. MCL cells were divided into four groups: control group, CAL-101 group, BTZ group, and CAL-101/BTZ group. Te expression of PI3K-p110σ, AKT, ERK, p-AKT and p-ERK were detected by Western blot. Te apoptosis rates of CAL-101 group, BTZ group, and combination group were detected by fow cytometry. Te location changes of nuclear factor kappa-B (NF-κB) of 4 groups was investigated by NF-κB Kit exploring. Western blot was applied to detect the levels of caspase-3 and the phosphorylation of AKT in diferent groups.
Results: CAL-101 dose- and time-dependently induced reduction in MCL cell viability. CAL-101 combined with BTZ enhanced the reduction in cell viability and apoptosis. Western blot analysis showed that CAL-101 signifcantly blocked the PI3K/AKT and ERK signaling pathway in MCL cells. Te combination therapy contributed to the inactivation of NF-κB and AKT in MCL cell lines. However, cleaved caspase-3 was up-regulated afer combined treatment.
Conclusion: Our study showed that PI3K/p110σ is a novel therapeutic target in MCL, and the underlying mechanism could be the blocking of the PI3K/AKT and ERK signaling pathways. Tese fndings provided a basis for clinical evaluation of CAL-101 and a rationale for its application in combination therapy, particularly with BTZ.
CAL-101; bortezomib (BTZ); phosphatidylinositol-3-kinase (PI3K); mantle cell lymphoma (MCL)
Introduction
Mantle cell lymphoma (MCL), an aggressive disease that accounts for 5%-10% of all lymphomas, is incurable with conventional therapies1-3. Te prevalence of MCL has been increasing over the last few decades, particularly among elderly patients4,5. Despite various therapeutic options, MCL remains an incurable disease with a median survival time of less than 3 years6.
Phosphatidylinositol-3-kinases (PI3Ks) are enzymes that mediate signals from cell surface receptors. Class I PI3K isozymes (PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ) regulate various cellular functions by producing phosphatidylinositol-3,4, 5-triphosphate7,8. Generation of phosphatidylinositol-3,4, 5-triphosphate activates downstream serine/threonine kinases, namely, Akt and mammalian target of rapamycin (mTOR), which positively afects cell survival, proliferation, growth, and metabolism9,10. Dysregulation of the PI3K/Akt/mTOR pathway is important in the etiology of human malignancies11. Of several PI3K isoforms, PI3Kδ plays a pivotal function in B-cell signaling in response to chemokinesand cytokines. CAL-101, also named GS-1101, is a novel oral PI3Kδ-specific inhibitor that demonstrates activity in other types of B-cell cancers12-14. In this study, we characterized the effect of CAL-101 on MCL cells to provide translational support for PI3Kδ inhibition as a novel strategy for MCL treatment.
Bortezomib (BTZ), a boronic anhydride that functions as a reversible proteasome inhibitor, was the frst clinically available drug and approved for refractory multiple myeloma (MM)15. BTZ preferentially targets malignant cells16, and the mechanisms underlying the proteasome inhibitor lethality of BTZ include misfolded protein accumulation, endoplasmic reticulum stress induction, oxidative injury, nuclear factor kappa-B (NF-κB) inhibition (by IκBα accumulation), pro-apoptotic protein up-regulation, p53 and JNK stabilization, and DNA repair interference. Although several factors support the strategy of combining CAL-101 with proteasome inhibitors in non-Hodgkin’s lymphoma (NHL), including MCL, the effect of CAL-101 when combined with BTZ on MCL cells remains unknown. Therefore, we aim to clarify whether a synergistic efect exists between CAL-101 and BTZ in MCL.
