Geriatric oncology: problems with under-treatment within this population
2015-12-29DivyaSwaminathanVikramSwaminathan
Divya Swaminathan, Vikram Swaminathan
1Norwich Medical School, University of East Anglia, Norwich NR4 7TJ, UK;2Health Education North West, Liverpool L3 4BL, UK
Geriatric oncology: problems with under-treatment within this population
Divya Swaminathan1, Vikram Swaminathan2
1Norwich Medical School, University of East Anglia, Norwich NR4 7TJ, UK;2Health Education North West, Liverpool L3 4BL, UK
We are currently faced with an aging population, which is rapidly growing worldwide. Two thirds of cancer occurs in the over 65-year age group. Societal conceptions from the past have created ageist stereotypes; old age is associated with frailty and the elderly are perceived to be destined for deterioration and loss of independence. Cancer within the elderly is also subject to these stereotypes, with elderly cancer patients considered by some not as likely to recover as younger patients with cancer. We summarise and review the current concerns regarding elderly management and treatments utilised for the management of oncological disease in the elderly, and discuss the impact of under-treatment within this population.
Cancer; elderly; management; geriatric oncology
Introduction
Te expressions elderly and geriatric are used widely in modern medicine and are defined as terms describing the old or aged person1. However, when asked to specifcally defne old age, there is no universally accepted interpretation in place2. The World Health Organisation (WHO) proposes that most countries define elderly as being over the age of 65 years, generally recognised as retirement age3. In our society, this demographic is unfortunately susceptible to misconceptions and ageist stereotypes, creating complexities in the medical management of the eldly.
We are currently faced with an aging population, which is rapidly growing worldwide. Healthy life expectancy is increasing and people are living for longer periods of time4. In 2010 the number of over 65’s was estimated to be around 534 million, the equivalent of 8% of the worlds’ population5. By 2021, this is predicted to rise to approximately 10%6. Moreover, by 2050, this is anticipated to almost triple in number to 1.5 billion; 16% of the world’s population will be of retirement age2. As a result of these demographic changes, it could be argued that our accepted definition of elderly and the associated age of 65 years is no longer medically relevant and it should be changed to an age of at least 75 years and over; perhaps this cut of would provide greater meaning. Nonetheless in the current era of geriatric medicine, age alone is no longer enough to characterise an individual6.
Age is an indirect risk factor for cancer; extended life duration is coupled with increased exposure to carcinogenic factors alongside greater time for the accumulation of genetic changes, which could eventually result in the generation of a tumor. Two thirds of cancer occurs in the over 65-year age group, a noteworthy proportion of individuals6. Statistics collected between 2009 and 2011 by Cancer Research UK present the male to female ratio of diagnosis as 1.06:1 per year (60,828:57,221) within the elderly aged over 75 years7,8. About 70% of cancer related deaths occur within this population9. The prevalence of certain cancers varies with gender. In order of decreasing incidence, prostate, lung, and bowel cancers contribute to 57% of cancers diagnosed in elderly males. Females are most commonly afected by breast cancer (21%), followed by lung, bowel, stomach, and uterine cancer10.
Societal conceptions from the past have created ageiststereotypes; old age is associated with frailty and the elderly are perceived to be destined for deterioration and loss of independence11. Cancer within the elderly is also subject to these stereotypes, with elderly cancer patients considered by some not as likely to recover as younger patients with cancer. However, this is not necessarily the case and currently elderly members of the community are ofen able to work and play an active role in society12. Ageism is defned as ‘an atitude that discriminates, separates, stigmatises or otherwise disadvantages older adults on the basis of chronological age’13. In healthcare, it alludes to the idea that considerable deterioration is always a normal part of aging and that older individuals may not beneft as much as a younger individual from certain treatment, and therefore should perhaps not be offered it. Under-treatment in the geriatric patient is reinforced by the ageist approach to care and although this approach is built on the basis of avoiding harm, it does not follow the principles of consent, capacity and acting in a patient’s best interest14. We summarise and review the current concerns regarding elderly management and treatments utilised for the management of oncological disease in the elderly, and discuss the impact of under-treatment within this population.
Issues associated with the management of the elderly
The management of geriatric patients does not come without challenges. The aging process is accompanied by multisystem physiological changes in the body, including deterioration of vision and hearing, reduced kidney function, reduced gastrointestinal motility, reduced efficiency of the cardiovascular and respiratory systems, decreased bone density, and reduced mobility to mention a few15. Although these changes are natural they render the elderly less able to manage, with a reduced reserve for stresses placed on the body and more susceptible to pharmacokinetic and pharmacodynamics interactions, which may lead to toxicity2.
Any psychological or cognitive decline can alter the amount of patient support required, which may be accompanied by difcult social or financial circumstances16. Limited social support and difficulty accessing transport and healthcare create further hurdles in the pathway to obtaining treatment for geriatric patients. All of these factors may contribute to the efectiveness of treatment and a patients’ prognosis. Evidence suggests that good family support networks increase patient tolerance towards more extensive treatment regimens1. It is clear that even elderly patients of good health still have diferent health needs compared to younger patients8.
