高温液态水预处理木质纤维素
2015-12-28赵孟姣李国民徐琴琴银建中
赵孟姣,李国民,徐 刚,徐琴琴,张 昱,3,银建中
(1.大连理工大学化工机械学院,辽宁大连116024;2.辽宁石油化工大学机械工程学院,辽宁抚顺113001;3.内蒙古工业大学化工学院,内蒙古呼和浩特010051)
在燃料乙醇生产过程中可发酵糖(木糖与葡萄糖)的制取很关键。自然状态下,木质纤维素细胞壁的结构很复杂,既含结晶聚合物又含无定型聚合物,而且半纤维素与木质素紧紧包裹着纤维素,阻碍了酶与纤维素的接触,糖收率低。采用预处理可增大纤维素与酶的接触面积(可及度),提高糖收率及酶解效率。预处理方法包括化学法和物理法。化学法是采用酸、碱、高温液态水[1]以及有机溶剂等进行预处理;物理法是采用气爆与球磨等进行预处理。酸、碱预处理效率高,但腐蚀设备,产物中含有对发酵起抑制作用的物质;有机溶剂价格昂贵,有毒。与其它预处理方法相比,高温液态水在预处理过程中既是溶剂又是反应剂,无需添加其它物质,且价格低,绿色环保,因此成为研究热点。
作者在此总结了高温液态水的物化性质,在此基础上,从木质纤维素预处理时的溶解情况以及半纤维素水解影响因素、机理、强化等方面,详细叙述了木质纤维素高温液态水预处理工艺的研究进展。
1 高温液态水的物化性质
高温液态水是指温度在180~350 ℃(Tc=374 ℃,Pc=22 MPa)、压力高于饱和压力的液态水。随着温度升高,水的物理化学性质会发生改变,如图1所示。
由图1可看出,水的介电常数、黏度及密度均随温度的升高而降低,在近临界点处迅速下降,进入超临界区后缓慢下降。
在25 ℃、0.1 MPa下,水的介电常数与密度分别为78.46F·m-1、1.0g·cm-3;当温度升至350 ℃、20 MPa时,其值分别降至14.07F·m-1和0.60g·cm-3[2-3];继续升温至400 ℃、25 MPa时,其介电常数与密度已降至5.9F·m-1和0.17g·cm-3。
较低的介电常数使高温液态水在一定条件下表现出类似于极性有机溶剂的性质,能够大量地溶解气体和有机小分子,并且在超临界态下还可以与某些有机物以任意比例互溶[4-5]。应用这种溶解性质可以从植物中提取抗氧化剂[6],去除固体中的有机污染物[7]及金属[8]。另一方面,高温液态水使某些盐(如二类盐Na2CO3、K2SO4等)的溶解性下降,致使析出的盐造成管路堵塞[9]。高温液态水较低的密度提高了水本身的扩散性能,较低的黏度减小了扩散阻力。较低的介电常数、黏度和密度共同促进了有机物在高温液态水中迅速、大量地溶解[4]。
高温液态水最独特的性质是它的离子积(Kw)。标准状态下水的离子积是10-14[10],温度升高,Kw增大(图1b)。在250 ℃时,Kw达到最大值10-11,比标准状态下水的离子积大了3 个数量级,当温度升至374 ℃时,Kw陡降,在超临界区域缓慢降低。高温液态水可提供较多的H+和OH-,表现出酸碱催化的性能,用于催化(促进)反应。高温液态水既支持自由基反应又支持离子基反应:当Kw<10-14时,以自由基反应为主;当Kw>10-14时,以离子基反应为主[11]。水中H+的浓度不足以促进某些反应时,较高的温度则进行了弥补。