Methods
Cells and reagents
The human MCL cell lines, namely, Z138, HBL-2, and Jeko-1, were provided by the Moffitt Cancer Center. These cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Hyclone), 1% (v/v) penicillin (100 U/mL), and streptomycin (100 U/mL) at 37 ℃ in an incubator with 5% CO2under standard humidity. The specific proteasome inhibitor BTZ (also named Velcade and formerly known as PS-341) was provided by the Department of Hematology and Bone Marrow Transplantation of Tianjin Medical University Cancer Institute and Hospital. CAL-101 was purchased from Selleck Company (Catalog No. S2226). CAL-101 was dissolved in dimethyl sulfoxide (DMSO) as a stock solution, stored at 80 ℃, and diluted with serum-free RPMI 1640 prior to use. The final DMSO concentration should not exceed 0.1% for all experiments.
Cell viability assay
Cell viability was quantifed using MT assay. Z138, HBL2, and Jeko-1 cells were seeded in 96-well plates (8,000 cells/well) and incubated for 0, 24, 48, and 72 h with different CAL-101 concentrations (0, 20, 40, 60, 80, and 90 μmol/L) with or without BTZ (0, 0.06, 0.12, 0.18, 0.24, and 0.3 μmol/L). The cells were added with MTT reagent (Sigma-Aldrich) and then incubated at 37 ℃ for 3 h. Te solution was decanted, and 100 μL of DMSO was added to dissolve purple formazan crystals. Te absorbance of the resulting solution was determined at 570 nm with a microplate reader (Synergy 2, Bio Tek, VT, USA). Each dose was tested in triplicate. Dose efect and combination index (CI) curves were constructed using the CalcuSyn software. Data were confrmed by performing at least three independent experiments.
Apoptosis analysis
About 5×105Z138 and HBL2 cells were cultured in six-well plates. The cells were exposed to four groups: CAL-101 group (45 μmol/L CAL-101), BTZ group (0.3 μmol/L BTZ), CAL-101/BTZ combination group (45 μmol/L CAL-101 and 0.3 μmol/L BTZ), and control group (no drug added but supplement with RPMI-1640 medium) for 48 h. Afer treatment, the cells were washed with PBS and suspended in 100 μL of 1× binding bufer. Annexin V-FITC (5 μL) and PI (5 μL) were added. After the cells were incubated at room temperature for 15 min in dark, apoptosis rate was assessed with FACS (Becton Dickinson, USA). Data were analyzed using the FlowJo 2.7.4 sofware program (Tree Star, USA).
NF-κB activity
About 5×105Z138 and HBL2 cells were cultured in six-well plates. The cells were exposed to four groups: CAL-101 group (45 μmol/L CAL-101), BTZ group (0.3 μmol/L BTZ), CAL-101/BTZ combination group (45 μmol/L CAL-101 and 0.3 μmol/L BTZ), and control group (no drug added but supplemented with RPMI-1640 medium) for 48 h. After the cells were treated, nuclear protein was extracted using a nuclear extraction kit (Active Motif, Carlsbad, CA, USA). NF-κB activity was determined using the TransAM NF-κB p65 transcription factor assay kit (Active Motif) according to the manufacturer’s instruction.
Western blot analysis
About 5×105Z138, HBL2, and Jeko-1 cells were cultured in six-well plates and treated with various CAL-101 concentrations (0, 10, 20, and 40 μmol/L) for 48 h. In combination experiments, the cells were divided into four groups: CAL-101 group (45 μmol/L CAL-101), BTZ group (0.3 μmol/L BTZ), CAL-101/BTZ combination group (45 μmol/L CAL-101 and 0.3 μmol/L), and control group (no drug added butsupplemented with RPMI-1640 medium) for 48 h. Proteins were extracted from the MCL cell lines and analyzed through SDS-PAGE. Immunoblot analysis was performed using antibodies against p-ERK, p-AKT, AKT, ERK, caspase-3 (Cell Signaling Technology), and β-actin (Abcam, USA).
Statistical analysis
Te SPSS19.0 sofware program was used for statistical analysis. Results were presented as mean ± standard deviation (SD) and examined for significant differences using Student’s t test. An alpha value of P<0.05 was considered statistically significant difference. Synergistic drug interactions were tested through median dose-effect analysis with a commercially available sofware program (CalcuSyn, Biosof, MO).