Financial burden of cancer care in the elderly
The cost of treatments can vary globally, depending on the intervention, the site, and the stage (severity) of cancer being treated. If treatment costs continue to rise annually by 2%; breast and prostate cancers will produce the largest increases in expense, 32% and 42% relatively, simply due to the fact that these cancers will remain most prevalent in the population2.
According to Macmillan Cancer Support, the fnancial impact of cancer on an individual is most considerably infuenced by age, the stage of their cancer, and their social class (employment and income)17. In addition to treatment costs, these patients must be prepared to fund transportation to and from appointments and perhaps to specialist centres. Cancer may also result in increased costs for the family of the patient; addition funding is required to support the elderly patient, due to declining ability for the patient to manage their required activities of daily living.
A report published by the department of health in 2014 presented figures regarding emergency hospital admissions. The years 2012/2013 saw 82,600 admissions due to cancer, representing 3.7% of all emergency admissions. Geriatric patients may sometimes be referred to as ‘hazards of hospitalisation’, simply due to their greater risk of hospital related complications. These include malnutrition, pressure ulcers, falls, delirium and hospital acquired infection18. Emergency admissions to hospital can be expensive and elderly patients may have a higher occurrence of re-admission. Prevention of this is therefore a collective objective in the health service.
With a growing bank of people within the elderly demographic and an inevitable rise in the prevalence of cancer, we need to work towards providing optimal treatment in a cost effective manner. With limited resources and funding, there is a need to provide this optimal care to everyone, including the geriatric population.
Management of geriatric oncology patients
Tere is plenty of evidence to suggest that under-treatment of the elderly is a very real issue. Tis under-treatment arises for many reasons. Firstly, the lack of elderly patients in clinical trials has led to insufcient evidence and gaps in our knowledge as to what are the most efective treatments for geriatric oncology patients. Tis in turn results in a lack of guidelines to support evidence-based decisions, and so patients may not receive the most effective treatment and are undertreated. In addition, iatrogenic factors play a role. Te ageist approach to care of geriatric patients givesrise to bias and misconceptions as to what is best for the patient and which treatments they can tolerate. Lower risk and less aggressive interventions may appear a more attractive option to physicians, in order to avoid patient morbidity or mortality. Nonetheless, 15,000 older cancer patients die prematurely due to this approach and under-treatment leads to a reduced 5-year patient survival1,2.
A retrospective evaluation reported data involving 212 patients over 80 years old with newly diagnosed breast cancer at the University of Texas MD Anderson Cancer Centre between 1989 and 2004. Those treated only with primary endocrine therapy in comparison to endocrine therapy in combination with surgery had a signifcant reduced survival (P=0.001). Te study also illustrated that 57% of these patients with a huge proportion, were under-treated when consulting the existing guidelines19. The conclusions from this study held great importance. It not only highlighted the benefits of multidisciplinary therapy in the elderly, but also emphasised that under-treatment leads to reduced cancer survival and impaired prognosis1.
Difuse large B-cell lymphoma is a subtype of non-Hodgkin’s lymphoma and a malignancy that is increasing in prevalence throughout the elderly population. It is manageable and potentially curable, like several other cancers. In concordance with the reasons already mentioned, geriatric patients are largely under-represented in clinical trials and so treatment decisions for this cancer is generally based on evidence from younger patients. As a result some patients are given sub-optimal therapy in an attempt to avoid toxicity, perhaps having negative implications on prognosis. Alternative regimens may be considered for geriatric patients which would be deemed as under-treatment in younger populations. The issue of geriatric under-treatment is therefore very apparent. Te international society of geriatric oncology (SIOG) recently chose to review the treatment of these patients and outline more effective guidelines for management to target this potentially detrimental care20.
There are an increasing number of treatments and potential options now available for the medical and surgical oncological management of the geriatric patient. Current treatment pathways available include surgery, radiotherapy, chemotherapy, biological therapies, pharmacological treatment and endocrine therapy. Tere are a handful of further treatments also available that are not regarded as frontline. All of these treatments may be used individually as monotherapy, or in collaboration with adjuvant supportive treatment.
Surgical
According to the Royal College of Surgeons England, there were 4.7 million surgical admissions in 2013-201421. The most common procedures performed were hernia repairs (120,198) followed by hip replacements (115,758) and knee replacements (81,590). Despite the prevalence of cancer within the population, cancer related surgery did not appear amongst the top 10 surgeries performed.
