Relationship between gene polymorphisms and prostate cancer risk
2015-12-23QianHeHanZhongJieShanJianTingHuNanZhangXuePeiZhang
Qian-He Han, Zhong-Jie Shan, Jian-Ting Hu, Nan Zhang, Xue-Pei Zhang
1Department of Urology, People’s Hospital of Zhengzhou, Zhengzhou, China
2Department of Urology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Relationship between gene polymorphisms and prostate cancer risk
Qian-He Han1, Zhong-Jie Shan1, Jian-Ting Hu1, Nan Zhang1, Xue-Pei Zhang2*
1Department of Urology, People’s Hospital of Zhengzhou, Zhengzhou, China
2Department of Urology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
ARTICLE INFO
Article history:
Received 15 April 2015
Received in revised form 20 May 2015
Accepted 15 June 2015
Available online 20 July 2015
Prostate cancer
Case-control study
p27 V109G polymorphism
LNcap cells
Objective: To investigate the relationship between genetic factor and prostate cancer (Pca) risk and the possible cause in it. Methods: The polymorphisms of cytochrome P450 family 17 (CYPl7) rs743572, p27 V109G and androgen receptor (AR) gene CAG repeat length in peripheral blood from 70 cases and 70 controls were detected through the polymerase chain reaction-restriction fragment length polymorphism technique or short tandem repeatpolymerase chain reaction technique. Then, according to the results of case-control study, the recombinant plasmids containing the wild/mutant p27 gene were constructed and transfected Pca LNcap cells. After 24 and 72 h of transfection, the cell proliferative activity was determined by MTT method, cell cycle distribution and apoptosis was detected by flow cytometry, and the expression level of bcl-2, caspase-3 and p27 protein was determined by Western-blot. Results: In three target polymorphisms, only p27 V109G polymorphism was related to Pca risk (P=0.030, OR=0.202, 95% CI=0.042-0.973). Pca risk of p27-109G allele was lower than -109V allele (P=0.006, OR=0.285, 95% CI=0.110-0.737). Cells transfected with wild/mutant p27 gene both showed the higher cells apoptosis rate and the lower cell proliferative activity than mock cells (P<0.05 or 0.01), the regulatory effect of mutant p27 on cell proliferation and apoptosis was stronger than the wild p27 (P<0.05). Conclusions: p27-109G allele that could cause higher p27 protein expression than -109V allele in LNcap cells, maybe is the protective factor of Pca.
1. Introduction
As a common urological malignancy, thousands of studies related to prostate cancer (Pca) are reported every year. It is known that the only established risk factors of Pca are age, ethnicity and family history of Pca[1]. And numerous studies have confirmed that genetic factor is one the main causes of high incidence of Pca[2,3]. Therefore, it is important for understanding the effect of genetic polymorphisms in people of different ethnic backgrounds.
In many large-scale and genome-wide genetic studies, single nucleotide polymorphisms (SNPs) have been found to be associated with the increased risk of Pca[4,5]. Cytochrome P450 family 17 (CYPl7) rs743572 is the MspA1 polymorphism (T→C) in 5’-promotor region at 34 base pair upstream of the initiation of translation, A1 (wild allele) and A2 (variant allele) are the alleles. Published data on the association between rs743572 polymorphism and risk of Pca showed inconclusive results[6]. For example, Haiman et al found that A2 allele could increase the efficiency of transcription, then the androgen synthesis was elevated and consequently the risk of Pca was raised[7], but Wadelius and Habuchi reported that A1 allele may increase the risk of Pca[8,9]. P27 is a key regulator of the progression from G1 to S phase of the cell cycle and acts by binding and inhibiting the Cyclin E-CDK2 complex in the G1phase[10]. P27 V109G polymorphism had been suggested to confer the risk of advanced Pca in Caucasian males, but a hospitalbased case-control study reported that there was no significant association between p27 V109G polymorphism and Pca risk[11].The androgen receptor (AR) plays a central role in the normal development of the prostate gland, in prostate carcinogenesis, and in the progression of Pca to advanced metastatic disease[12]. The CAG repeat length polymorphism exists in the first exon of AR gene, and it varies in different races or populations, such as 23-25 repeats in the Hispanic population, 21-22 in the Caucasian population and the shortest average CAG repeats (19-20) in the black population[13,14]. The variations of CAG repeat length suggest that shorter CAG repeat lenghs, which are known to be associated with higher AR activity, may be associated with higher Pca risk[15].