在上覆坝体压力及廊道自身重力作用下,廊道出现竖直向下的挠曲变形,在防渗墙的带动下发生向下游挠曲变形。两种变形组合后在廊道轴线方向产生较大的拉压应力,廊道两端上游受拉,下游受压,河床中部上游面受压、下游面受拉。因坝基岩体的约束,基岩面处出现了明显的应力集中现象,左右岸1/4跨位置上游面压应力较大,下游面拉应力较大。表1列出了静力条件下廊道沿各方向的变形和正应力极值。
高温液态水较大的离子积与较高的温度共同促进有机物的水解,反应条件较温和;而在超临界水中,有机物高效、快速地水解,反应可在几秒钟内完成[12]。相对于超临界水而言,高温液态水水解木质纤维素所需温度低,时间长,较容易控制。
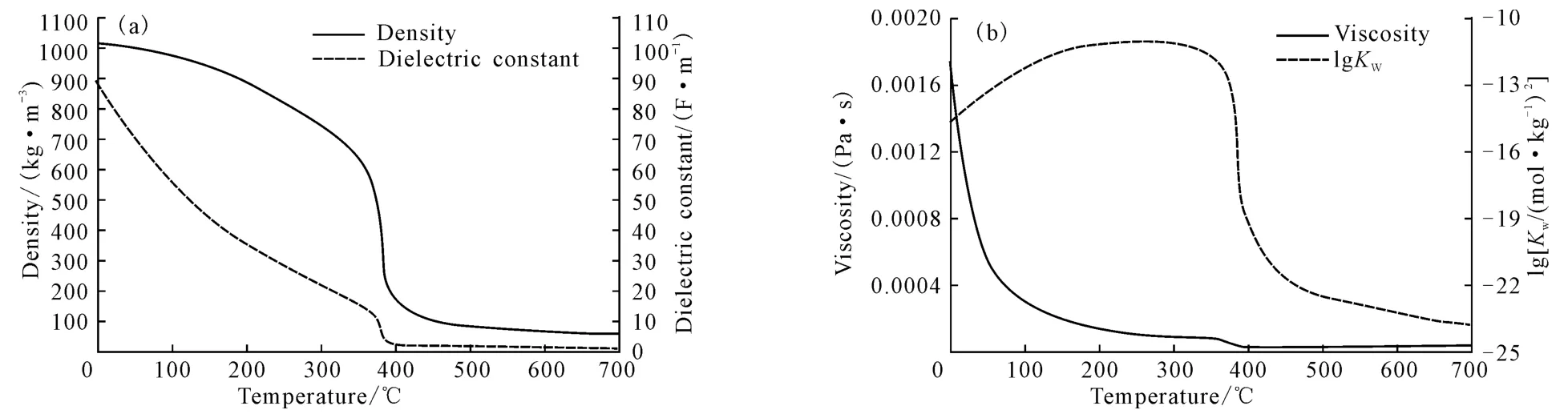
图1 25 MPa下,水的密度、介电常数(a)与黏度、离子积(b)随温度的变化曲线[9]Fig.1 Density and dielectric constant(a),viscosity and ion product(b)of water versus temperature at pressure of 25 MPa[9]
2 木质纤维素水解反应
木质纤维素在一定温度下可溶于高温液态水,其中半纤维素、纤维素在溶解状态下水解生成糖。它们水解的起始温度分别为180 ℃、230 ℃[13-14]。Mok等[15]指出热液解可作为酶解纤维素前的预处理。与爆破预处理破坏木质纤维素细胞壁不同[16],高温液态水预处理的实质是利用热量和自身酸催化能力使全部半纤维素及少量木质素溶解并发生水解反应,重新定位大部分木质素分布,增大纤维素与酶的接触面积[17-18],提高酶解效率和糖收率,同时回收70%~90%半纤维素衍生糖。
2.1 半纤维素、木质素的溶解
半纤维素在纤维素的表面通过氢键纤维丝交联。半纤维素去除越多,纤维素酶解效果越好[19-21]。高温液态水可溶解全部半纤维素[22-23]。Mok等[15]采用不同种类的木本和草本植物在200~230 ℃的高温液态水中处理0~15min,半纤维素100%溶解,经后续处理得到90%的单糖。
在木质纤维素水解还原糖的研究中,对木质素单独的研究较少。当高温液态水的温度升至木质素熔溶温度后,它透过细胞壁并在细胞壁表面沉降下来[24],重新分布,同时少量木质素脱落,最后生成木质素衍生物[25],木质纤维素溶解越多,酶解糖收率越大[26-27]。
木质纤维素溶解最多的条件未必是最佳预处理条件。评价预处理的效果有两方面,即半纤维素糖收率与纤维素的可及度。目前,利用高温液态水预处理木质纤维素以获得最大总还原糖收率,预处理工艺及半纤维素水解机理是主要的研究方向。表1为不同木质纤维素原料在高温液态水中不同反应条件下的糖收率。