Surgery is perhaps the branch of treatment that causes most debate. It is perhaps misconceived by some health professionals and certainly by the public that old age is contra-indication to oncological surgery. Surgery is invasive and inevitably there are greater risks associated, including lengthy hospital stays, ICU admissions and undesirable complications such as premature mortality. Despite this, surgery in an older patient can provide similar success with cancer treatment as in younger patients with well-regarded evidence to support the feasibility of surgery within this age group1,22. It is important to remember that surgical therapy can be curative; surgery is reported as ‘the most efective cancer-ablative therapy’22. Surgical resection of cancer can reduce costs by removing the need for ongoing long-term treatments to manage the cancer in elderly patients.
Surgical interventions come in several forms, ranging from simple local excision to the removal of entire glands such as in a mastectomy23. As patients remain healthier for longer, the breadth of patients within the surgical group is increasing. Lung cancer is characterised by an association with age22. Over 65% of patients with lung cancer are over the age of 65 years when they are initially diagnosed. Furthermore, 25% of patients diagnosed are 75 years or older with 30% of patients who die from lung cancer within this latter demographic. A lung lobectomy is the current standard procedure for patients of any age, and carries an operative mortality risk of 1.4%22. Results from randomised trials suggest that there is no increased mortality risk related to this surgery with proceeding age24. Further research is required to identify peri-operative mortality rates that are representative for the elderly population but these fgures suggest that the elderly patient should not be excluded from surgical interventions on the basis of age alone.
Between the years 1989 and 1999, Bouchardy et al.25performed a study reviewing 407 breast cancer patients over 80. Te study demonstrated that under-treatment of the elderly was a very real issue and that treatment provided was independently associated with age. Of all the participants, 12% of women had no treatment, 33% had a mastectomy, 32% received hormone therapy in the form of tamoxifen, 14% had breast conserving surgery with adjuvant therapy and 7% had breast conserving surgery alone25. The primary outcome (5-year specific breast cancer survival) produced notable findings. Five-year survival fgures were presented as 46% for women with no treatment and51% for those given tamoxifen. Contrasted with an 82% 5-year survival from the mastectomy patients and a 90% survival from those who had breast conserving surgery and adjuvant treatment, not only do these results suggest that under-treatment leads to reduced survival and worse prognosis, they highlight the benefts of surgical intervention for geriatric patients.
Nevertheless although surgery should be considered optimistically, it remains a high-risk intervention. The overall peri-operative mortality rate stands at 1.2% for the general population within 30 days of surgery and this rate substantially rises with increasing age26-29. Te risk of death rises signifcantly to 19.8% with major surgery in the over 90s age group30. It is important to remember that proposing surgery to prolong life still carries a considerable risk (Table 1).
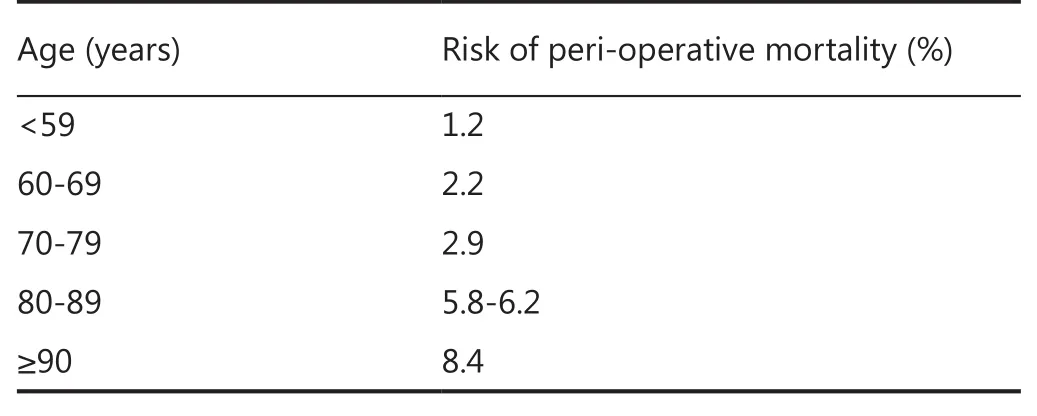
Table 1 Mortality rates during surgery split by age groups (34-38)
The mortality associated with surgical intervention is not only associated with age but with the nature of the surgery itself. Te risk associated with aggressive, invasive surgery such as pancreatic or oesogastric surgery is not comparable to the risks associated with breast or lung cancer surgery. However, a study driven by the current changing demographics and elderly population reviewed 438 patients over a 9-year period following a pancreatic resection. Te study concluded that elderly patients can safely endure invasive pancreatic surgery and that age alone should not exclude them from this group. Nevertheless there was still significant risk of morbidity, poor prognosis and reduced quality of life as a result31.
A further concern is the issue of screening for cancer in the elderly population. Perhaps the importance of early diagnosis and prevention is overlooked and not stressed sufciently within the geriatric population. Earlier diagnosis could reduce the need to high risk emergency surgery in this population.