Results
CAL-101 and BTZ synergistically suppressed MCL cell viability
The cytotoxic effects of single-agent CAL-101 or BTZ were studied, and IC50 was evaluated in three diferent MCL cell lines through MT assay. Te IC50 of single-agent CAL-101 and BTZ were 56.96 and 0.335 μmol/L in Z138 cells, respectively, 62.99 and 0.46 μmol/L in HBL-2 cells, respectively, and 77.82 and 0.388 μmol/L in Jeko-1 cells, respectively (Figure 1A). To determine the effect of combined exposure to both agents on cell viability, we incubated the cells with various concentrations of CAL-101 and BTZ for 48 h. Fractional effect-CI curves were constructed (Figure 1B). The CI values of CAL-101-BTZ werelower than 1 for the three cell lines, which demonstrated the synergistic inhibition of the drug combination. Furthermore, CAL-101 exhibited a time-dependent inhibitory efect on the viability of Z138, HBL-2, and Jeko-1 cells. CAL-101 and BTZ concentrations were fxed at 60 and 0.18 μmol/L, respectively (Figure 1C).
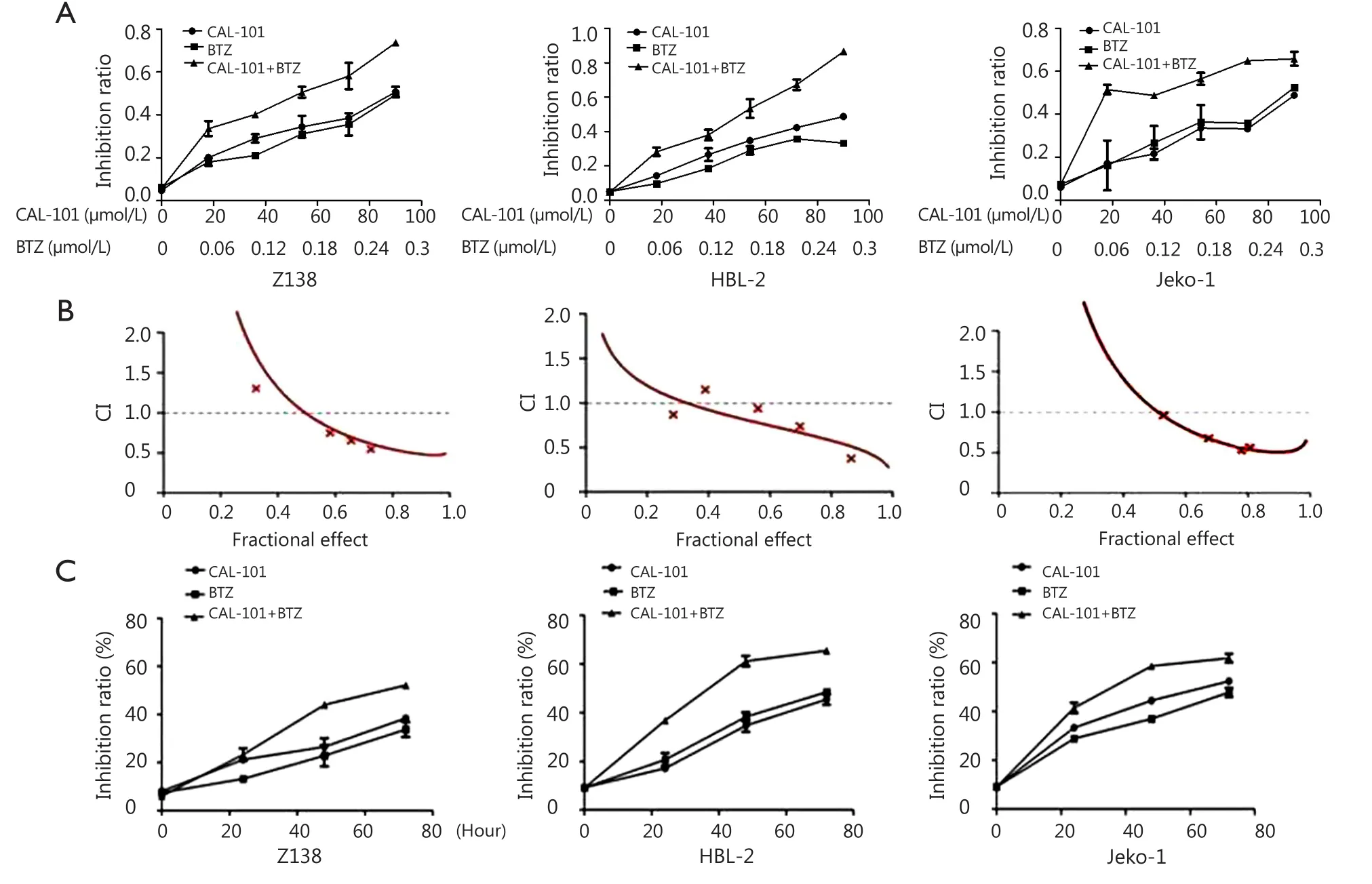
Figure 1 CAL-101 and bortezomib synergistically interact in Z138, HBL-2, and Jeko-1 cells. (A) CAL-101/bortezomib combination significantly suppressed viability of MCL cells compared with single-agent CAL-101 or BTZ through MTT. (B) CAL-101/bortezomib combination induced significantly synergistic cytotoxicity in MCL cells by CalcuSyn software calculation [Combination index (CI) <1.0]. CI values <1.0 indicates a synergistic interaction and CI values>1.0 indicates antagonistic drug effects. A straight line at CI =1.0 represents additive effects of both drugs. (C) Inhibitory effect of CAL-101 on the viability of Z138, HBL-2, and Jeko-1 cells was time dependent.