2.2 半纤维素水解的影响因素
2.2.1 木质纤维素种类
半纤维素是植物细胞壁中除纤维素以外的杂聚多糖的总称。不同种类木质纤维素中的半纤维素含量与组成不同,因此预处理效果也不同[35-36]。采用6种农业废物制取低聚木糖,其产量与半纤维素含量及半纤维素支链上的乙酰基有关,可能是因为乙酰基脱落生成的乙酸促进了半纤维素水解[37]。但有实验证明其无影响[38-39],甚至在去乙酰基的木质纤维素中加入过量乙酸(使用量超过脱掉的乙酰基量),其水解效果不如自然进料[40]。乙酰基脱落生成的乙酸对半纤维素的水解是否具有催化作用与木质纤维素的种类相关,需要进一步探讨半纤维素水解机理才能得出定论。
2.2.2 工艺条件
在反应温度、反应时间及反应压力3个参数中,反应温度对半纤维素水解影响最大[41];反应时间依赖于反应温度;反应压力不仅能使水在预处理温度下保持液态,而且能使木质纤维素的结构发生变化[26]。在高温液态水预处理木质纤维素过程中,通常采用Overend与Chornet推导的反应强度系数[42],即R0=t×描述反应温度与反应时间对半纤维素水解的共同影响[19]:半纤维素衍生糖收率是反应温度与反应时间共同作用的结果。反应温度升高,半纤维素水解速率加快;在同一反应温度下,随反应时间的延长,糖收率先升高后降低,即半纤维素首先水解生成寡糖,寡糖再生成单糖,单糖进一步降解成副产物[43],当半纤维素水解速率大于单糖降解速率时,糖收率升高,反之则降低。反应温度越高,反应时间越长,单糖降解的副产物越多[44]。在200~220 ℃的高温液态水中预处理某些草本及木本生物质0~10 min,即可得到最高半纤维素衍生糖收率[45]。固液比(1.0%~10.0%)对半纤维素水解糖收率没有影响[46];原料颗粒越小,与水接触的面积越大,半纤维素水解越容易,效果越好[47],但原料颗粒小到一定程度时,其粉碎作用相当于球磨,将减少半纤维素的含量[48]。
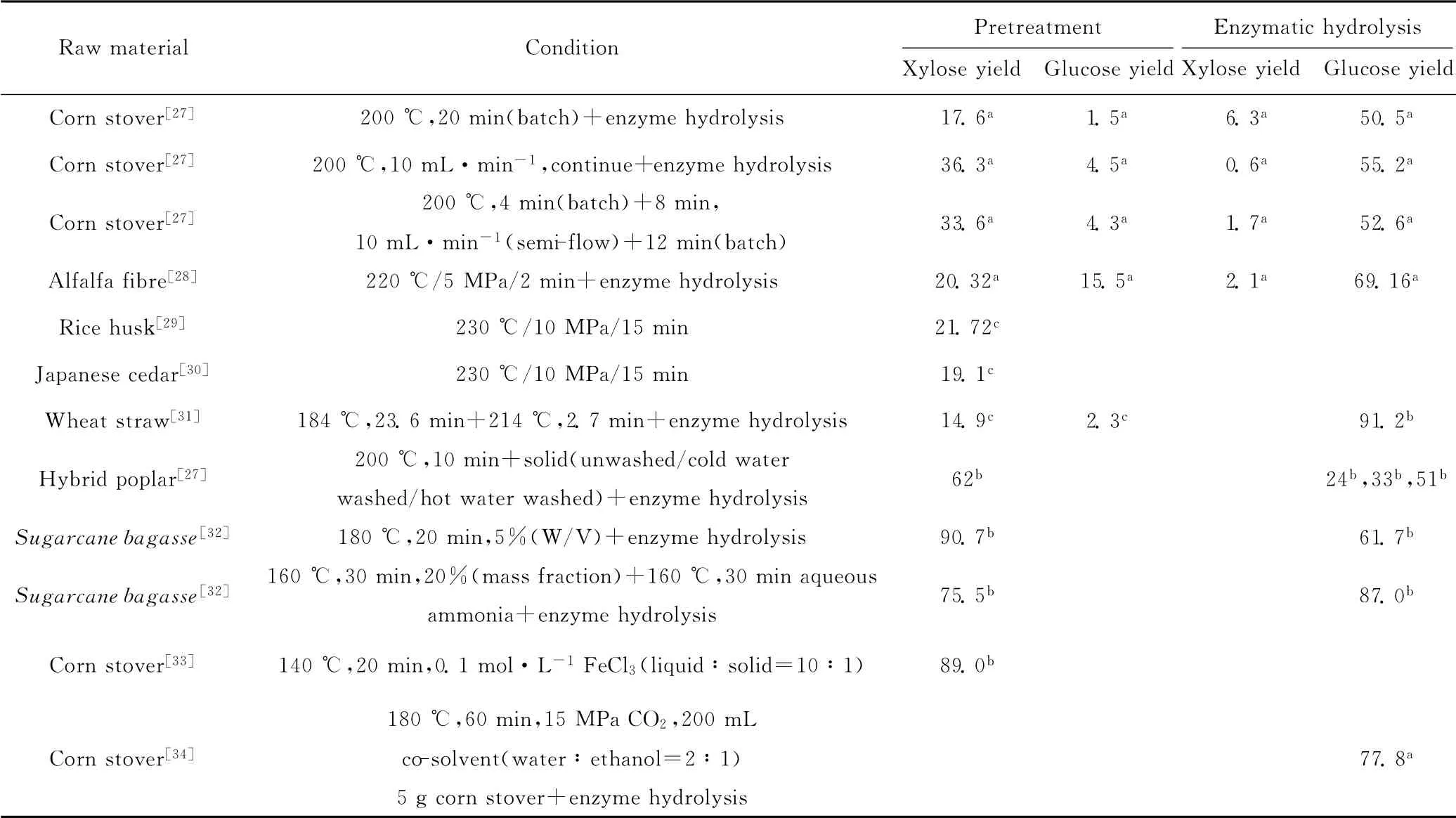
表1 不同木质纤维素原料在高温液态水中不同反应条件下的糖收率/%Tab.1 Sugar yields for different types of lignocellulose materials in hot liquid water under different reaction conditions/%
预处理常用设备有间歇釜式反应器与半连续釜式反应器。在间歇釜式反应器中,水解产物单糖在反应器内停留时间较长,降解副产物多,但水解液糖浓度高,可实现较大的固液比;在半连续釜式反应器中,流水的冲刷使水解液中木质纤维素表面的长链聚合物与水分子形成的“冰层”变薄,有利于水渗入到木质纤维素内部,加速水解反应进行,并且生成的寡糖与溶出的木质素被水带出反应器,减少单糖的降解,提高半纤维素衍生糖收率与酶解糖收率,但水解液糖浓度较小,用水量大,能耗高[39,49]。近年来,有学者采用半连续固定床反应器提高了原料固液比,有效地抑制水解产物的降解,降低能耗,且不需要对原料进行粉碎[50]。因此,研制出高效、节能的反应器型式是提高预处理效果、降低用水量及能耗的重要途径。