In an era where cancer is becoming increasingly by prevalent and diverse, there is no longer room for a ‘one criterion fts all’parameter in the form of an age threshold. Te surgical approach should arguably never be excluded despite patients’ age. Patients can be individually assessed and evaluated for their fitness for surgery using various tools. Using a more holistic approach and considering a combination of factors (including health assessment), many elderly individuals may be deemed fit for surgical treatment of their cancer. Fear associated with surgical outcomes in addition to a lack of guidelines leads to surgical options being inadequately considered, resulting in undertreatment of these geriatric cancer patients.
Medical
Te UK budget for the cancer drug fund was raised from 200 million pounds in 2013/2014 to an estimated 340 million pounds in April 201532. However, earlier in 2015 several cancer drugs were removed from The National Health Service (NHS) funding simply due to their expense. Examples include lapatinib, eribulin, and everolimus, all used in the treatment of breast cancer; pemetrexed, which is used to treat lung cancer, and cabazitaxel, used for late-stage hormone resistant prostate cancer. Tese cut backs are estimated to affect almost 8,000 patients in the UK, many of them with advanced disease and perhaps likely to be geriatric33.
With advancements in the medical management of cancer, recent years have seen a decline in the incidence of mortality due to breast cancer in younger women, however, this trend is not been observed in older women1. Advancements in pharmaceutical treatments created by the drug industry drive the development of more effective medicinal therapies for use in oncology. However, this progression in efcacy is inescapably coupled with increasing expense and financial repercussion. An example is traztuzumab (Herceptin) which is used in the treatment of breast cancer. A course of traztuzumab costs on average approximately 25,000 pounds with the cost efectiveness estimated at 400,000 pounds per recurrence prevented6. We will become ever more reliant on these drugs in the future but unsurprisingly many patients may not be able to aford to fund these treatments privately.
Evidence suggests that endocrine therapy should not be used in geriatric patients at all, particularly with those that are frail or those with tumors that are at low risk34. Tis is because these hormonal therapies may cause several unfavourable side efects such as deep vein thrombosis; bone loss and musculoskeletal conditions. Tese negative efects largely outweigh the potential therapeutic benefit in elderly patients. However, as with all treatments there are exceptions to the rule and the decision must be made on an individual basis. Medical management of oncology in the elderly should not be dismissed purely on an impression of ability to tolerate side effects, as this can lead to under-treatment within this population. For example, with thetreatment of breast cancer, hormonal therapy can be used in both young and older patients; it remains the adjuvant therapy most readily used in older women due to the predominance of cases with estrogen receptor (ER) expression. Tamoxifen would perhaps be recommended for use in geriatric care. Tamoxifen also helps to prevent reduced bone density, a physiological change associated with old age. The use of any drug in the geriatric patient requires close monitoring35.
Chemotherapy
The cost of chemotherapy or radiotherapy per episode, which may be comprised of 3-4 sessions, can be as overwhelming as 35,000 pounds36. Unsurprisingly, the use of chemotherapy in geriatric oncology has the smallest evidence base. Te potential toxicity of treatment also can make clinical trials unethical for the geriatric population. The narrow therapeutic index of chemotherapeutic drugs makes dose selection particularly complex. An additional physiological change associated with aging is reduced function of the CYP2 enzyme9. This biological catalyst plays a key role in the metabolic pathway involved in the action of chemotherapeutic drugs, important to minimising toxicity. The current lack of evidence creates a barrier in establishing the efectiveness of this treatment in older patients. Current practice suggests that adjuvant chemotherapy is indicated only afer careful consideration of a combination of factors, such as recurrence risk and concurrent mortality risk26. In one study, 318 patients ranging from age 80 to 92 years were reviewed between 2005 and 2010. All participants were receiving chemotherapy for solid malignant tumors and the consequences of this treatment were investigated. The primary outcome of the study was discontinuation of chemotherapy due to toxicity and there were several secondary outcomes regarding adverse effects. Chemotherapy was first line for 89% of participants; 41% ordered an upfront dose reduction, 32% withdrew from treatment due to the toxicity and notably 32% of patients were hospitalised27. Inspite of a small study, this evidence quite clearly implies that chemotherapy in older patients poses quite serious risks and the requirement for effective methods of patient selection are emphasised. Clinicians must question whether the risks presented are worthwhile for the therapeutic benefit achieved from chemotherapy treatment of oncology in geriatric patients.
Several randomised trials have been published regarding metastatic colorectal cancer to investigate reduced-dose chemotherapy options and assess predictors of outcome in frail patients with advanced colorectal cancer. A study published in 2011 once more highlighted that although often treated with chemotherapy, the elderly are under-represented in such clinical trials. FOCUC2 aimed to investigate reduced-dose chemotherapy options and assess outcomes in frail patients with advanced colorectal cancer37. Similarly further trials include FFCD 2001-02, AVEX and PRODIGE 20. Trials are ongoing and therefore display that progress is being made to improve the evidence base for oncological treatment of geriatric patients.