In the study, we explored the relationship between the polymorphisms in CYP17, p27 and AR gene and the risk of Pca in Chinese men. And then, through transfecting the recombinant plasmids containing wild/mutant target gene, we tried to investigate the possible causes of polymorphism which influence Pca risk in vitro.
2. Materials and methods
2.1. Subjects
The case group was the local hospitalized patients with Pca diagnosed by histopathology after 2008. The patients in control group were those without tumors selected from Digestive System Department, Respiratory Department and Otorhinolaryngology Department of the same hospital. All the subjects were enrolled from People’s Hospital of Zhengzhou. The matching condition was as follows: the same age group, the same nationality (Han) and the same type of residence. The control was the patients with other diseases, excluding the diseases of urinary system and endocrine system, coronary artery disease and malignant tumors. The base characteristics of both groups were similar.
2.2. Blood collection and extraction of DNA in plasma
After informed consent, 3 mL peripheral venous blood of all the subjects was collected. Then, EDTA was added for anticoagulating, the plasma and erythrocytes were separated and placed in -80 ℃fridge. After that, the plasma DNA was extracted, the polymorphisms of p27 V109G and rs743572 in CYP17 gene were detected by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. AR gene CAG repeat length was detected by short tandem repeat-polymerase chain reaction technique.
The authors state that the protocol for the research project had been approved by the Ethical Committee of the Medical Ethics Committee of People’s Hospital of Zhengzhou. Before the study, the purpose and procedures of the surveys were explained to all the participants, and a written informed consent was obtained from each participant.
2.3. PCR primers and reaction condition
The primers of CYP17, p27 and AR gene were designed by using Primer Premier 5 software. The sequences of primers were seen in Table 1 and they were synthesized by Shanghai Sangon Biotechnology co., Ltd. The CYP17 PCR procedures were as follows: an initial denaturation step at 93 ℃ for 2 min, followed by an amplification step for 35 cycles at 93 ℃ for 30 s, 58 ℃ for 30 s and 72 ℃ for 30 s, and followed by a final extension step at 72 ℃for 10 min. The PCR products were digested with restriction enzyme MspAⅡ (Promega Corp., USA) and separated by 3% agarose gel electrophoresis. The A1 genotype is identified by the presence of 414 bp, A1/A2 genotype by 414, 290, 124 bp and the A2 genotype by 290 and 124 bp.
AR gene PCR procedures were as follows: an initial denaturation step at 95 ℃ for 10 min, followed by an amplification step for 35 cycles at 94 ℃ for 30 s, 60 ℃ for 30 s and 72 ℃ for 45 s, and followed by a final extension step at 72 ℃ for 10 min. The PCR products were separated by agarose gel electrophoresis. After the electrophoresis, the DNA was detected by ABI PRISM 3770 sequencer and the data was analyzed by GeneScan Analysis 3.7 and Genotyper 3.7 software for identifying the genotype.
P27 gene PCR procedures were as follows: an initial denaturation step at 94 ℃ for 5 min, followed by an amplification step for 35 cycles at 94 ℃ for 45 s, 60 ℃ for 30 s and 72 ℃ for 60 s, and followed by a final extension step at 72 ℃ for 10 min. The PCR products were digested with the restriction enzyme Bgl Ⅰ (New England BioLabs Inc, USA). The V/V genotype is identified by the presence of 76, 378 bp, V/G genotype by 76, 113, 265, 378 bp and the G/G genotype by 76, 113 and 265 bp.
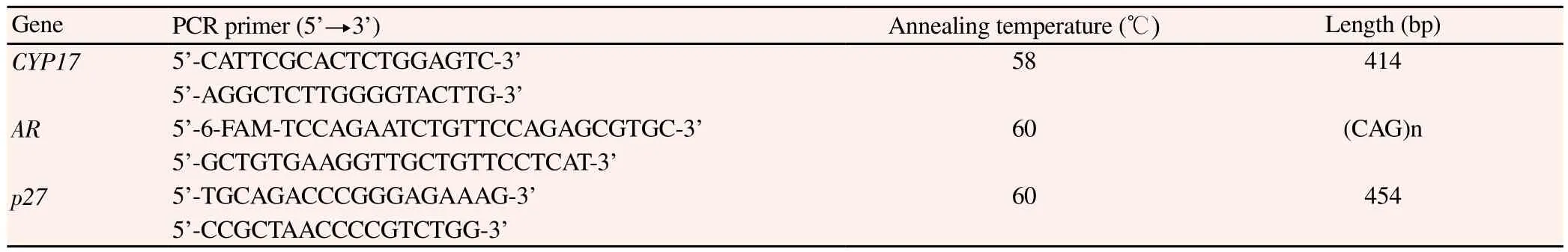
Table 1 PCR primers sequences.