在半连续操作中,水的高速流动对半纤维素水解的促进作用只在反应初期有效而末期效果却不明显,因而Liu等[51]提出部分流动工艺:先在200 ℃下间歇反应4min,然后在连续流动中反应8min,最后再进行间歇反应12min。木糖收率84%~89%,纤维素的酶消化性88%~90%,木质素去除了40%~45%,总糖收率达到了90%~92%,比单纯采用连续流动操作节省了60%的用水量。在间歇操作过程中,最高半纤维素衍生糖收率与最高纤维素酶解糖收率的预处理条件不一致,因此采用两步法预处理:第一步水解获取最高半纤维素衍生糖收率,第二步使纤维素可及度最大,此时,所得木质纤维素总糖收率最高[25,31]。此外,将水pH 值控制在4~7 之间[26],可使半纤维素水解生成的糖以寡糖的形式存在,减少单糖的生成与降解,提高半纤维素衍生糖收率[52]。
2.2.3 水解过程强化
采用CO2加压的方式使水保持液相的同时形成碳酸,Savage等根据CO2-H2O 二元系统的热力学性质建立了预测富含CO2高温液态水的pH 值的模型。计算证明,添加的CO2可以使高温液态水的pH 值降低几个单位[53]。CO2可催化某些木质纤维素中半纤维素水解,显著提高木糖和呋喃的浓度,抑制水解液中有机酸的累积,泄压之后,CO2逸出,水的pH 值又可恢复正常[53-54]。采用富含高压CO2的液态水在较低温度下(105~110 ℃)预处理甘蔗渣,酶解糖收率与经高温预处理后的酶解糖收率相似[55];但是,CO2对山杨[56]和黑麦稻秆的水解[54]却没有效果,木糖的收率也未提高。此外,向富含CO2的高温液态水中加入少量乙醇,可溶解更多的木质素,酶解糖收率提高[34]。
木质纤维素的灰分中含有少量的Ca2+、K+、Mg2+、Na+、Al3+、Fe3+,含有这些阳离子的无机盐对木质纤维素的气化过程[57]及半纤维素水解有催化作用,对水解液产品具有选择性[33,58-59]。高温液态水联合其它预处理不仅可以处理高固液比原料[32],还可以减少后续水解纤维素的酶用量[60]。
2.3 半纤维素水解机理
半纤维素水解产物受反应器、工艺参数、添加剂以及木质纤维素本身的影响,而且水解产物复杂,除木(单)糖之外还有不同聚合度的低聚糖、有机酸及糠醛等[61]。高温液态水中的H+由水提供,并表现出稀酸催化作用,因此,通常把稀酸催化半纤维素水解的一阶连串动力学模型应用于高温液态水中,并采用阿伦尼乌斯方程预测动力学参数。最初的半纤维素动力学模型是以纤维素动力学模型为基础而建立的,如模型(1):
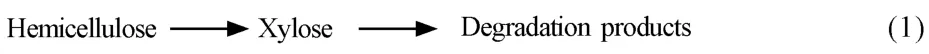