In addition to this, a promising instrument developed to predict chemotherapy toxicity by the Cancer and Ageing research group has been recently trialled and supported in several clinical environments including geriatric oncology. Also, the first prospective clinical trial regarding elderly women and ovarian cancer is being supported by the Elderly Taskforce of the Gynaecological Oncology group. The American Society of Clinical Oncology (ASCO) plays an active role in geriatric oncology, introducing a geriatric oncology issue exploration team, establishing further educational materials, creating the B.J Kennedy Award for Excellence in Geriatric Oncology and establishing a geriatric oncology element to the Cancer Education Committee. All these interventions aim to provide evidence based guidance for the management of oncology in the geriatric population, aiming to improve the future of geriatric oncology29.
Radiotherapy
Evidence suggests than any benefits of radiotherapy appear to decrease as age increases1. Radiotherapy can be very debilitating, particularly to older patients, due to the effect on the entire body, as well as complications locally where applied. Radiotherapy treatment can be an ongoing commitment, requiring multiple sessions over a long period of time. This can be physically taxing, and is accompanied by the financial burden of retreatments. This may lead to a lack of compliance or withdrawal from therapy, as patients cannot tolerate the treatment. A recent publication in the European Geriatric Medicine journal re-evaluated some retrospective data regarding the treatment of breast cancer patients over 65 years of age. The authors investigated 79 patients who had been treated and followed-up in the Cumburiyet University Medical Oncology Department and found that 66.6% of patients receiving chemotherapy and/or radiotherapy had related side efects38. However, the study concluded that neither therapy was more toxic in elderly patients compared to younger patients, therefore treatment of each age group should be equivalent38.
Appropriate health assessment and treatment for geriatric oncology patients
The treatment of geriatric patients may be complicated by a spectrum of physical, psychological and social challenges. The factor of age alone cannot be just justified as the reason for treatment selection or dismissal. As with any age group, each patient is different and must be individually evaluated to establish the most effective management. Within the general population, standard methods of medical evaluation are efective but are not sufcient for assessment of elderly patients, as they do not account for factors such as cognitive decline, reduced mobility or iatrogenic disorders1.
Acknowledgement of the importance of the initial evaluation in the medical community has led to the creation of several tools for bespoke assessment, which play a key clinical role. A useful example is the comprehensive geriatric assessment (CGA). The use of this evaluation enables treatment selection acting in the patient’s best interest as it removes any ageist bias. This standardised tool reviews several domains (Table 2)39.
A recent study evaluating the use of the CGA on patients after hospital admission came to several statistically significant conclusions40. The meta-analysis found several benefits of performing a CGA including a reduction in patient deterioration and a decreased incidence of death (P=0.001). Evidence was also presented showing that elderly patients were more likely to survive hospital admission, return home, and remain alive for the return home and remain alive for the following year if a CGA was performed during their inpatient stay. Tis research clearly highlights the efectiveness of geriatric assessment. In addition to these advantages an overall beneft in cognitive measures was also shown (P=0.02)40. Tese conclusions were reinforced by another study, which also presented benefts of an inpatient CGA vs. no CGA in 10,427 participants from a varied elderly population41. Twenty randomised control trials were included together in a systematic review. In addition to the benefits expressed in the previous study, this review concluded that ‘for every 100 patients receiving a CGA, three more will be alive in their own homes’41. It is interesting how the use of such a simple methodological tool can make such a diference to quality of life. Additional recently adopted and regularly used tools include the mini-geriatric and G8 score. There is a wealth of evidence to suggest that these methods of evaluation are particularly effective. A study evaluating the G8 and fTRST screening tools in geriatric care concluded that both measures were efective for assessing patients and determining their prognosis with regards to functional decline and overall survival. A total of 937 patients aged over 70 years were included. Tese individuals had a malignant tumor and a new cancer event requiring a treatment decision42.
Similar positive results were shown by a small study carried out involving elderly patients with diferent digestive cancers. Te study aimed to assess the feasibility of mini geriatric assessment (MGA) in the adaptation of anticancer treatments and help make treatment decisions in these geriatric patients. The study concluded that the assessment could help gastroenterologists adapt treatment plans for oncological treatment43. There are also further methods of frailty assessment such as the Charlson Comorbidity Index and the ‘Multidimensional assessment for cancer in the elderly’ also known as MACE. Through consideration of several factors, these tools can assist in determining prognosis.
Frailty assessment has been readily adopted into professional practice in geriatric medicine. Macmillan Cancer Support, backed by Age UK has collaborated with the department of health to fund further studies on older cancer patients, an important step towards valuable research and making progress6. On the basis of the clear advantages of geriatric assessment, funds will also be dedicated towards establishing new means of evaluation to improve selection of cancer treatment. In addition, Macmillan aims to address current issues of ageist prejudice.All these methods of geriatric assessment have resulted in changes in treatment proposals and have been particularly effective in evaluating whether a patient is fit for surgery22,41. Patient health assessment in the geriatric population allows for a comprehensive evaluation of patient health and frailty, helping to overcome the issue of under-treatment due to health perception based on age alone.
