Preparation of Melamine Formaldehyde Resin Coated Red Phosphorus by Inverse Suspension Polymerization
2015-12-23ao
-, , ao-, -
(College of Chemistry and Chemical Engineering, Key Lab of Sustainable Resources Processing and Advanced Materials, Hunan Normal University, Changsha 410081, China)
Preparation of Melamine Formaldehyde Resin Coated Red Phosphorus by Inverse Suspension Polymerization
YUZhi-peng,WANGXi,ZHANGXiao-min,SUSheng-pei
(College of Chemistry and Chemical Engineering, Key Lab of Sustainable Resources Processing and Advanced Materials, Hunan Normal University, Changsha 410081, China)
AbstractMicroencapsulated red phosphorus(MRP) was prepared through inverse suspension polymerization of melamine and formaldehyde (MF) on the surface of red phosphorus (RP) powder, and its structure was characterized by Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS). The limiting oxygen index (LOI) for MRP composites with polypropylene (PP) or polyamide 6 (PA6) was measured. The thermal property of microencapsulated red phosphorus (MRP) was evaluated and the fire retardant efficiency was tested in PP and PA6 matrix. Experimental results from IR, SEM and XPS confirmed the coating of MF resin onto the surface of RP powder. In the flame retardancy of PA6 and PP, the experimental results showed that the flame retarded PA6 had the LOI>28.2% when the loading of microencapsulated red phosphorus was 15wt%, and the flame retarded PP had the LOI>22.4% when the loading of microencapsulated red phosphorus was 15wt%.
Key wordsinverse suspension polymerization; melamine formaldehyde resin; microencapsulated coating; red phosphorus
Red phosphorus (RP) has become one of the efficient, ecological and the most harmless additives for fire retardant polymers[1]. As a fire retardant additive, the phosphorus is required to be in fine particle size to have less negative effect on the mechanical properties of fire retardant polymer composites. However, phosphorus with very fine particle is highly flammable especially at elevated temperature in the presence of moisture and atmospheric oxygen. In addition, surface-modification is also required to improve the interfacial interactions between polymer matrix and phosphorus. The most widely used technique is inorganic or polymeric microencapsulation[2-9]. In polymeric microencapsulation, the thermosetting resins including epoxy resin, melamine formaldehyde, urea formaldehyde and phenolic formaldehyde resins were preferably used[10-12]. Typically, phosphorus powders were suspended in a polymeric aqueous solution at higher temperature; once the temperature was cooled down, suspended phosphorus particles were coated with the precipitated polymers. The encapsulated phosphorus was obtained after filtration. In this process, a large quantity of water with dissolved polymers needs to be treated[13]. In this study, inverse suspension polymerization method was applied in the preparation of melamine formaldehyde resin coated red phosphorus. Higher coating efficiency of polymers and lower waste water were expected in this method.
1Experimental
1.1Materials
Red phosphorus powder with a particle size of about 600 mesh was provided by Luxi Songgong Hi-Tech Co., Ltd (China); Soybean oil was provided by Xinsha cereals and oils Co., Ltd (China); Melamine and formaldehyde were purchased from Tianjin Hengxing reagent Co., Ltd (China). Chemicals including Span 80, acetic acid, ammonia aqueous solution and ethyl acetate were purchased from Tianjin Fengchuan chemical reagent Technology Co., Ltd (China); PA6 was purchased from Yueyang Petrol-Chemical Co., Ltd (China); PP was purchased from Chinese Petrol-Chemical Co., Ltd (China).