2.4. Construction of the recombinant plasmids containing p27 gene
In order to obtain the mutant p27 gene, the pcDNA3.1-p27 (V/V) was set as the template, the primers were as follows: F-5’-GCAG GAGAGCCAGGATGGCAGCGGGAGCCGCCCG-3’, and R-5’-CGGGCGGCTCCCGCTGCCATCCTGGCTCTCCTGC-3’. PCR procedures were as follows: an initial denaturation step at 95 ℃ for 1 min, followed by an amplification step for 18 cycles at 95 ℃ for 45 s, 60 ℃ for 60 s and 68 ℃ for 7 min, and followed by a final extension step at 72 ℃ for 10 min. After the reaction, 1 μL Dpn I restriction enzyme was added into the PCR reaction system, mixed and incubated for 1h. After the digestion, the products were used for transforming and cloning. Finally, the plasmid containing the correct sequence was named pcDNA3.1-p27 (G/G).
2.5. Cell proliferative activity, cell cycle and apoptosis
The pcDNA3.1-p27(G/G), pcDNA3.1-p27(V/V) and null vector pcDNA3.1 was respectively transfected the LNcap cells in 96-well plate, and the transfected cells were grouped as the p27 G-allele cells, p27 V-allele cells and mock cells, respectively. The cell proliferative activity was determined by MTT method after 24, 48 and 72 hours of transfection. The untreated cells were regarded as blank cells, the OD value at 490 nm represented the proliferative activity.
Cell cycle and apoptosis rate were determined by using flow cytometry. LNcap cells were seeded in 6-well plate at 1×106cells/ mL and transfected according to the above method. After 24 or 72 h of transfection, cells were collected and fixed with 70% ethanol for 48 hours at 4 ℃, rehydrated by ice-cold PBS, and stained by 50 μg/ mL propidium iodide (PI). The percentage of cells in each cell cycle phase was analyzed by Elite flow cytometer (Coulter Cytometry, Inc., USA). Similarly, the cell apoptosis rate at different time points (24, 48 and 72 hours post-transfection) was also detected.
2.6. Expression levels of apoptosis-related proteins
LNcap cells were collected after 24, 72 hours of the transfection, then the RIPI lysis buffer was added. β-actin served as the endogenous control, the expression level of bcl-2, caspase-3 and p27 was semiquantitatively analyzed by Western-blot. Antibodies to bcl-2, caspase-3, p27 and β-actin as well as HRP-labeled secondary anti-rabbit antibodies were obtained from Abcam Co., UK.
2.7. Statistical analysis
The results were analyzed by SPSS 16.0 software. Differences in genotype frequency distribution between case and control group was done by using x2test. Comparisons among multiple groups were analyzed by One-way ANOVA. Post hoc analysis was carried out, whenever appropriate, by the Bonferroni test. All the experiments in vitro repeated at least three times. When P<0.05, the difference was considered to be statistically significant.
3. Results
3.1. Relationship between polymorphisms and Pca risk
Polymorphisms of CYP17 rs743572 and AR gene CAG sequence repeat were not associated with Pca risk, only p27 V109G polymorphism was related to it (P<0.05). Compared with the p27 V/V genotype carriers, the G/G genotype carriers showed the lower Pca risk (P=0.030, OR=0.202, 95% CI=0.042-0.973); and -109G allele could reduce Pca risk (P=0.006, OR=0.285, 95% CI=0.110-0.737) (Table 2).
