Expression of transferrin in hematoma brain tissue at different stages after intra cerebral hemorrhage in rats
2015-12-23LongChenXueGangJinJianFangZhuHuiJuanLiYanPingWangYouXinZhouJianWangWenHuaWang
Long Chen, Xue-Gang Jin, Jian-Fang Zhu, Hui-Juan Li, Yan-Ping Wang, You-Xin Zhou, Jian Wang, Wen-Hua Wang*
1Department of Neurosurgery, Kunshan Hospital, Nanjing University of Traditional Chinese Medicine, Suzhou 215300, China
2Department of Endocrinology, Second People’s Hospital, Yangzhou University, Suzhou 205331, China
3Department of Neurosurgery, Huai’An Hospital, Nanjing Medical University, Huai’An 223300, China
4Department of Neurosurgery, First People’s Hospital, Suzhou University, Suzhou 215006, China
Expression of transferrin in hematoma brain tissue at different stages after intra cerebral hemorrhage in rats
Long Chen1, Xue-Gang Jin1, Jian-Fang Zhu1, Hui-Juan Li2, Yan-Ping Wang3, You-Xin Zhou3, Jian Wang1, Wen-Hua Wang1*
1Department of Neurosurgery, Kunshan Hospital, Nanjing University of Traditional Chinese Medicine, Suzhou 215300, China
2Department of Endocrinology, Second People’s Hospital, Yangzhou University, Suzhou 205331, China
3Department of Neurosurgery, Huai’An Hospital, Nanjing Medical University, Huai’An 223300, China
4Department of Neurosurgery, First People’s Hospital, Suzhou University, Suzhou 215006, China
ARTICLE INFO
Article history:
Received 15 April 2015
Received in revised form 20 May 2015
Accepted 15 June 2015
Available online 20 July 2015
Intracerebral hemorrhage
Objective: To explore the expression of transferrin (Tf) and transferrin receptor (TfR) in hematoma brain tissue at different stage after intracerebral hemorrhage (ICH) in rats. Methods: ICH rats model were established by collagenase method, and rats were sacrificed at 24 h, 72 h, 7 d and 14 d after operation. The levels of Tf and TfR in different periods of rats were detected by immunohistochemical method, and correlation between two groups was analyzed. Results: Tf, TfR-positive cells at each time after operation in observation group were significantly higher than that in control group (P<0.05). Tf, TfR-positive cells began to increase from 24 h after the operation and reached the peak 72 h-7 d after surgery, but then gradually decreased. Tf was mainly expressed in nucleus and cytoplasm of neurons and glial cells around the hematoma, but TfR was mainly expressed in nucleus and cytoplasm of neurons and choroid plexus endothelial cells. Correlation analysis showed that the Tf-positive cell was significantly positively correlated with TfR-positive cell expression (r=0.447, P=0.022). Conclusions: Tf and TfR were important transporters in brain tissue excessive load iron transport after ICH, and detecting the expression levels of the two indicators can provide a reference for prognosis treatmentin ICH.
1. Introduction
Intracerebral hemorrhage (ICH) refers to primary parenchymal hemorrhage, which contributes to 10% to 30% of overall brain death. Approximately 80% ICH occurs in cerebral hemisphere, mainly in basal ganglia region, and the rest occurs in brain-stem and cerebellum[1-3]. Later-onset cerebral edema may appear in ICH, meanwhile, accompanies with bleeding, hemolysis and mass iron accumulation. Thereafter, many pathways of iron transfer and metabolism are activated. A previous study[4] shows that post ICH edema is associated with neurotoxin which produced by hemoglobin. However, currently, few studies have reported on iron transfer mechanism of ICH edema. We inferred that transferrin (Tf) levels in different stages detection would be helpful for prognosis of ICH because Tf is the main transport iron structure[5]. In order to verify the assumption, we established ICH rat models, then observed Tf and transferring receptor (TfR) dynamic changes and correlations in hematoma tissue at different stages post ICH with the aim to reveal the Tf molecular mechanism in ICH edema and provide theory basis for ICH prognosis and effective treatment.
2. Materials and methods
2.1. Animals and grouping
A total of 80 male SD rats were chosen for this experiment(provided by Suzhou University Experiment Center), weighing 230 g to 320 g [mean eight, (287.5 ±35.3) g]. Based on random number table, all 80 rats were divided into observation group (n=40), also as ICH group and control group (n=40). Rats in both groups were killed at 24 h, 72 h, 7 d and 14 d after surgery (10 rats every period) respectively.
2.2. Model establishment
In the observation group, collagen Ⅳ type enzyme was used to establish ICH rat model[6]. At first, the rats was weighed and fixed them at prone position on stereotaxic apparatus under chloral hydrate anesthesia (300 mg/kg). Then a 0.8-sized incision was made under scalp and exposed the bregma. The oriental device was readjusted to ensure the front and back bregma at a plane. Drilled hole was at the dorsal part of the skull post bregma 0.2 mm median line side 2.9 mm site. Micro-syringe fixed on the stereotaxic apparatus was used to inject normal saline contained Ⅳ type collagen into the hole, and then the injection syringe (syringe needle diameter is about 0.7 mm) was inserted along the hole with 6mm depth (caudate nucleus position).Injection was finished within 5min and the needle retained for 5min then withdrew it slowly. Finally, the skin incision sutured and sterilized. Postoperative physical sign and neurological sign of rats were observed, and Menzies methods were employed to assess whether the model was successful or not. Cases of death and hemiplegia were removed. The same as the observation group, equivalent normal saline was injected in the control group.