1.2Microencapsulation of RP
For a typical encapsulation process, 6.4 g of melamine and 3.8 g of paraformaldehyde was dispersed in 150 mL of water and was magnetically stirred at 85 ℃ for 0.5h until a clear solution was formed while the pH of the mixture was adjusted to 8~9 with aqueous ammonia. And then 40 g of red phosphorus was added into the mixture. After kept stirring for another 20 min, a well dispersed mixture was obtained. Meanwhile, 450 mL of soybean oil and 2 g of Span 80 were stirred at 85 ℃ for 10 min. Then, the mixture of phosphorus was added dropwise into the mixture of soybean oil. And then the red phosphorus/melamine formaldehyde/soybean oil mixture was kept stirring for another 30 min to obtain a stable dispersion while the temperature was kept at 85 ℃. The pH of the dispersion was then adjusted to 4~5 with glacial acetic acid and the mixture was kept stirring at a constant temperature of 85 ℃ for another 2 h. After cooled to room temperature, the mixture was filtered, and the solid was washed three times with ethyl acetate, then the microencapsulated red phosphorus (MRP) was obtained after dried to constant weight in vacuum at 75 ℃.
1.3Preparation of PP/MRP and PA6/MRP composites
The PP/MRP and PA6/MRP composites were prepared by the melt blending method respectively. PP (PA6) and MRP at a loading of 15 wt% were premixed in a high-speed mixer, and then the mixture was extruded by a twin-screw extruder. Finally, the bars for LOI testing were prepared by injection molding in a size of 100 mm×30 mm× 6 mm.
1.4Characterization
1.4.1Ignition point and water absorption[14]One gram of pristine RP or microencapsulated RP sample was placed into a 10 mL porcelain crucible, then put into an electric furnace and heated to observe ignition point with a heating rate of 2 ℃/min.
Two grams of RP or microencapsulated RP sample were placed in a thermo-hydrostatic chamber at a constant temperature of 65 ℃ and a relative humidity of 95%. The samples were weighed before and after placed in the chamber for 5 days to calculate the percentage of increasing weight.
1.4.2Recovery of MFThe recovery of MF(Y) was calculated using Eq 1 as follows:
Y=(m1-m2)/m3
(1)
whereYis the recovery of MF,m1is the mass of MRP,m2is the mass of red phosphorus, andm3is the mass of MF.
1.4.3FT-IR and XPS spectroscopyFT-IR spectra were recorded in the range from 4 000 to 400 cm-1with 50 scans and at a resolution of 4 cm-1using KBr background at room temperature on Perkin-Elmer System 2000 FT-IR spectrometer. XPS (X-ray photoelectron spectroscopy) data was obtained with a K-Alpha 1063 electron spectrometer from VG Scientific using 72 W Al Kαradiation. The base pressure was about 10-9mbar.
1.4.4SEM microscopySurface morphology of pristine RP and microencapsulated RP were examined by scanning electron microscopy (SEM) after coated with gold. SEM micrographs were taken on a HITACH TM3030 field emission scanning electron microscopy at an operating voltage of 12 kV.
1.4.5The limiting oxygen index (LOI)The LOI measurements were carried out on an oxygen index instrument according to GB 2406-80.
2Results and discussion
2.1Chemical composition analyzed by IR and XPS
A typical FT-IR spectrum of the product from this process was shown in Fig.1. The absorption bands at 1 556, 1 489, 1 351 and 813 cm-1were attributed to the vibrations of melamine rings and indicated the presence of melamine formaldehyde resin[15].
To confirm that the polymer resin was coated on the surface of phosphorus, XPS was applied to analyze the elements in the samples. XPS spectra obtained from RP and MRP were shown in Fig.2. The peaks at 188.0 eV and 129.0 eV were assigned to P(2s) and P(2p) binding energy of phosphorus atom; the peak at 532.3 eV was assigned to O(1s) binding energy; the peak at 399.1 eV was assigned to the N(1s) binding energy; the peak at 284.0 eV was assigned to C(1s) binding energy. It was obvious that there were peaks at 284.0 eV, 399.1 eV and 532.0 eV while there was no peak at 188.0 eV and 129.0 eV in the spectrum of MRP, indicating the surface of red phosphorus was well coated by the melamine-formaldehyde resin.

Fig.1 FT-IR spectrum obtained from MRP

Fig.2 XPS spectra obtained from: (a)RP; (b)MRP
2.2Morphology characterization by SEM
In this work, SEM was used to observe the surfaces of pristine RP and MRP. It was obvious that the surface of RP was smooth while the surface of MRP was rough and uneven, indicating the achievement of polymer coatings on the surface of red phosphorus[16].
