Effect of subarachnoid nerve block anesthesia on glutamate transporter GLAST and GLT-1 expressions in rabbits
2015-12-23KeQingXiaoMeiXiaoLiMengXiangYangDuJingHuBaoFengGaoWenQiangYuXinJieWangYanLinBan
Ke-Qing Xiao, Mei Xiao, Li Meng, Xiang-Yang Du, Jing Hu, Bao-Feng Gao, Wen-Qiang Yu, Xin-Jie Wang, Yan-Lin Ban
1Department of Anesthesiology, Shandong Provincial Hospital, Jinan, Shandong 250031, China
2Division of Preventive Medicine, Third People's Hospital of Pingdu city , Qingdao, Shandong 266753, China
3Shandong Province Chest Hospital, Department of Respiratory Diseases of Jinan city , Shandong 250 013, China
4Department of Anesthesiology, Qianfoshan Hospital affiliated to Shandong University in Shandong Province, Jinan, Shandong 250014, China
Effect of subarachnoid nerve block anesthesia on glutamate transporter GLAST and GLT-1 expressions in rabbits
Ke-Qing Xiao1*, Mei Xiao2, Li Meng1, Xiang-Yang Du1, Jing Hu1, Bao-Feng Gao1, Wen-Qiang Yu1, Xin-Jie Wang3, Yan-Lin Ban4
1Department of Anesthesiology, Shandong Provincial Hospital, Jinan, Shandong 250031, China
2Division of Preventive Medicine, Third People's Hospital of Pingdu city , Qingdao, Shandong 266753, China
3Shandong Province Chest Hospital, Department of Respiratory Diseases of Jinan city , Shandong 250 013, China
4Department of Anesthesiology, Qianfoshan Hospital affiliated to Shandong University in Shandong Province, Jinan, Shandong 250014, China
ARTICLE INFO
Article history:
Received 15 April 2015
Received in revised form 20 May 2015
Accepted 15 June 2015
Available online 20 July 2015
Subarachnoid nerve block anesthesia
Glutamate transporter
GLAST
GLT-1
Objective: To observe the effect of subarachnoid nerve block anesthesia on glutamate transporter glutamate-aspartate transporter (GLAST) and GLT-1 expressions in rabbits, and to investigate the effect of peripheral nerve anesthesia on the morphology and function of the spinal cord. Methods: Twenty healthy New Zealand white rabbits were randomly divided into two groups: the experimental group and control group; with 10 rabbits in each group. For spinal nerve anesthesia, 5 g/L of bupivacaine was used in the experimental group, and sterile saline was used in the control group. After 30 min of cardiac perfusion, GLAST and GLT-1 protein expression in spinal neurons were detected by immunohistochemistry and immunofluorescence staining. Results: GLAST and GLT-1 protein-positive cells increased in neurons in the experimental group, compared with the control group (P<0.05). Conclusions: After subarachnoid nerve block anesthesia, rabbit glutamate transporter GLAST and GLT-1 expression is increased; and spinal cord nerve cell function is inhibited.
1. Introduction
Subarachnoid nerve block anesthesia (lumbar anesthesia) is an anesthetic widely used in clinical practice. This method is simple, provides reliable results, and has few side effects. However, its effect and mechanism in blocking nerve cells remains unclear. Amino acid neurotransmitters are important neurotransmitters in the central nervous system of mammals, and the most important excitatory neurotransmitters are glutamate (Glu) and aspartate (Asp)[1]. When the nerve is excited, glutamate concentrations in the synaptic cleft elevate and act on the postsynaptic membrane of the glutamate receptor; and glutamate in the synaptic cleft could be uptakeby glutamate transporters to terminate its efficacy of synaptic transmission. Glutamate transporters accurately regulates glutamate to ensure proper nerve excitatory synaptic transmission[2,3]. The current study shows that glutamate transporters is related to various brain and spinal pathological processes and play an important role in the process of glutamate transporters[4,5]. In the present study, New Zealand white rabbits were used in preparing the spinal anesthesia model to observe the expressions of astrocytes and microglial cells in the two types of glutamate transporters, Glutamate-aspartate transporter (GLAST) and GLT-1, after anesthesia and to investigate the potential regulatory mechanisms of anesthesia in spinal nerve cell function for guidance in clinical practice.
2. Materials and methods
2.1. Animals and groupings
Twenty male 6-8 month New Zealand white rabbits, weighing 2.5-3.5 kg, were provided by the Experimental Animal Center, Zhongnan Hospital of Wuhan University.