Kobayashi等[62]用稀酸水解硬木,发现半纤维素可以分为两部分水解:快速水解部分和慢速水解部分,快速水解部分约占30%。研究发现,在半纤维素向单糖转变过程中还存在寡糖,如模型(2)[46]:
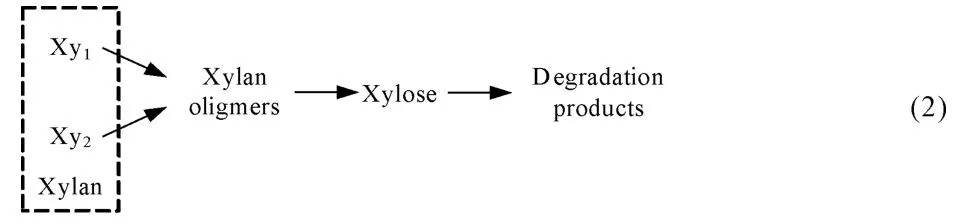
Nabarlatz等[63]将寡糖水解产物细化成3 种单体,即乙酸、木糖和阿拉伯糖,而后单糖再降解成糠醛等副产物,3种单体收率的理论计算值与实验值相符,但寡糖构成随时间与温度变化的理论计算值与实验值存在偏差,如模型(3):

余强等[64]认为半纤维素主要发生链间的断裂,产物以木聚糖为主,支链上的基团会脱落生成阿拉伯糖、乙酸和葡萄糖醛酸等,低聚木糖会进一步水解为木糖、小分子酸类,如甲酸是糠醛和乙醇酸的进一步降解产物,并将木聚糖和单糖放在一起考虑,如模型(4):
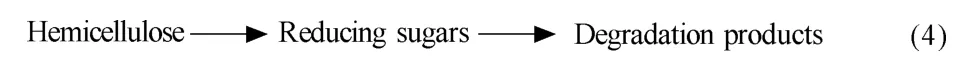
应用该模型预测水稻秆与棕榈秆的水解,糖产率的实验值与理论计算值较吻合[45]。不同原料、不同反应条件、不同模型的水解反应动力学参数如表2所示。
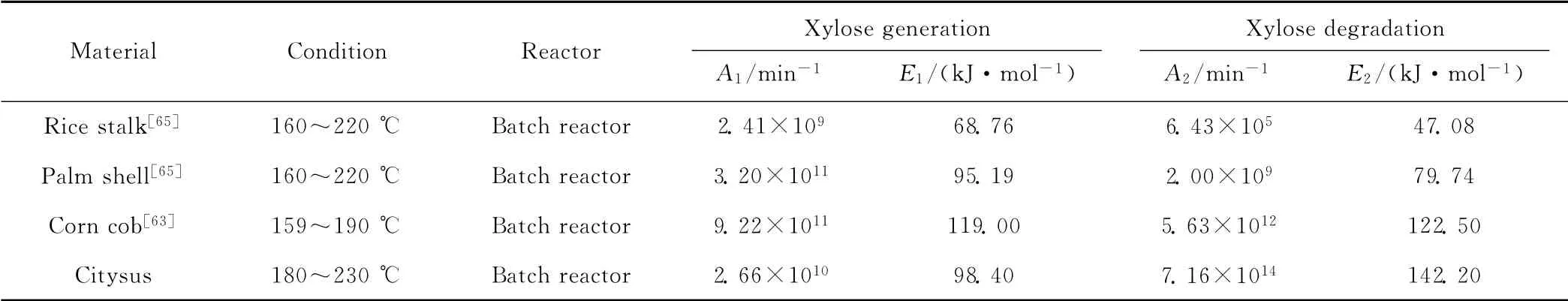
表2 不同木质纤维素原料半纤维素高温液态水水解反应动力学参数Tab.2 Kinetic parameters for hydrolysis of hemicellulose in hot liquid water with different lignocellulose materials
3 纤维素水解反应
在半纤维素水解的同时还有一部分纤维素——无定型纤维素与半纤维素、木质素同时水解[14,66]。研究表明,无定型纤维素在180℃开始水解,结晶纤维素则在>230 ℃水解[67-68]。在240~290 ℃时,纤维素分解成低聚物、单糖和降解产物[69]。在320~350 ℃时,纤维素的水解率与寡糖的降解率随温度升高而增大,纤维素水解速率小于葡萄糖和纤维二糖的分解速率,产品主要是葡萄糖降解产物;在350~400 ℃时,反应速率更快,400 ℃时反应速率比320~350 ℃时快两个数量级,此时,纤维素水解速率大于葡萄糖与纤维二糖的分解速率,产品是单糖与低聚糖[70]。
预处理的主要目标是溶解半纤维素和木质素,保留纤维素并最大限度地将其暴露出来。因此,预处理温度应低于结晶纤维素的水解温度(<230 ℃)。
4 结语
高温液态水预处理是一种高效、绿色的方法,增大了木质纤维素中纤维素与酶的接触面积,提高了酶水解效率及还原糖收率,且预处理过程中无需添加其它化学制剂。目前,预处理工艺存在水解液糖浓度较低、进料液固比较高、水解产物复杂、糖收率不高等问题。因此,应根据木质纤维素种类并结合酶水解效果共同研究合理的预处理工艺及反应器,提高预处理效果,并进一步研究半纤维素的水解机理,建立准确的动力学模型,从而指导和控制水解过程。
[1]赵岩,王洪涛,陆文静,等.秸秆超(亚)临界水预处理与水解技术[J].化学进展,2007,19(11):1832-1838.