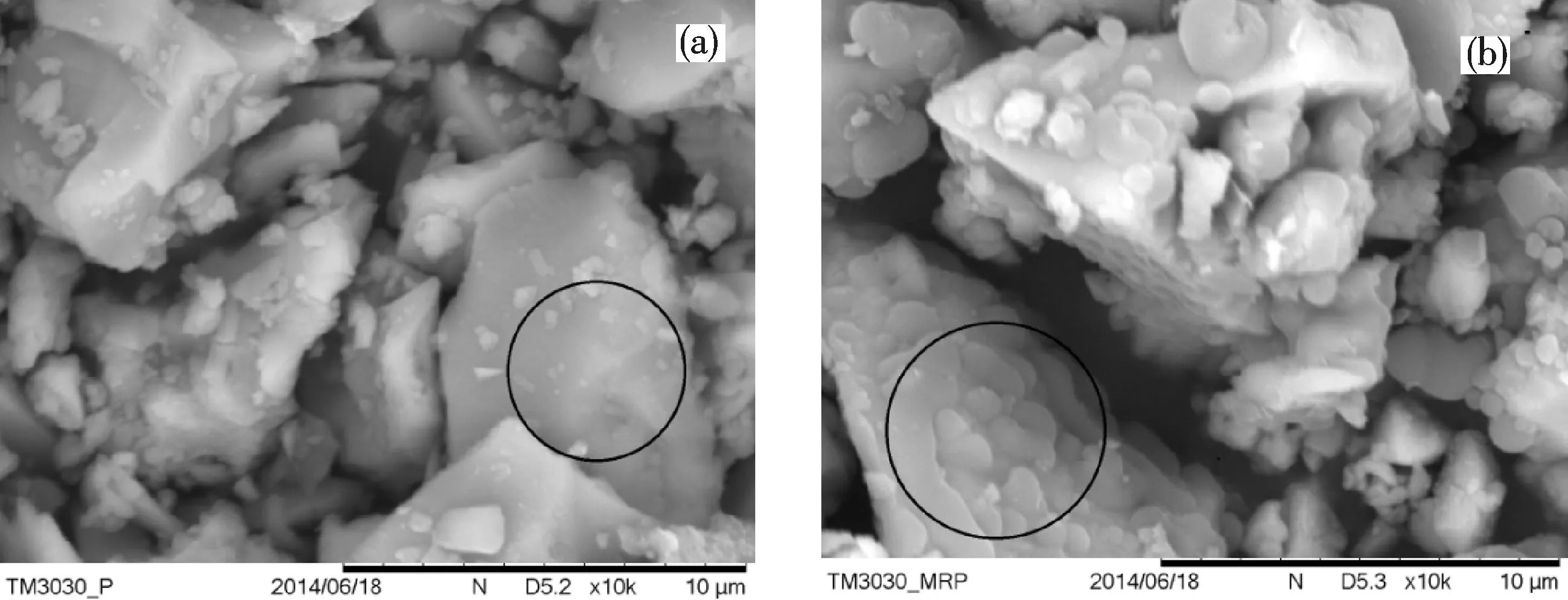
Fig.3 SEM images obtained from: (a)RP; (b)MRP
2.3Water absorption and ignition point
The water absorption and ignition point of MRP are directly related to the surface coverage of microcapsules, the pristine RP is easily ignited and the oxidized surface of phosphorus is the main cause of water absorption. The data for water absorption and ignition point of RP and MRP were listed in Tab.1. MRP exhibited lower water absorption and higher ignition point compared to that of RP which indicated that the surface of MRP was well coated[15].