Co-administration of CAL-101 and BTZ synergistically enhanced apoptosis in MCL cells
To determine the effect of combined exposure to both agents on apoptosis, we treated the cells with CAL-101 with or without BTZ for 48 h. Annexin staining for apoptosis was then performed. Te result showed that treatment with CAL-101 and BTZ resulted in a pronounced increase in apoptosis in Z138 and HBL-2 cells (Figure 2). The results of dose-response studies further revealed that cell exposure to 0.3 μmol/L BTZ and 45 μmol/L CAL-101 for 48 h resulted in significant increase in cell death. Tese fndings suggested that combined treatment of CAL-101 and BTZ potently induced apoptosis in MCL cell lines.
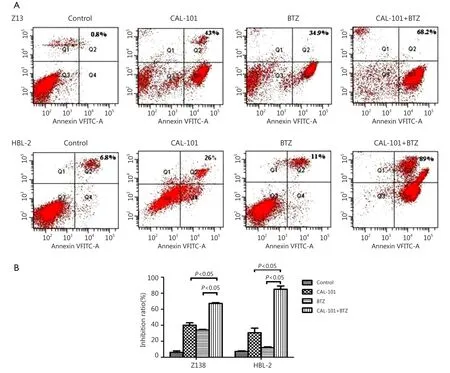
Figure 2 Combined treatment with CAL-101 and bortezomib synergistically induces apoptosis in MCL cells. (A) Annexin-V/PI assay indicated that CAL-101/BTZ combination obviously induced cell apoptosis in two cell lines. (B) Bar chart showed that the combined treatment produced enhanced apoptosis in both cell lines.
CAL-101 inhibited the PI3K/AKT and ERK pathways in MCL cells
We demonstrated that PI3K-p110σ was expressed in Z138, HBL-2, and Jeko-1 cells (Figure 3A). After the cells were treated with CAL-101, AKT and ERK phosphorylation were dose-dependently inhibited (Figure 3B). Tis fnding indicated that PI3K/AKT and ERK signaling was inhibited.