[2]UEMATSU M,FRANK E U.Static dielectric constant of water and steam[J].Journal of Physical and Chemical Reference Data,1980,9(4):1291.
[3]TOOR S S,ROSENDAHL L,RUDOLF A.Hydrothermal liquefaction of biomass:A review of subcritical water technologies[J].Energy,2011,36(5):2328-2342.
[4]KRUSE A,DINJUS E.Hot compressed water as reaction medium and reactant[J].The Journal of Supercritical Fluids,2006,41(3):361-379.
[5]AKIYA N,SAVAGE P E.Roles of water for chemical reactions in high-temperature water[J].Chemical Reviews,2002,102(8):2725-2750.
[6]HERRERO M,CIFUENTES A,IBANEZ E.Sub-and supercritical fluid extraction of functional ingredients from different natural sources:Plants,food-by-products,algae and microalgae[J].Food Chemistry,2006,98(1):136-148.
[7]HAWTHORNE S B,YANG Y,MILLER D J.Extraction of organic pollutants from environmental solids with sub-and supercritical water[J].Analytical Chemistry,1994,66(18):2912-2920.
[8]BRUNNER G,MISCH B,FIRUS A,et al.Supercritical Water and Supercritical Carbon Dioxide for Cleaning of Soil Material[M]//Treatment of Contaminated Soil,Berlin:Springer-Veerlag:491-517.
[9]HODES M,MARRONE P A,HONG G T,et al.Salt precipitation and scale control in supercritical water oxidation—Part A:Fundamentals and research[J].The Journal of Supercritical Fluids,2004,29(3):265-288.
[10]MARSHALL W L,FRANCK E U.Ion product of water substance,0~1000 ℃,1~10000bars:New international formulation and its background[J].J Phys Chem Ref Data,1981,10(2):295-340.
[11]SAVAGE P E.Organic chemical reactions in supercritical water[J].Chemical Reviews,1999,99(2):603-621.
[12]刘慧屏,银建中,徐刚.超/亚临界水两步法水解玉米秸秆制备还原糖[J].化学与生物工程,2010,27(11):47-50.
[13]ANDO H,SAKARI T,KOBUSHO T,et al.Decomposition behavior of plant biomass in hot-compressed water[J].Industrial&Engineering Chemistry Research,2000,39(10):3688-3693.
[14]HASHAIKEH R,FANG Z,BUTLER I S,et al.Hydrothermal dissolution of willow in hot compressed water as a model for biomass conversion[J].Fuel,2006,86(10):1614-1622.
[15]MOK W S L,ANTAL M J.Uncatalyzed solvolysis of whole biomass hemicellulose by hot compressed liquid water[J].Industrial&Engineering Chemistry Research,1992,31(4):1157-1161.
[16]YIN J Z,HAO L D,YU W,et al.Enzymatic hydrolysis enhancement of corn lignocellulose by supercritical CO2combined with ultrasound pretreatment[J].Chinese Journal of Catalysis,2014,35:763-769.
[17]KRISTENSEN J B,THYGESEN L G,FELBY C,et al.Cell-wall structural changes in wheat straw pretreated for bioethanol production[J].Biotechnol Biofuels,2008,1(1):5.
[18]HANSEN M A T,KRISTENSEN J B,FELBY C,et al.Pretreatment and enzymatic hydrolysis of wheat straw(TriticumaestivumL.)—The impact of lignin relocation and plant tissues on enzymatic accessibility[J].Bioresource Technology,2010,102(3):2804-2811.
[19]YANG B,WYMAN C E.Effect of xylan and lignin removal by batch and flow through pretreatment on the enzymatic digestibility of corn stover cellulose[J].Biotechnology and Bioengineering,2004,86(1):88-98.
[20]ZENG M J,MOSIER N S,HUANG C P,et al.Microscopic examination of changes of plant cell structure in corn stover due to hot water pretreatment and enzymatic hydrolysis[J].Biotechnology and Bioengineering,2007,97(2):265-278.
[21]XIAO X,BIAN J,LI M F,et al.Enhanced enzymatic hydrolysis of bamboo(DendrocalamusgiganteusMunro)culm by hydrothermal pretreatment[J].Bioresource Technology,2014,159:41-47.
[22]LASER M,SCHULMAN D,ALLEN S G,et al.A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol[J].Bioresource Technology,2002,81(1):33-44.
[23]SASAKI M,ADSCHIRI T,ARAI K.Fractionation of sugarcane bagasse by hydrothermal treatment[J].Bioresource Technology,2002,86(3):301-304.
[24]DONOHOE B S,DECKER S R,TUCKER M P,et al.Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment[J].Biotechnology and Bioengineering,2008,101(5):913-925.
[25]YU Q,ZHUANG X S,YUAN Z H,et al.Two-step liquid hot water pretreatment ofEucalyptusgrandisto enhance sugar recovery and enzymatic digestibility of cellulose[J].Bioresource Technology,2009,101(13):4895-4899.