2.2. Rabbit subarachnoid nerve block anesthesia
Rabbits were injected with 30 mg/kg sodium pentobarbital (20 g/ L) from the ear. Rabbits were fixed in a prone position and a needle was inserted into the subarachnoid space at the side edges of the L6-L7 spinous process. After the needle was secured, 0.8 mL of 0.5% bupivacaine (Shanghai Harvest Pharmaceutical Co., Ltd.) was injected in rabbits in the experimental group, while saline was injected in rabbits in the control group.
2.3. Prepared sections after routine perfusion
After 30 minutes of anesthesia, rabbits were fixed on backand the chest was opened. After exposing the heart, the left ventricle was punctured from the apex with a syringe and then fixed with hemostatic forceps, cut a small opening in the right atrium for bloodletting, 500 mL normal saline was injected into the left ventricle until the right ventricle outflow of transparent liquid, reperfusion in 1L of 4% paraformaldehyde, and the L6-L7 spinal cord segments were cut and obtained. Then, corresponding segments of the spinal cord were taken out from rabbits in the normal control group as described above.
2.4. HE staining and observation
The L6-L7 spinal cord segment was obtained, embedded in paraffin, cross-section sliced with a thickness of about 5 μm, HE-stained, and the form of soft meninges of the spinal cord segments were observed under a microscope.
2.5. Immunohistochemical staining and analysis
L6-L7 spinal cord segments were placed and fixed in 4% paraformaldehyde for three hours, and dehydrated in 30% sucrose solution overnight. After tissue masses were sunk to the bottom, a transverse plane was made by frozen section machine, and sliced with a thickness of about 6-7 μm. Slices were placed in 3% hydrogen peroxide for 15 minutes to inactivate endogenous peroxidase, and washed three times with 1× PBS. Segments were divided into three groups: experimental group and control group. Segments were separately added in anti-GLAST (1:500, Beijing Bioss company) and GLT-1 (1:400, CST company), and incubated at 4 ℃ overnight; then, shaking and rinsed three times, every 5 minutes. Added second antibody (1:200, Beijing ZSGB-Biotechnology Co., Ltd.), incubated at 37 ℃ for 30 minutes, and washed three times with 1 × PBS. Added SABC and incubated at 37 ℃ for 20 minutes. Then, DAB stained after washing with PBS. Dehydrated by 70%, 90%, 100% alcohol, Xylene Ⅰ,Ⅱ, Ⅲ transparent medium, mounted with neutral gum, and dried. Positive cell with brown granules were microscopically observed, and each section was randomly observed by a five high power field film. Image-Pro plus 6.0 software was used to analyze the integrated optical density (IOD) value of the positive expression; wherein, the higher the IOD value, the stronger the protein expression.
2.6. Immunofluorescence staining and analysis of astrocytes and microglial cells
After sections were stained with blocking serum, different primary and secondary antibodies were added: astrocytes were stained with GFAP antibody (1:100, Sigma Company) and microglial cells were stained with OX-42 antibody (1:100, AbD Serotec company). At the same time, sections were separately stained with GLAST (1:100, CST company) and GLT-1 (1:100, CST company) antibodies to detect GLAST and GLT-1 expressions in the two cells. Fluorescence microscopy film (Olympus, Japan) was used to take a photograph and Image-pro plus 6.0 software were used to analyze the double stained positive area of the region and double positive area (DPA), with its average value as the measurement value.
2.7. Statistical analysis
Data were measured using SPSS 13.0 software and were represented as mean ± standard deviation, groups were compared using t test, and P<0.05 was considered as significant difference.
3. Results
3.1. Observation of rabbit spinal pia mater morphology
Spinal cord sections in the experimental and control groups were detected by HE staining, and the morphology of the spinal pia mater was observed, as shown in Figure 1. Compared with the control group, the spinal pia mater in the experimental group had some stratificationand slight edema.
3.2. Influence of spinal nerve block anesthesia in GLAST and GLT-1 expressions in spinal cord neurons
In the normal control group, immunohistochemical assay revealed that GLAST and GLT-1 were mainly expressed in the gray matter in the transverse section of the spinal cord. Brown particle density was evenly distributed, deeply stained, and the nucleolus was concentrated dark brown yellow. In the experimental group, GLAST and GLT-1 expressions significantly increased (P<0.05), as shown in Table 1 and Figure 2.