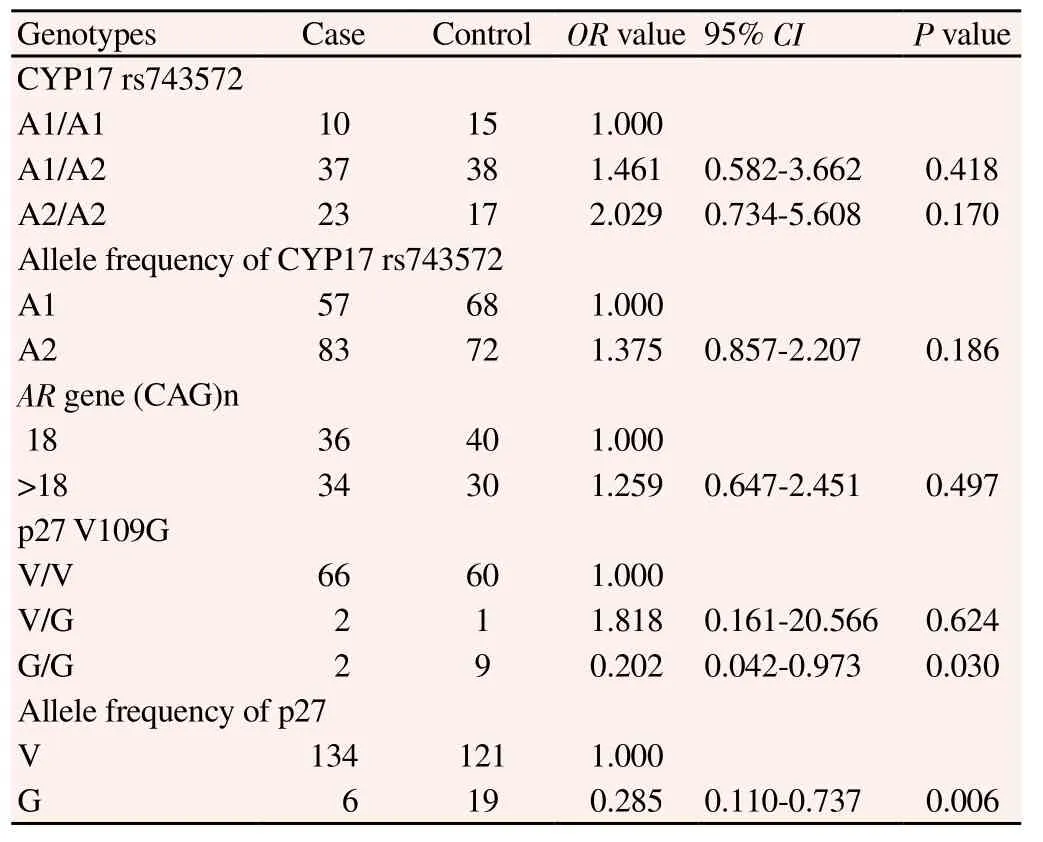
Table 2 Univariate analysis of the relationship of the polymorphisms and Pca risk.
3.2. Influence of p27 V109G polymorphism on LNcap cell proliferative activity, cell cycle distribution and apoptosis
Figure 1 showed that the cell proliferative activity of V-allele cells and G-allele cells were both lower than mock cells after 48 h of transfection (P<0.05 or 0.01). Moreover, the cell proliferative activity of p27 G-allele cells was lower than V-allele cells 72 h posttransfection (P<0.05). Cell cycle analysis (Table 3) showed that transfection with recombinant plasmids could cause obvious G0/ G1cell cycle arrest. In p27 V-allele and G-allele cells, significantly higher number of cells was in G0/G1stage as well as significantly lower number of cells in S and G2/M stages, as compared to the mock cells (P<0.05 or 0.01); compared with the p27 V-allele cells,the p27 G-allele cells manifested significantly higher number of cells in G0/G1stage as well as significantly lower number of cells in S stage after 72 h of transfection (P<0.05) .
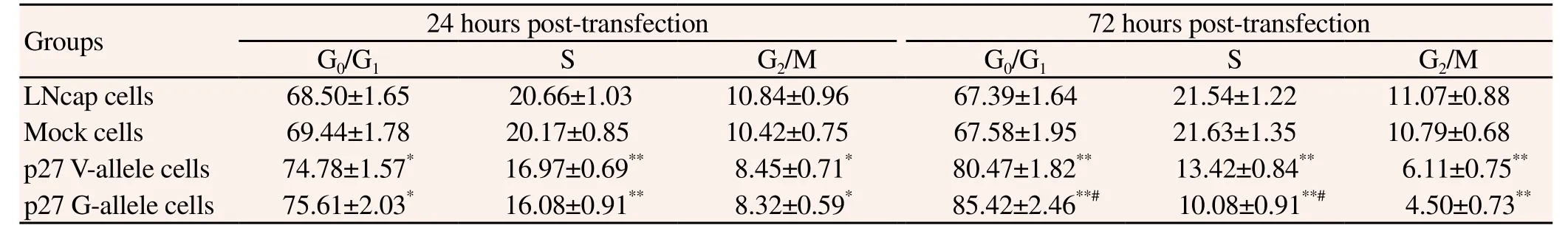
Table 3 Cell cycle distribution after 24 or 72 hours of transfection (%).