[26]ALVIRA P,TOMAS-PEJO E,BALLESTEROS M,et al.Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis:A review[J].Bioresource Technology,2009,101(13):4851-4861.
[27]KIM Y,MOSIER N S,LADISCH M R.Enzymatic digestion of liquid hot water pretreated hybrid poplar[J].Biotechnology Progress,2009,25(2):340-348.
[28]SREENATH H K,KOEGEL R G,MOLDES A B,et al.Enzymic saccharification of alfalfa fibre after liquid hot water pretreatment[J].Process Biochem,1999,35(1-2):33-41.
[29]PHAIBOONSILPA N,OGURA M,YAMAUCHI K,et al.Twostep hydrolysis of rice(Oryzasativa)husk as treated by semiflow hot-compressed water[J].Industrial Crops and Products,2013,49:484-491.
[30]PHAIBOONSILPA N,YAMAUCHI K,LU X,et al.Two-step hydrolysis of Japanese cedar as treated by semi-flow hot-compressed water[J].Journal of Wood Science,2010,56(4):331-338.
[31]PEREZ J A,BALLESTEROS I,BALLESTEROS M,et al.Optimizing liquid hot water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production[J].Fuel,2008,87(17):3640-3647.
[32]YU Q,ZHUANG X S,YUAN Z H,et al.Pretreatment of sugarcane bagasse with liquid hot water and aqueous ammonia[J].Bioresource Technology,2013,144:210-215.
[33]LIU L,SUN J S,CAI C Y,et al.Corn stover pretreatment by inorganic salts and its effects on hemicellulose and cellulose degradation[J].Bioresource Technology,2009,100(23):5865-5871.
[34]LÜ H S,REN M M,ZHANG M H,et al.Pretreatment of corn stover using supercritical CO2with water-ethanol as co-solvent[J].Chinese Journal of Chemical Engineering,2013,21(5):551-557.
[35]PHAIBOONSILPA N,SAKA S.Hydrolysis behaviors of lignocellulosics as treated by two-step semi-flow hot-compressed water[J].Cellulose,2012,48(44.8):p.31.3.
[36]IMMAN S,ARNTHONG J,BURAPATANA V,et al.Autohydrolysis of tropical agricultural residues by compressed liquid hot water pretreatment[J].Applied Biochemistry and Biotechnology,2013,170(8):1982-1995.
[37]NABARLATZ D,EBRINGEROVA A,MONTANE D.Autohydrolysis of agricultural by-products for the production of xylooligosaccharides[J].Carbohydrate Polymers,2006,69(1):20-28.
[38]LIU C,WYMAN C E.The effect of flow rate of compressed hot water on xylan,lignin,and total mass removal from corn stover[J].Industrial & Engineering Chemistry Research,2003,42(21):5409-5416.
[39]BOBLETER O,BONN G,PRUTSCH W.Steam explosion-hydro-thermolysis-organosolv.A comparison[C]//Steam Explosion Techniques.Gordon and Breach,Philadelphia,1991:59-82.
[40]STUHLER S L.Effects of solids concentration,acetylation,and transient heat transfer on uncatalyzed batch pretreatment of corn stover[D].Dartmouth College,2002.
[41]PEREZ J A,GONZALEZ A,OLIVA J M,et al.Effect of process variables on liquid hot water pretreatment of wheat straw for bioconversion to fuel-ethanol in a batch reactor[J].Journal of Chemical Technology and Biotechnology,2007,82(10):929-938.
[42]OVEREND R P,CHOMET E,GASCOIGNE J A.Fractionation of lignocellulosics by steam-aqueous pretreatments[J].Philosophical Transactions of the Royal Society of London A,1987,321(1561):523-536.
[43]LI X,CONVERSE A O,WYMAN C E.Characterization of molecular weight distribution of oligomers from autocatalyzed batch hydrolysis of xylan[J].Applied Biochemistry and Biotechnology,2003,107(1):515-522.
[44]ROGALINSKI T,INGRAM T,BRUNNER G.Hydrolysis of lignocellulosic biomass in water under elevated temperatures and pressures[J].The Journal of Supercritical Fluids,2008,47(1):54-63.
[45]ZHUANG X S,YUAN A H,MA L L,et al.Kinetic study of hydrolysis of xylan and agricultural wastes with hot liquid water[J].Biotechnology Advances,2009,27(5):578-582.
[46]JACOBSEN S E,WYMAN C E.Xylose monomer and oligomer yields for uncatalyzed hydrolysis of sugarcane bagasse hemicellulose at varying solids concentration[J].Industrial &Engineering Chemistry Research,2002,41(6):1454-1461.
[47]KROGELL J,KOROTKOVA E,ERANEN K,et al.Intensification of hemicellulose hot-water extraction from spruce wood in a batch extractor—Effects of wood particle size[J].Bioresource Technology,2013,143:212-220.