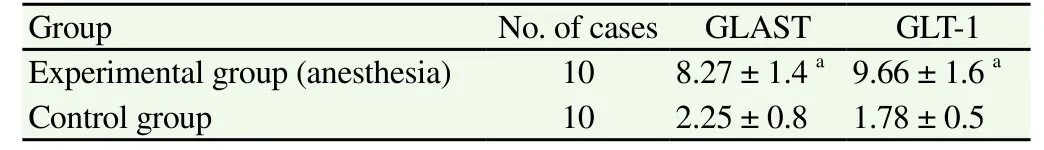
Table 1 Immunohistochemistry results of GLAST and GLT-1 in spinal cord neurons of rabbits (IOD, ××104, mean±sd).
3.3. Comparison of GLAST and GLT-1 expressions in astrocytes and microglial cells
GLAST and GLT-1 expressions in astrocytes and microglial cells were detected using immunofluorescence double staining method, as shown in Figure 3. Results revealed that the GLAST and GFAP double labeled positive area in the transverse section in the spinal cord between the experimental group and control group had no significant changes. However, GLT-1 and GFAP double positive areas significantly increased (P<0.05); namely, GLT-1 protein expression was upregulated and GLAST expression remained unchanged in astrocytes in the anesthesia group. In microglial cells, GLAST and OX-42 double positive area expressions was upregulated in the experimental group, compared with the control group; and there was a statistical difference (P<0.05). Further, the double positive area of GLT-1 and OX-42 significantly increased. Results revealed that GLAST and GLT-1 protein expressions in microglial cells increased in the anesthesia group.
4. Discussion
Glutamate is one of the most important neurotransmitters in the nervous system, and glutamate concentration in the synaptic cleft is precisely regulated mainly by the glutamate transporter. Under normal physiological conditions, glutamate is mainly distributed in the presynaptic vesicle area; and when the presynaptic membrane is released into the synaptic cleft excited due to depolarization, it plays a role by acting on postsynaptic membrane receptors. When glutamate is released into the synaptic cleft, it is necessary to be promptly removed to ensure the normal conduction of excitement[6,7]. Glutamate uptake mainly rely on it location in neurons, and high affinity glial cell membranes and force glutamate transporters. Glutamate transporters are mainly divided into five subtypes: excitatory amino acid transporter-1 (EAAT-1) (GLAST), EAAT2, E AAT3, E AAT4 and EAAT5[8,9]. Among them, GLAST and GLT-l are mainly expressed in astrocytes and microglia; and approximately 80%-90% of the total amount of their glutamate transporter are transported[10,11]. GLAST, also known as EAAT-1, is mainly expressed in the mammalian brains and spinal cord astrocytes and microglial cells. Some studies on surface excitatory amino acids, particularly glutamate, plays a key role in the central sensitization process, particularly in pain[12,13]; and its mechanism in the synaptic cleft of the spinal cord is to regulate the large number of glutamate accumulation. When normal rats were intrathecally injected with glutamate transporter antagonists, rats screamed, trembled, showed behavioral disorders, as well as other pain responses in a dosedependent manner[14]. Overall, glutamate transporters, with respect to excitatory amino acid circulation, are particularly important for terminating excitatory signals and protecting nerve cells.
Currently, the effect and mechanism of nerve blocks produced by anesthetics in nerve cells remains unclear; and there are some studies on the surface of local anesthetics for blocking nerve conduction through sodium channel inactivation. All five kinds of glutamate transporters are Na+dependent. However, specifically, after intrathecal nerve block anesthesia, whether it can affect nerve cellular functions and glial transporter expressions remains unclear. After intrathecal nerve block anesthesia, by immunohistochemistry and immunofluorescence, we found that GLAST and GLT-1expressions in microglial cells significantly increased; and in astrocytes, GLAST expressions did not have any significant change, while GLT-1 expressions were upregulated. These results suggest that intrathecal nerve block anesthesia could regulate glutamate transporter GLAST and GLT-1 expressions, upregulating these expressions and promoting synaptic cleft glutamate uptake, while promptly reducing glutamate acting on glutamate receptors; thereby, inhibiting the activation of nerve conduction and pain pathways. Anesthesia should be a safe and reversible process. In experiments, we found that after spinal anesthesia, there was a slight stratification in rabbit spinal cord meninges and phenomenon of edema. This prompted for strict control of anesthetic quality and concentration in clinical applications to avoid soft meninges caused by vasodilation and edema after anesthetics act on the spinal pia mater.