Similarly, the apoptosis rate of V-allele cells and G-allele cells were both obviously higher than mock cells since 24 h of transfection (P<0.01), and that of G-allele cells was also lower than V-allele cells since 24 h of transfection (P<0.05).
3.3. Influence of p27 V109G polymorphism on the expression of bcl-2, caspase-3 and p27 protein
Results in Figure 2 showed that transfection with wild and mutant p27 gene both could down-regulate bcl-2 expression and up-regulate the expression of caspase-3 and p27, and this regulatory effect caused by G-allele was more obvious than that caused by V-allele (P<0.05 or 0.01).
4. Discussion
In the study, through the case-control study, we found that only p27 V109G polymorphism was related to Pca risk, and p27 G/ G genotype could reduce the risk. Based on these results, in vitro, we found cells transfected with p27 G-allele displayed a lower proliferative activity and expression level of caspase-3 and p27 protein and a higher apoptosis rate than cells transfected with V-allele, though cells transfected with mutant and wild p27 gene both manifested lower proliferative activity and higher apoptosis rate than the mock cells.
Several clinical studies and laboratory experiments have demonstrated that p27 is an important tumor suppressor gene in Pca etiology. Huang et al detected the p27 V109G polymorphism in 190 Pca patients and 292 controls, their findings showed there was no difference of G allele frequency between the case and control group; in general, they believed p27 V109G polymorphism was nothing with Pca risk in the tested Taiwanese[11]. Chang et al conducted a family-based test and their subjects were hereditary prostate cancer probands and their family members, they found the C allele of -79C/ T was over transmitted from parents to their affected offspring and evidence for this association was primarily contributed by affected offspring whose age at diagnosis was <65 years[16]. Another study in which the subjects were European-American population was similar to the present study about the results, they pointed that the p27 genotype VV was associated with an increased risk of advanced Pca (OR, 1.95; 95% CI, 1.09-3.47)[17]. In the above studies, their results were not usually in keeping with this study, which may be attributed to the hereditary heterogeneity of subjects, different polymorphisms of p27 gene and so on. Moreover, the sample size possibly influenced the results.
Currently, the reports about the relationship between this polymorphism and Pca risk were contradictory. For example, Sekiya reported that the common polymorphism rs2066827 may play a role in corticotropinoma susceptibility and tumorigenesis through a molecular mechanism not fully understood thus far[18]. The results of a case-control study showed that the GG genotype of p27 V109G polymorphism was significantly higher in ovarian cancer patientsthan in normal individual (P=0.02)[19]. In our study, mutant p27 protein expression was higher than the wild, therefore, the effect on accelerating cell apoptosis of mutant p27 was more obvious.
However, because the incidence of Pca was quite low in Chinese population, the cases collected in the last three years were still not enough, which may affect the performance statistics and the reliability of the results.
In summary, we proved that p27-109G allele was the protective factor of Pca. Mutant p27 protein could more obviously promote Pca LNcap cell apoptosis than the wild, which may be caused by the higher expression level of mutant p27 protein than the wild protein.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This work was supported by grant from the General Financial Grant from the China Postdoctoral Science Foundation (2012M521410).
[1] Chan JM, Holick CN, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes Control 2006; 17(2): 199-208.
[2] Schaid DJ. The complex genetic epidemiology of prostate cancer. Hum Mol Genet 2004; 13 Spec No 1: R103-R121.
[3] Shen FR, Yan CY, Liu M, Feng YH, Chen YG. Relationship between multidrug resistance 1 polymorphisms and the risk of prostate cancer in Chinese populations. Genet Mol Res 2013; 12(3): 3806-3812.
[4] Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci 2006; 11: 1388-1413.
[5] Edwards SM, Eeles RA. Unravelling the genetics of prostate cancer. Am J Med Genet C Semin Med Genet 2004; 129C(1): 65-73.