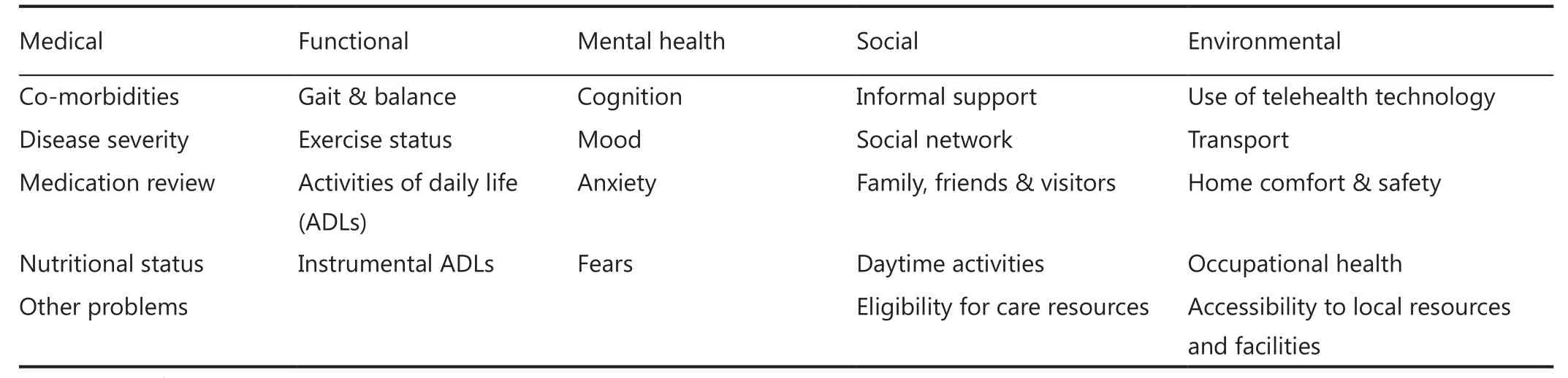
Table 2 Domains reviewed in CGA
Appropriate treatment of geriatric cancer patients can lead to prognostic improvements. Two cohorts of patients aged 70 years or older were compared in a study contrasting the outcomes and effectiveness of oncogeriatric care vs. standard care. The cohorts had similar baseline characteristics such as their stage of breast cancer. Te results presented how patients receiving standard care obtained fewer modalities of treatment, particularly less surgery, chemotherapy and hormone therapy (P<0.001). More importantly, the three-year mortality rate for the group receiving standard care was 71% (95% CI, 61%-83%) in comparison to 58% (95% CI, 42%-75%) for patients in the oncogeriatric care group. This study concluded that more bespoke, cancer-specific care is beneficial in reducing mortality rates and provides an improved prognosis. Te study showed how more appropriate care could change survival and emphasised the requirement for further research to quantify the extent of these improvements44.
Future care
Fortunately some progress has been made in the right directions. A number of recent trials by the National Cancer Institute (NCI) have demonstrated the improvement in clinical research regarding oncogeriatric medicine. Te institute acknowledges the necessity and clinical requirement for further focused research on geriatric patients45. However, barriers still exist for research in geriatric cancer patients. A study published in 2003 by the ASCO investigated the barriers to clinical trial participation by the older women with breast cancer. Te study aimed to establish whether the under-representation was due to fewer older women being asked to take part or conversely more of them declining to participate. Seventy-seven pairs were assigned consisting of one older woman aged 65 or above and one younger woman under 65 and these pairs were interviewed about their reasons for participation or refusal. Clinicians offering trials were also questioned about their motives for ofering or not ofering a trial to an individual. About 68% of younger patients were offered a trial compared to 34% of patients over 65 (P=0.004). The most infuential reasons for trial ofering appeared to be age and stage of cancer. Once offered a trial, there was no significant difference between participation (P=0.67), 56% of younger patients accepted and 50% of older patients did the same46. Te study concluded that the largest obstacle for older women for contributing to a trial was the physicians’ perception about age and tolerance for potential toxicity, both infuenced by an ageist approach.
Conclusion
Current opinions and evidence regarding geriatric oncology and under-treatment all appear to draw similar conclusions. It is universally agreed that there is a lack of evidence for management of elderly cancer patients. Involvement of more elderly patients in clinical trials will allow evidence-based decisions to be made when considering the most effective management for geriatric oncology patients. The risks associated with the treatment options for cancer are present regardless of age; awareness of age is an important factor, but this prejudice needs to be removed. The use of tools enabling geriatric and frailty assessment, such as the CGA, helps to surpass exclusion of treatment due to any age discrimination. Every patient should be educated about all available treatment options to allow them to make an informed decision. In order to make this informed decision and consent, an individual must have capacity. This means they must be able to understand, retain and weigh up information and then communicate a decision28. Assumptions should never be made regarding capacity based on age and it should be presumed that every patient has capacity until proven otherwise. Elderly patients should be fully informed about treatment and no information should be withheld or overlooked on the basis of their age. Such presumptions and perceived lack of capacity can lead to under-treatment19.