Results of this present study revealed that intrathecal nerve block anesthesia could promote the upregulation of transporter GLAST and GLT-1 expressions, and may contribute a role of avoiding excessive glutamate uptake in glutamate receptors; thereby, inhibiting nerve conduction. In this process, whether there is a corresponding cytokine or other regulatory molecules remains unclear. Studies have revealed that some pathological processes of the ERK1/2 signaling pathway after spinal cord injury could affect the expression of glutamate transporters[15]. Further research is needed to determine how GLAST and GLT-1 transporters are regulated.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Pinto MC, Lima IV, Da CF, Rosa DV, Mendes-Goulart VA, Resende RR, et al. Glycine transporters type 1 inhibitor promotes brain preconditioning against NMDA-induced excitotoxicity. Neuropharmacology 2015; 89: 274-281.
[2] Krzyzanowska W, Pomierny B, Filip M, Pera J. Glutamate transporters in brain ischemia: to modulate or not? Acta Pharmacol Sin 2014; 35(4): 444-462.
[3] Verdon G, Oh S, Serio RN, Boudker O. Coupled ion binding and structural transitions along the transport cycle of glutamate transporters. Elife 2014; 3: e2283.
[4] Zuidema JM, Hyzinski-Garcia MC, Van Vlasselaer K, Zaccor NW, Plopper GE, Mongin AA, et al. Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-L-lactic acid fibers. Biomaterials 2014; 35(5): 1439-1449.
[5] Grewer C, Gameiro A. How do glutamate transporters function as transporters and ion channels? Biophys J 2014; 107(3): 546-547.
[6] Jackson JG, O'Donnell JC, Takano H, Coulter DA, Robinson MB. Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J Neurosci 2014; 34(5): 1613-1624.
[7] Huang H, Li Q, Hong YG, Wang DM. MrgC receptor activation reverses chronic morphine-evoked alterations of glutamate transporters and nNOS in rats. Sheng Li Xue Bao 2014; 66(4): 449-456.
[8] Karki P, Smith K, Johnson JJ, Aschner M, Lee EY. Genetic dys-regulation of astrocytic glutamate transporter EAAT2 and its implications in neurological disorders and manganese toxicity. Neurochem Res 2015; 40(2): 380-388.
[9] Bianchi MG, Bardelli D, Chiu M, Bussolati O. Changes in the expression of the glutamate transporter EAAT3/EAAC1 in health and disease. Cell Mol Life Sci 2014; 71(11): 2001-2015.
[10] Nakagawa T, Kaneko S. SLC1 glutamate transporters and diseases: psychiatric diseases and pathological pain. Curr Mol Pharmacol 2013; 6(2): 66-73.
[11] Gegelashvili G, Bjerrum OJ. High-affinity glutamate transporters in chronic pain: an emerging therapeutic target. J Neurochem 2014; 131(6): 712-730.
[12] Bjornsen LP, Hadera MG, Zhou Y, Danbolt NC, Sonnewald U. The GLT-1 (EAAT2; slc1a2) glutamate transporter is essential for glutamate homeostasis in the neocortex of the mouse. J Neurochem 2014; 128(5): 641-649.
[13] Schreiner AE, Durry S, Aida T, Stock MC, Ruther U, Tanaka K, et al. Laminar and subcellular heterogeneity of GLAST and GLT-1 immunoreactivity in the developing postnatal mouse hippocampus. J Comp Neurol 2014; 522(1): 204-224.
[14] Qu X, Xu C, Wang H, Xu J, Liu W, Wang Y, et al. Hippocampal glutamate level and glutamate aspartate transporter (GLAST) are upregulated in senior rat associated with isoflurane-induced spatial learning/ memory impairment. Neurochem Res 2013; 38(1): 59-73.
[15] Zhang X, Shi M, Bjoras M, Wang W, Zhang G, Han J, et al. Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front Pharmacol 2013; 4: 152.
*Corresponding author: Ke-Qing Xiao, Department of Anesthesiology, Shandong Provincial Hospital, Jinan, Shandong 250031, China.
Tel: 18660168668
E-mail: Doctorxiao1115@foxmail.com
Foundation project: It is supported by Natural Science Foundation of Shandong Province (Y2006C02).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Risk factors of polycystic ovarian syndrome among Li People
- Influence of overexpression of SOCS2 on cells of DN rat
- Effect of salinomycin on metastasis and invasion of bladder cancer cell line T24
- Expression of transferrin in hematoma brain tissue at different stages after intra cerebral hemorrhage in rats
- Relationship between gene polymorphisms and prostate cancer risk
- Anti-tumor effect of LTA combined with 5-FU on H22 tumor bearing mice