[48]TILLMAN L M,LEE Y Y,TORGET R.Effect of transient acid diffusion on pretreatment/hydrolysis of hardwood hemicellulose[J].Applied Biochemistry and Biotechnology,1990,24(1):103-113.
[49]LU X,YAMAUCHI K,PHAIBOONSILPA N,et al.Two-step hydrolysis of Japanese beech as treated by semi-flow hot-compressed water[J].Journal of Wood Science,2009,55(5):367-375.
[50]INGRAM T,ROGALINSKI T,BOCKEMUHL V,et al.Semicontinuous liquid hot water pretreatment of rye straw[J].The Journal of Supercritical Fluids,2008,48(3):238-246.
[51]LIU C G,WYMAN C E.Partial flow of compressed-hot water through corn stover to enhance hemicellulose sugar recovery and enzymatic digestibility of cellulose[J].Bioresource Technology,2005,96(18):1978-1985.
[52]MOSIER N,HENDRICKSON R,HO N,et al.Optimization of pH controlled liquid hot water pretreatment of corn stover[J].Bioresource Technology,2005,96(18):1986-1993.
[53]HUNTER S E,SAVAGE P.Quantifying rate enhancements for acid catalysis in CO2-enriched high-temperature water[J].AIChE Journal,2008,54(2)516-528.
[54]van WALSUM G P,SHI H.Carbonic acid enhancement of hydrolysis in aqueous pretreatment of corn stover[J].Bioresource Technology,2004,93(3):217-226.
[55]GURGEL L V A,PIMENTA M T B,da SILVA CURVELO A A.Enhancing liquid hot water(LHW)pretreatment of sugarcane bagasse by high pressure carbon dioxide(HP-CO2)[J].Industrial Crops and Products,2014,57:141-149.
[56]McWILLIAMS R C,van WALSUM G P.Comparison of aspen wood hydrolysates produced by pretreatment with liquid hot water and carbonic acid[J].Applied Biochemistry and Biotechnology,2002,98-100:109-121.
[57]VARHEGYI G,ANTAL M J,SZEKELY T,et al.Simultaneous thermogravimetric-mass spectrometric studies of the thermal decomposition of biopolymers.1.Avicel cellulose in the presence and absence of catalysts[J].Energy & Fuels,1988,2(3):267-272.
[58]LIU C G,WYMAN C E.The enhancement of xylose monomer and xylotriose degradation by inorganic salts in aqueous solutions at 180 ℃[J].Carbohydrate Research,2006,341(15):2550-2556.
[59]YU Q,ZHUANG X S,YUAN Z H,et al.The effect of metal salts on the decomposition of sweet sorghum bagasse in flowthrough liquid hot water[J].Bioresource Technology,2011,102(3):3445-3450.
[60]INOUE H,YANO S,ENDO T,et al.Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus[J].Biotechnology Biofuels,2008,1(1):2.
[61]金强,张红漫,严立石,等.生物质半纤维素稀酸水解反应[J].化学进展,2010,22(4):654-662.
[62]KOBAYASHI T,SAKAI Y B.Hydrolysis rate of pentosan of hardwood in dilute sulfuric acid[J].Agr Chem Soc Japan,1956,20(1):1-7.
[63]NABARLATZ D,FARRIOL X,MONTANE D.Kinetic modeling of the autohydrolysis of lignocellulosic biomass for the production of hemicellulose-derived oligosaccharides[J].Industrial&Engineering Chemistry Research,2004,43(15):4124-4131.
[64]余强,庄新姝,袁振宏,等.高温液态水中甜高粱渣半纤维素水解及其机理[J].化工学报,2012,63(2):599-605.
[65]徐明忠,庄新姝,袁振宏,等.农业废弃物高温液态水水解动力学[J].过程工程学报,2008,8(5):941-944.
[66]ALLEN S G,KAM L C,ZEMANN A J,et al.Fractionation of sugar cane with hot,compressed,liquid water[J].Industrial &Engineering Chemistry Research,1996,35(8):2709-2715.
[67]YU Y,WU H.Significant differences in the hydrolysis behavior of amorphous and crystalline portions within microcrystalline cellulose in hot-compressed water[J].Industrial & Engineering Chemistry Research,2010,49(8):3902-3909.
[68]YU Y,WU H.Evolution of primary liquid products and evidence of in situ structural changes in cellulose with conversion during hydrolysis in hot-compressed water[J].Industrial &Engineering Chemistry Research,2010,49(8):3919-3925.
[69]KAMIO E,SATO H,TAKAHASHI S,et al.Liquefaction of cellulose in hot compressed water under variable temperatures[J].Industrial & Engineering Chemistry Research,2006,45(14):4944-4953.
[70]SASAKI M,FANG Z,FUKUSHIMA Y,et al.Dissolution and hydrolysis of cellulose in subcritical and supercritical water[J].Industrial & Engineering Chemistry Research,2000,39(8):2883-2890.