Co-exposure of MCL cells to CAL-101 and BTZ inactivated the NF-κB and AKT pathways
The effects of the combination of CAL-101 and BTZ on NF-κB activation were assessed in Z138, HBL-2, and Jeko-1 cells by using an ELISA TransAM NF-κB p65 transcription factor assay kit (Active Motif). NF-κB activity was significantly less afer combined exposure to both agents than that afer exposure to single agent in Z138, HBL-2, and Jeko-1 cells. This finding suggested that the combination of CAL-101 and BTZ enhanced NF-κB inactivation (Figure 4A). We demonstrated that PI3K/AKT signaling may also be inhibited by CAL-101. The cells were treated with the combined treatment, and the results showed the induced p-AKT down-regulation in Z138, HBL-2, and Jeko-1 cells (Figure 4B). Cleaved caspase-3, which is the downstream kinase of the AKT pathway, was up-regulated afer the combined treatment (Figure 4C).
Discussion
MCL, a distinct subset of B-cell NHL, is characterized by the t(11;14)(q13;q32) chromosomal translocation. Several survival pathways involving NF-κB, phosphatidylinositol-4, and PI3K have been reported to be associated with MCL17,18. Conventional treatment with immunochemotherapy and autologous stem cell transplantation has shown improved outcomes, and recent advances in MCL treatment are promising.
CAL-101, a selective inhibitor of PI3K/p110σ, exhibits excellent clinical activity in CLL, difuse large B cell lymphoma, and MM19-21. The PI3K survival pathways are deregulated in several malignancies, including MCL. The inhibition of the PI3K pathway by CAL-101 (GS-1101, idelalisib) blocks survival signals, induces apoptosis, and disrupts signals from the tumor microenvironment, resulting in B-cell malignancies.
CAL-101 exhibits cytotoxic activity on MCL cells. Previous studies demonstrated that AKT is constitutively activated in an MCL subset22-27. AKT activation promotes cell survival, proliferation, and apoptosis inhibition through multiple mechanisms, including NF-κB activation28-30. As CAL-101 is a PI3K inhibitor, we then tested PI3K