Te future of geriatric oncology involves taking a large stride away from the traditional paternalistic approach to treatment, moving to a more patient-centered, tailored approach with well-integrated standardised holistic assessment2. Guidelines implemented by institutions such as the SIOG are beginning to recognise the importance of an individualistic methodology not infuenced by age. Tey are endorsing steps towards a more equivalent approach to care. Without such guidelines we are lef with a spectrum of care offered which often results in undertreatment of elderly cancer patients and rarely provides the optimal care geriatric patients need.
Conflict of interest statement
No potential conficts of interest are disclosed.
1. Oxford Dictionary. Defnition of Elderly in English from the Oxford Dictionary. Available online: htp://www. oxforddictionaries.com/defnition/english/elderly [Accessed 22nd August 2015].
2. Swaminathan V, Audisio R. Geriatric oncology: a problem and solution. Oncology News 2012:7:12-13.
3. WHO World Health Organisation. Defnition of an older or elderly person. Available online: htp://www.who.int/healthinfo/survey/ ageingdefnolder/en/ [Accessed 22nd August 2015].
4. Te World Bank. Life expectancy at birth, total (years). Available online: htp://data.worldbank.org/indicator/SP.DYN.LE00.IN
5. National institute on aging, national institutes of health, u.s. department of health and human services. Global health and aging. available online: htp://www.who.int/ageing/publications/ global_health.pdf
6. Swaminathan V, Audisio R. Cancer in older patients: an analysis of elderly oncology. Ecancermedicalscience 2012;6:243.
7. Cancer research UK: Cancer incidence statistics. Available online: htp://www.cancerresearchuk.org/health-professional/cancerstatistics/incidence [Accessed on 22nd August 2015].
8. Vicini E, Swaminathan V, Audisio R. Management of breast cancer in elderly patients. Clin Pract 2014:11:59-69.
9. Walko CM, McLeod HL. Personalizing medicine in geriatric oncology. J Clin Oncol 2014;32:2581-2586.
10. Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581-592.
11. Masse M, Meire P. Is ageism a relevant concept for health care practice in the elderly? Geriatr Psychol Neuropsychiatr Vieil 2012;10:333-341.
12. Meyer H. Te Guardian. Older, healthier and working: britons say no to retirement. Available online: htp://www.theguardian.com/ society/2013/aug/24/working-britons-retirement [Accessed 23rd August 2015].
13. Medical dictionary. Defnition of ageism by medical dictionary. Available online: htp://medical-dictionary.thefreedictionary. com/ageism [Accessed on 22ndAugust].
14. Good Medical Practice. Consent: Patients and doctors making decisions together. general medical council. Available online: htp://www.gmc-uk.org/guidance/ethical_guidance/consent_ guidance_index.asp [Accessed 22nd August 2015].
15. Raza A, Raza SA, Qamar MF, Llaqat A. Human brain; physiological alterations occurring underlying process of aging. Professional Med J 2015;22:522-526.
16. Wilkinson R, Marmot M, editors. Social Determinants of Health: Te Solid Facts. Second Edition. Denmark: World Health Organisation; 2003.
17. Macmillan Cancer Support. Cancers Hidden Price Tag: Revealing the cost behind the illness. Available online: htp:// www.macmillan.org.uk/Documents/GetInvolved/Campaigns/ Costofcancer/Cancers-Hidden-Price-Tag-report-England.pdf
18. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118:219-223.
19. August DA, Rea T, Sondak VK. Age-related diferences in breast cancer treatment. Ann Surg Oncol 1994;1:45-52.
20. Morrison VA, Hamlin P, Soubeyran P, Stauder R, Wadhwa P, Aapro M, et al. Approach to therapy of difuse large B-cell lymphoma in the elderly: the International Society of Geriatric Oncology (SIOG) expert position commentary. Ann Oncol 2015;26:1058-1068.
21. Royal College of Surgeons. Surgery and the NHS in Numbers. Available online: htps://www.rcseng.ac.uk/media/mediabackground-briefngs-and-statistics/surgery-and-the-nhs-innumbers [Accessed 20th August 2015].
22. Korc-Grodzicki B, Downey RJ, Shahrokni A, Kingham TP, Patel SG, Audisio R. Surgical considerations in older adults with cancer. J Clin Oncol 2014;32:2647-2653.
23. Swaminathan V, Spiliopoulos MK, Audisio R. Choices in surgery for older women with breast cancer. Breast Care (Basel) 2012;7:445-451.
24. Allen MS, Darling GE, Pechet T, Mitchell JD, Herndon JE 2nd, Landreneau RJ, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Torac Surg 2006;81:1013-1019; discussion 1019-1020.