[6] Wang Y, Zhang Y, Meng H, Hou X, Li Z, Liu Q, et al. Quantitative assessment of the association between CYP17 rs743572 polymorphism and prostate cancer risk. Cell Biochem Biophys 2015; 71(2): 983-991.
[7] Haiman CA, Stampfer MJ, Giovannucci E, Ma J, Decalo NE, Kantoff PW, et al. The relationship between a polymorphism in CYPl7 with plasma hormone levels and prostate cancer. Cancer Epidemiol Biomarkers Prev 2001; 10(7): 743-748.
[8] Wadelius M, Andersson AO, Johansson JE, Wadelius C, Rane E. Prostate cancer associated with CYP17 genotype. Pharmacogenetics 1999; 9(5): 635-639.
[9] Habuchi T, Liqing Z, Suzuki T, Sasaki R, Tsuchiya N, Tachiki H, et al. Increased risk of prostate cancer and benign prostatic hyperplasia associated with a CYP17 gene polymorphism with a gene dosage effect. Cancer Res 2000; 60(20): 5710-5713.
[10] Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev 1997; 11(11):1464-1478.
[11] Huang SP, Yu CC, Liu CC, Wu TT, Huang CH, Wu MT. CDKN1B V109G polymorphism frequency and prostate cancer risk in Taiwan. Urol Int 2008; 81(1): 36-40.
[12] Koochekpour S, Buckles E, Shourideh M, Hu S, Chandra D, Zabaleta J, et al. Androgen receptor mutations and polymorphisms in African prostate cancer. Int J Biol Sci 2014; 10(6): 643-651.
[13] Balic I, Graham ST, Troyer DA, Higgins BA, Pollock BH, Johanson-Pais TL, et al. Androgen receptor length polymorphism associated with prostate cancer risk in Hispaic men. J Urol 2002; 168(5): 2245-2248.
[14] Lange EM, Sarma AV, Ray A, Wang Y, Ho LA, Anderson SA, et al. The androgen receptor CAG and GGN repeat polymorphisms and prostate cancer susceptibility in African-American men: results from the Flint Men’s Health Study. J Hum Genet 2008; 53(3): 220-226.
[15] Mao X, Li J, Xu X, Boyd LK, He W, Stankiewicz E, et al. Involvement of different mechanisms for the association of CAG repeat length polymorphism in androgen receptor gene with prostate cancer. Am J Cancer Res 2014; 4(6): 886-896.
[16] Chang BL, Zheng SL, Isaacs SD, Wiley KE, Turner A, Li G, et al. A polymorphism in the CDKN1B gene is associated with increased risk of hereditary prostate cancer. Cancer Res 2004; 64(6): 1997-1999.
[17] Kibel AS, Suarez BK, Belani J, Oh J, Webster R, Brophy-Ebbers M, et al. CDKN1A and CDKN1B polymorphisms and risk of advanced prostate carcinoma. Cancer Res 2003; 63(9): 2033-2036.
[18] Sekiya T, Bronstein MD, Benfini K, Longuini VC, Jallad RS, Machado MC, et al. p27 variant and corticotropinoma susceptibility: a genetic and in vitro study. Endocr Relat Cancer 2014; 21(3): 395-404.
[19] Mohamed FZ, Hussien YM, AIBakry MM, Mohamed RH, Said NM. Role of DNA repair and cell cycle control genes in ovarian cancer susceptibility. Mol Biol Rep 2013; 40(5): 3757-3768.
*Corresponding author: Xue-Pei Zhang, Department of Urology, First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
E-mail: xpzhang70@163.com
Foundation project: This research was supported by Science and Technology Plan Projects of Henan Province (132102310513).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Andrographolide effect on both Plasmodium falciparum infected and non infected RBCs membranes
- Immunogenicity and efficacy of recombinant 78 kDa antigen of Leishmania donovani formulated in various adjuvants against murine visceral leishmaniasis
- Oral administration of Sauce llorón extract to growing lambs to control gastrointestinal nematodes and Moniezia spp.
- Hepatoprotective and proapoptotic effect of Ecballium elaterium on CCl4-induced hepatotoxicity in rats
- Evaluation of protective effect of cactus pear seed oil (Opuntia ficusincida L. MILL.) against alloxan-induced diabetes in mice
- Antimicrobial activity and synergism of Sami-Hyanglyun-Hwan with ciprofloxacin against methicillin-resistant Staphylococcus aureus