activity afer treatment with CAL-101. We found that CAL-101 inhibited AKT and ERK phosphorylation (p-AKT and p-ERK), which demonstrated that CAL-101 cytotoxicity in MCL cells was mediated via an inhibitory efect on the PI3K/AKT pathway and ERK signaling.This finding supported that p110σ, p-AKT, and p-ERK were involved in cell growth and proliferation.
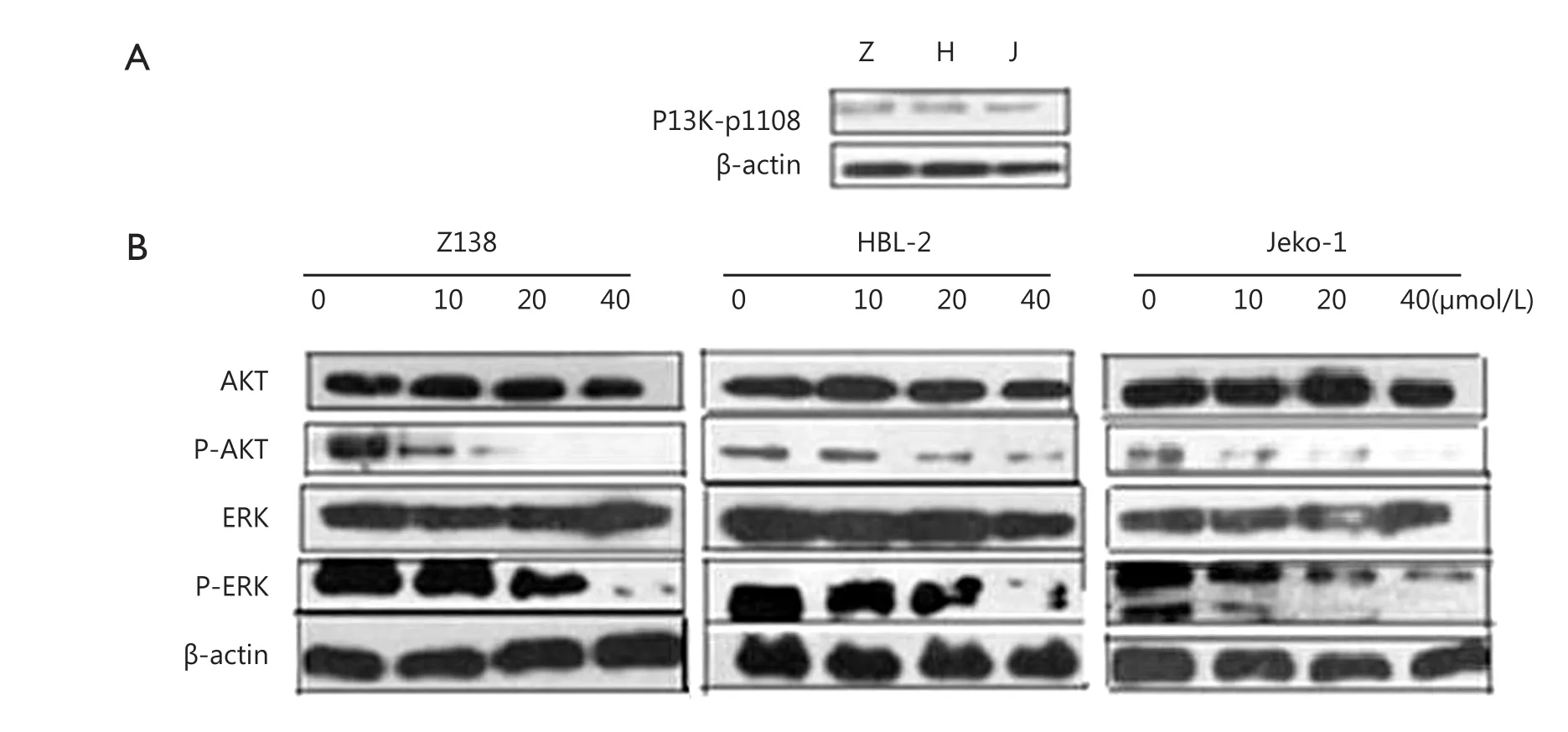
Figure 3 CAL-101 inhibits the PI3K/AKT and ERK pathway. (A) PI3K-p110σ was expressed in MCL cell lines. (B) The expression of P-AKT and P-ERK decreased with increasing concentration of CAL-101.
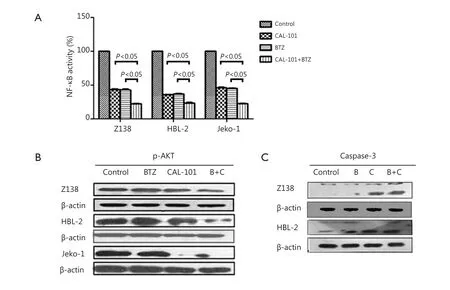
Figure 4 (A) NF-κB Inactivation is enhanced by CAL-101/Bortezomib combination. (B) Combined treatment induced marked downregulation of p-AKT in MCL cells. (C) Caspase-3 was up-regulated after combined treatment in MCL cell lines.
BTZ, a reversible proteasome inhibitor, was approved for refractory MCL, but this drug has limited single-agent capacity31-34. The AKT and ERK pathways are important for malignant cell survival35, and interruption of each pathway potentiates proteasome inhibitor lethality in cancer cells36,37. Tese fndings supported the feasibility of using the combination of CAL-101 and BTZ for MCL. We found that CAL-101 and BTZ synergistically induced cell death in MCL cells, induced inhibition of AKT and NF-κB activity, and significantly enhanced cell apoptosis.
In summary, we reported that CAL-101 was cytotoxic to human MCL cells and potently enhanced the activity of the proteasome inhibitor BTZ. The underlying mechanism could be the targeting of NF-κB and AKT activity. In line with the emerging or established evidence of the activity of these agents in MCL, this regimen must be further investigated in vivo. Overall, we provide a basis for clinical trials of CAL-101 in MCL and a rationale for its use in combination therapy with BTZ.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 30672208, 81270603, and 31301161) and Tianjin Natural Science Foundation of China (Grant No. 13JCYBJC22800).