25. Bouchardy C, Rapiti E, Fioreta G, Laissue P, Neyroud-Caspar I, Schäfer P, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol 2003;21:3580-3587.
26. Bernardi D, Errante D, Gallligioni E, Crivellari D, Bianco A, Salvagno L, et al. Treatment of breast cancer in older women. Acta Oncol 2008;47:187-198.
27. Te Royal College of Surgeons of England. Access all ages: Assessing the impact of age on access to surgical treatment. Available online: htps://www.rcseng.ac.uk/publications/docs/ access-all-ages
28. General Medical Council. Consent guidance: part 3: capacity issues. Available online: htp://www.gmc-uk.org/guidance/ ethical_guidance/consent_guidance_part3_capacity_issues.asp [Accessed: 22nd August 2015].
29. Lichtman S, Hurria A, Jacobsen P. Geriatric oncology: an overview. J Clin Oncol 2014;32:2521-2522.
30. Jenkins K, Baker AB. Consent and anaesthetic risk. Anaesthesia 2003;58:962-984.
31. Oliveira-Cunha M, Maldr DJ, Aldouri A, Morris-Stif G, MenonVK, Malvern Smith A. Results of pancreatic surgery in the elderly: is age a barrier? HPB 2013;15:24-30.
32. NHS England 2015: News. NHS increases budget for cancer drugs fund from £280 million in 2014/15 to an expected £340 million in 2015/16. Available online: htps://www.england.nhs. uk/2015/01/12/cancer-drug-budget/
33. Donnelly L, Walton G. 25 cancer drugs to be denied on NHS. Available online: htp://www.telegraph.co.uk/news/ politics/11340860/25-cancer-drugs-to-be-denied-on-NHS.html [Accessed 22nd August 2015].
34. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Türlimann B, Senn HJ, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Terapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-1747.
35. Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, et al. Skeletal efects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol 2007;8:119-127.
36. NHS: what we give and what we get. Available online: htp:// news.bbc.co.uk/2/hi/programmes/breakfast/4898158.stm
37. Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 2011;377:1749-1759.
38. Seker MM, Yucel B, Seker A, Ay Eren A, Bahar S, Celasun G, et al. Treatment and prognosis of breast cancer in elderly: diferent from young patients? Eur Geriatr Med 2014;5:261-264.
39. British Geriatrics Society. Comprehensive assessment of the frail older patient. Available online: htp://www.bgs.org.uk/index. php?option=com_content&view=article&id=195
40. Ellis G, Whitehead MA, Robinson D, O'Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admited to hospital: meta-analysis of randomised controlled trials. BMJ 2011;343:d6553.
41. Ellis G, Langhorne P. Comprehensive geriatric assessment for older hospital patients. Br Med Bull 2005;71:45-59.
42. Kenis C, Decoster L, Van Puyvelde K, De Grève J, Conings G, Milisen K, et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol 2014;32:19-26.
43. Aparicio T, Girard L, Bouarioua N, Patry C, Legrain S, Soulé JC. A mini geriatric assessment helps treatment decision in elderly patients with digestive cancer. A pilot study. Crit Rev Oncol Hematol 2011;77:63-69.
44. van de Water W, Bastiaannet E, Egan KM, de Craen AJ, Westendorp RG, Balducci L, et al. Management of primary metastatic breast cancer in elderly patients--an international comparison of oncogeriatric versus standard care. J Geriatr Oncol 2014;5:252-259.
45. National Cancer Institute. Focusing on older cancer patients: a clinical need and a research necessity. Available online: htp:// www.cancer.gov/about-cancer/treatment/research/older-patients [Accessed 16/11/15].
46. Kemeny MM, Peterson BL, Kornblith AB, Muss HB, Wheeler J, Levine E, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 2003;21:2268-2275.
Cite this article as: Swaminathan D, Swaminathan V. Geriatric oncology: problems with under-treatment within this population. Cancer Biol Med 2015;12:275-283. doi: 10.7497/j.issn.2095-3941.2015.0081
Vikram Swaminathan
E-mail: d.swaminathan@uea.ac.uk
Received September 22, 2015; accepted November 24, 2015.
Available at www.cancerbiomed.org
Copyright © 2015 by Cancer Biology & Medicine
杂志排行
Cancer Biology & Medicine的其它文章
- Pathological clavicular fracture as first presentation of renal cell carcinoma: a case report and literature review
- Synergistic suppression of the PI3K inhibitor CAL-101 with bortezomib on mantle cell lymphoma growth
- Prognostic value of body mass index before treatment for laryngeal squamous cell carcinoma
- Mechanistic basis and clinical relevance of the role of transforming growth factor-β in cancer
- Dermatofibrosarcoma protuberans: from translocation to targeted therapy
- Upgrading the definition of early gastric cancer: better staging means more appropriate treatment