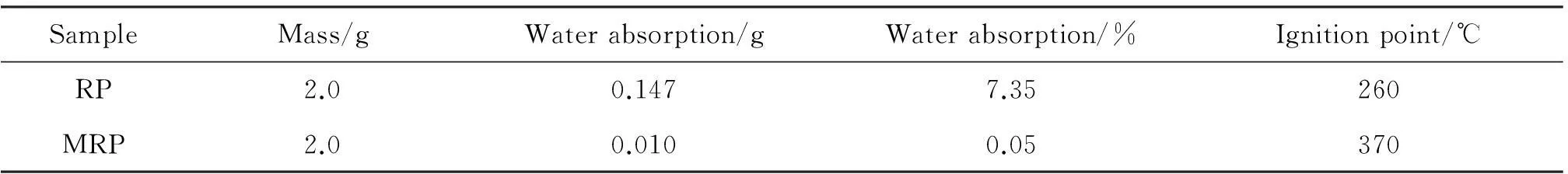
Tab.1 Water absorption and ignition point of pristine RP and MRP
2.4The recovery of MF prepared by in situ polymerization and inverse suspension polymerization
In a typical industrial process, waste treatment and costing were big issues. The encapsulation rates of MF on RP prepared by in situ polymerization[2]and inverse suspension polymerization method were compared, and the experiment data on the recovery of MF from MRP prepared by in situ polymerization and inverse suspension polymerization were 73% and 85%, respectively. This data indicated that fewer polymers were wasted in the inverse suspension polymerization, and only one third of water was used in the inverse suspension polymerization.
2.5The fire retardant efficiency of microencapsulated RP
To evaluate the flame retardant efficiency of the microencapsulated RP, PA6 and PP were selected as the polymers for MRP polymer composites and LOI was selected as measurement; the LOI results of composites were reported in Tab.2. The experimental results showed that the LOI data for PA6 and PP MRP composites are 28.2 and 22.4, respectively when the loading of microencapsulated red phosphorus was at 15 wt%. These data are very similar to that reported in reference [17].

Tab.2 Effect of different loading of MRP on the limiting oxygen index (LOI) of PP/MRP and PA6/MRP composites
3Conclusions
Melamine resin coated red phosphorus was obtained for the first time by inverse suspension polymerization. Experimental data from FT-IR, SEM, XPS and LOI for PP/MRP or PA6/MRP composites indicated the well-coated red phosphorus was obtained and this coated red phosphorus has high flame retardant efficiency in PP and PA6. Based on the evidences from experiments the treatment and application of melamine resin coated red phosphorus in this work was a promising project in the industrial production of coated red phosphorus due to cost-saving and environment benefits.
References:
[1]BRAUN U, SCHARTEL B. Effect of Red Phosphorus and Melamine Polyphosphate on the Fire Behavior of HIPS[J]. J Fire Sci, 2005,23(1):5-30.
[2]LIU Y, WANG Q. Melamine cyanurate-microencapsulated red phosphorus flame retardant unreinforced and glass fiber reinforced polyamide 66[J]. Polym Degrad Stab, 2006,91(12):3103-3109.
[3]RONALD L H, DALE E W, COLIN E D. Microencapsulated epoxy adhesive system: US 4536524[P].1985-08-20.
[4]MATSUO S, USAMI I, KURIHARA M,etal. Adhensive containing microcapsules: EP 0543675A1[P].1993-05-26.
[5]YUAN L, LIANG G Z, XIE J Q,etal. Preparation and characterization of poly(urea-formaldehyde) microcapsules filled with epoxy resins[J]. Polymer, 2006,47(15):5338-5349.
[6]YIN T, RONG M Z, ZHANG M Q,etal. Self-healing epoxy composites Preparation and effect of the healant consisting of microencapsulated epoxy and latent curing agent[J]. Compos Sci Technol, 2007,67(2):201-212.
[7]COSCO S, AMBROGL V, MUSTO P,etal. Properties of poly(urea-formaldehyde) microcapsules containing an epoxy resin[J]. J Appl Polym Sci, 2007,105(3):1400-1411.
[8]XIAO D S, RONG M Z, ZHANG M Q. A novel method for preparing epoxy-containing microcapsules via UV irradiation-induced interfacial copolymerization in emulsions[J]. Polymer, 2007,48(16):4765-4776.