Conflict of interest statement
No potential conficts of interest are disclosed.
1. Zhou Y, Wang H, Fang W, Romaguer JE, Zhang Y, Delasalle KB, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer 2008;113:791-798.
2. Salaverria I, Perez-Galan P, Colomer D, Campo E. Mantle cell lymphoma: from pathology and molecular pathogenesis to new therapeutic perspectives. Haematologica 2006;91:11-16.
3. Pileri SA, Falini B. Mantle cell lymphoma. Haematologica 2009;94:1488-1492.
4. Aschebrook-Kilfoy B, Caces DB, Ollberding NJ, Smith SM, Chiu BC. An upward trend in the age-specifc incidence paterns for mantle cell lymphoma in the USA. Leuk Lymphoma 2013;54:1677-1683.
5. Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood 2009;114:1469-1476.
6. Martin P, Coleman M, Leonard JP. Progress in mantle-cell lymphoma. J Clin Oncol 2009;27:481-483.
7. Klippel A, Kavanaugh WM, Pot D, Williams LT. A specifc product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol 1997;17:338-344.
8. So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem J 2012;442:465-481. 9. Castillo JJ, Furman M, Winer ES. CAL-101: a phosphatidylinositol-3-kinase p110-delta inhibitor for the treatment of lymphoid malignancies. Expert Opin Investig Drugs 2012;21:15-22.
10. Fruman DA, Rommel C. PI3Kdelta inhibitors in cancer: rationale and serendipity merge in the clinic. Cancer Discov 2011;1:562-572.
11. Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood 2006;107:4053-4062.
12. Kahl B, Byrd JC, Flinn IW, Wagner-Johnston N, Spurgeon S, Benson DM, et al. Clinical safety and activity in a phase 1 study of CAL-101, an Isoform-selective inhibitor of phosphatidylinositol-3-Kinase p110δ, in patients with relapsed or refractory non-Hodgkin lymphoma. Blood (ASH annual Meeting abstracts) 2010;116:abstract 1777.
13. Min YH, Eom JI, Cheong JW, Maeng HO, Kim JY, Jeung HK, et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia 2003;17:995-997.
14. Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM Jr, Wagner-Johnston ND, et al. CAL-101, an isoform-selective inhiBitor of phosphatidylinositol 3-kinase p110, demonstrates clinical activity and pharmacodynamic effects in patients with relapsed or refractory chronic lymphocytic leukemia. Blood (ASH annual Meeting abstracts) 2010;116:abstract 55.
15. Montagut C, Rovira A, Albanell J. Te proteasome: a novel target for anticancer therapy. Clin Transl Oncol 2006;8:313-317.
16. Chen KF, Yeh PY, Yeh KH, Lu YS, Huang SY, Cheng AL. Downregulation of phospho-Akt is a major molecular determinant of bortezomib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res 2008;68:6698-6707.
17. Tabe Y, Jin L, Konopleva M, Shikami M, Kimura S, Andreef M, et al. Class IA PI3K inhibition inhibits cell growth and proliferation in mantle cell lymphoma. Acta Haematol 2014;131:59-69.
18. Rahal R, Frick M, Romero R, Korn JM, Kridel R, Chan FC, et al. Pharmacological and genomic profiling identifies NF- kB-targeted treatment strategies for mantle cell lymphoma. Nat Med 2014;20:87-92.
19. Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood 2010;116:2078-2088.
20. Ikeda H, Hideshima T, Fulciniti M, Perrone G, Miura N, Yasui H, et al. PI3K/p110 is a novel therapeutic target in multiple myeloma. Blood 2010;116:1460-1468.
21. Dunleavy K, Pitaluga S, Czuczman MS, Dave SS, Wright G, Grant N, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of difuse large B-cell lymphoma. Blood 2009;113:6069-6076.
22. Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 2006;24:4867-4874.
23. Nguyen LX, Sesay A, Mitchell BS. Effect of CAL-101, a PI3Kδ inhibitor, on ribosomal rna synthesis and cell proliferation in acute myeloid leukemia cells. Blood Cancer J 2014;4:e228.
24. Orlowski RZ, Small GW, Shi YY. Evidence that inhibition of p44/42 mitogenactivated protein kinase signaling is a factor in proteasome inhibitor-mediated apoptosis. J Biol Chem 2002;277:27864-27871.
25. Jung HJ, Chen Z, Fayad L, Wang M, Romaguera J, Kwak LW, et al. Bortezomib-resistant nuclear factor kappaB expression in stem-like cells in mantle cell lymphoma. Exp Hematol 2012;40:107-118.e2.
26. Kim A, Park S, Lee JE, Jang WS, Lee SJ, Kang HJ, et al. Te dual PI3K and mTOR inhibitor NVP-BEZ235 exhibits anti-proliferative activity and overcomes bortezomib resistance in mantle cell lymphoma cells. Leuk Res 2012;36:912-920.
27. Yeramian A, Sorolla A, Velasco A, Santacana M, Dolcet X, Valls J, et al. Inhibition of activated receptor tyrosine kinases by Sunitinib induces growth arrest and sensitizes melanoma cells to Bortezomib by blocking Akt pathway. Int J Cancer 2012;130:967-978.
28. Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011;117: 6287-6296.
29. Viglieto G, Moti ML, Bruni P, Melillo RM, D’Alessio A, Califano D, et al. Cytoplasmic relocalization and inhibition of the cyclindependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med 2002;8:1136-1144.
30. Hay N. Te Akt-mTOR tango and its relevance to cancer. CancerCell 2005;8:179-183.
31. Alinari L, Christian B, Baiocchi R. Novel targeted therapies for mantle cell lymphoma. Oncotarget 2012;3: 203-211.
32. Pérez-Galán P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood 2011;117:26-38.
33. Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol 2003;171: 88-95 .
34. Palumbo A, Atal M, Roussel M. Shifs in the therapeutic paradigm for patients newly diagnosed with multiple myeloma: maintenance therapy and overall survival. Clin Cancer Res 2011;17:1253-1263.
35. Data SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cellintrinsic death machinery. Cell 1997;91:231-241.
36. Iyengar S, Clear A, Bödör C, Maharaj L, Lee A, Calaminici M, et al. P110α-mediated constitutive PI3K signaling limits the efcacy of p110δ-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood 2013;121:2274-2284.
37. So L, Yea SS, Oak JS, Lu M, Manmadhan A, Ke QH, et al. Selective inhibition of phosphoinositide 3-kinase p110α preserves lymphocyte function. J Biol Chem 2013;288:5718-5731.
Cite this article as: Qu FL, Xia B, Li SX, Tian C, Yang HL, Li Q, Wang YF, Yu Y, Zhang YZ. Synergistic suppression of the PI3K inhibitor CAL-101 with bortezomib on mantle cell lymphoma growth. Cancer Biol Med 2015;12:401-408. doi: 10.7497/j.issn.2095-3941.2015.0013
*These authors contributed equally to this article.
Yi-Zhuo Zhang
E-mail: yizhuozhang111@163.com
Received February 9, 2015; accepted April 20, 2015.
Available at www.cancerbiomed.org
Copyright © 2015 by Cancer Biology & Medicine
杂志排行
Cancer Biology & Medicine的其它文章
- Dermatofibrosarcoma protuberans: from translocation to targeted therapy
- PI3K/Akt/mTOR inhibitors in breast cancer
- Advances in drug delivery system for platinum agents based combination therapy
- Upgrading the definition of early gastric cancer: better staging means more appropriate treatment
- Prognostic value of body mass index before treatment for laryngeal squamous cell carcinoma
- Pathological clavicular fracture as first presentation of renal cell carcinoma: a case report and literature review