[9]谢建强,梁国正,袁莉,等.聚苯乙烯包覆环氧树脂微胶囊的研制[J].塑料工业, 2007,35(3):64-67.
[10]PECHT M, DENG Y L. Electronic device encapsulation using red phosphorus flame retardants[J]. Microelectron Reliab, 2006,46(1):53-62.
[11]WANG L J, HE X J, LU D H,etal. Flame retardancy of polypropylene (nano)composites containing LDH and zinc borate[J]. Polymers for advanced technologies, 2011,22(7):1131-1138.
[12]JOU W S, CHEN K N, CHAO D Y,etal. Flame retardant and dielectric properties of glass fibre reinforced nylon-66 filled with red phosphorous[J]. Polym Degrad Stabil, 2001,74(2):239-245.
[13]LIU Y, WANG Q. Melamine cyan rate-microencapsulated red phosphorus flame retardant unreinforced and glass fiber reinforced polyamide66[J]. Polym Degrad Stab, 2006,91(12):3103-3109.
[14]WU Q, LU J P, QU B J. Preparation and characterization of micro capsulated red phosphorus and its flame-retardant mechanism in halogen-free flame retardant polyolefin[J]. Polym Int, 2003,52(8):1326-1331.
[15]WANG H T, MENG X F, WEN B,etal. A simple route for the preparation of red phosphorus microcapsule with fine particle distribution[J]. Mater Lett, 2008,62(21-22):3745-3747.
[16]CHANG S K, ZENG C, YUAN W Z, REN J. Preparation and characterization of double-layerde microencapsulated red phosphorus and its flame retardance in poly(lactic acid)[J]. J Appl Polym Sci, 2012,125(4):3014-3022.
[17] LIU Y, WANG Q. Preparation of microencapsulated red phosphorus through melamine cyanurate self-assembly and its performance in flame retardant polyamide 6[J]. Polym Engineering Sci, 2006,46(11):1548-1553.
(编辑杨春明)
反相悬浮聚合法制备三聚氰胺甲醛树脂包覆红磷
于智鹏,王曦,张效敏,苏胜培*
(湖南师范大学化学化工学院 资源精细化与先进材料重点实验室,中国 长沙 410081)
摘要通过反相悬浮聚合法制备了三聚氰胺甲醛(MF)树脂微胶囊包覆红磷,采用红外光谱(FI-IR)、扫描电子显微镜(SEM)、X-射线光电子能谱(XPS)对其结构进行了表征,并对微胶囊红磷与聚丙烯(PP)及尼龙6(PA6)形成的复合物的极限氧指数进行了分析,评价了微胶囊红磷的热性能,测试了其在PP及PA6复合物中的阻燃效率.实验结果表明,三聚氰胺甲醛树脂被成功包覆在红磷颗粒的表面.当微胶囊红磷的添加质量分数为15%时,PA6/MRP的极限氧指数LOI大于28.2%,而PP/MRP的极限氧指数LOI大于22.4%.
关键词反相悬浮聚合; 三聚氰胺甲醛树脂;微胶囊包覆;红磷
中图分类号O641
文献标识码A
文章编号1000-2537(2015)05-0059-05
通讯作者*,E-mail:sushengpei@yahoo.com
基金项目:湖南师范大学潇湘学者启动资金资助课题(化050613)
收稿日期:2015-03-04
DOI:10.7612/j.issn.1000-2537.2015.05.010
猜你喜欢
杂志排行
湖南师范大学自然科学学报的其它文章
- 五强溪太湖新银鱼春群和秋群生长特性比较
- Studies of Iron-Porphyrin Complexes with Different Axial Ligands by Both Spin-Restrict and Spin-Polarized Density Functional Reactivity Theory
- One-Step Synthesis of Nano ZnO Decorated Macroporous Carbon with Enhanced Photocatalytic Properties
- 基于空间句法的城市交通空间形态研究——以长沙大学城为例
- 基于显示管理重载集的C++静态分派研究
- 重要启事