At four different postoperative periods as 24 h, 72 h, 7 d and 14 d, 10 rats were killed in each group. In brief, supine fixed the rats were supinely fixed after anesthesia, incised the chest wall and lavaged the heart with normal saline. Then, 10% triformol (PB) stationary liquid was used to perfusion controlling the perfusion speed (fast first then slow), then rats were decollated and took out brain until the rats died. 5-mm specimen were cut centered on the injection point and fixed in PB stationary liquid. One hour later, specimen were put into 75% ethyl alcohol and then stored in 0 ℃. Conventional dehydration and waxing were carried out, and then 5 μm thickness slices were made after paraffin embedding.
2.3. Immunohistochemical staining
Tf and TfR polyclonal antibodies were all bought from Xi’an Yunhong Biotechnology Ltd and the immunohistochemical kits were provided by Shanghai Baili Biotechnology Ltd. In brief, sections were deparafinated and incubated in 3% H2O2for 5-10 min, washing three times with distilled water. Slices were immersed in citrate salt buffer and heated with electric stove till boiling every 5 min for two times. Cooling slices were immersed in PBS for three times (5 min each). As they cooled, samples were put into PBS soaking for three times (5 min each time). Every slice was incubated with one drop antigen retrieval solution (50 g/L) for 30 min and with primary antibody overnight. After three washes with PBS (5 min each), slices were incubated with secondary antibodies for 30 min and washed three times with PBS (5 min each). Then the slices were covered with a fresh drop of DAB solution for 10 min under the microscope. Finally, sections were stained with hematoxylin when fully washed by running water, followed by dehydration, clearing and mounting. Stained brain sections were examined with a microscope. The number of positive cells was averaged over a 400× microscopic field from 5 randomly selected locations per section in the brain regions. Cells were defined as positive cells when yellow granules were found in nucleus or cytoplasm. The results were repeated for three times.
2.4. Statistical analysis
All data were analyzed using statistical software SPSS 16.0 and expressed as mean±sd refers to measurement data. Differences between two groups were analyzed using t-test. Pearson test was conducted for correlation analysis. P<0.05 was considered as statistically significant difference.
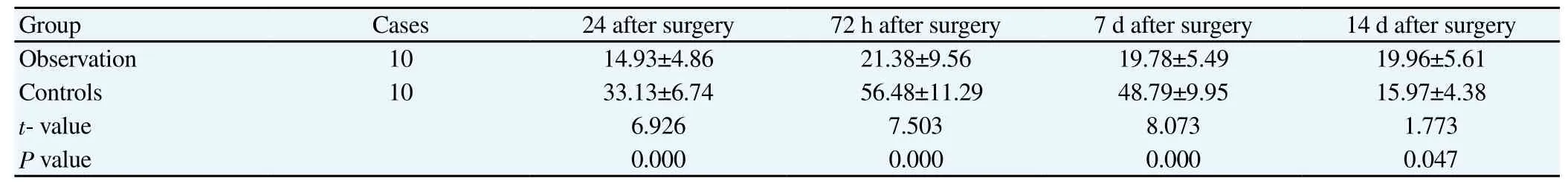
Table 2 TfR PCC of different periods in two groups (mean±sd).
3. Results
3.1. Tf-positive cell counts (PCC) at different stages
Tf PCC in the observation group was significantly higher than that of the control group and the difference was statistical significant (P<0.05); The Tf PCC started to increase 24 h after surgery and peaked at 72 h to 7 d then began to decrease. Microscope showed that Tf was mainly expressed around hematoma neuron, colloid cell nucleus and cytoplasm. All Tf was presented as yellow dyeing particles (Table 1 and Figure 1).
3.2. TfR PCC at different stages
TfR PCC in observation group was significantly increased compared with that of controls (P<0.05). In observation group, TfR PCC started to increase 24 h after surgery and peaked at 72 h to 7 d after surgery then began to decrease, but still remained notably higher than that of control at the same period (P<0.05). Microscope results showed that TfR was mainly expressed around hematoma neuron, choroid plexus endothelial cells and membrane. All presented as yellow dyeing particles (Table 2 and Figure 2).
3.3. Correlation analysis
It showed that Tf and TfR conformed to normal distribution. Moreover, Pearson correlation analysis showed that Tf-positive cell and TfR-positive cell expression had obviously positive relations (r=0.447, P=0.022).
4. Discussion
Dynamic balance of iron plays an important role in maintaining normal function of brain. Under normal circumstance, the central nervous system controls brain iron distribution and metabolism through iron transporter. Caliaperumal[7] has reported that plenty of iron would deposit in brain tissue as for ICH hematoma formation, by this time, a series of channels for transfer and iron metabolic would be activated to protect brain tissue from oxidative stress and secondary cerebral injury, but iron transporter and metabolic protein were needed in this process. Whereas, how brain tissue eliminated massive iron and relevant iron transfer mechanism were still unclear. One study showed that iron dynamic balance in brain tissue was mainly adjusted by Tf/TfR, hence, more and more studies began to focus on the effect of Tf on ICH iron transfer[8].
Encephaledema is an acute complication of ICH, and is also a major risk factor[9] for ICH aggravation. Generally, hematoma will occur 2h post ICH and aggravated gradually, peaks at 24h then gradually fades. Current study[10] considers that hematoma may be caused by blood clot coagulation. Within 2 h post ICH, thrombin and plasma protein in the brain tissue begin to aggregate[11], then erythrocyte dissolves and related decomposer hemoglobin produces toxic effect. There are plenty of iron contained in erythrocytes, and vast iron will deposit in the brain tissue particularly in the hematoma as the erythrocyte dissolves. Meng[12] showed brain edema and subsequent brain atrophy are positively correlated with around iron concentrations. Starke[13] has indicated that obvious hematoma forms in rat brain 24 h after injection of hemoglobin degradation product and metabolite, but iron chelator can reduce brain edema, indicating that iron chelator might reduce the sodium pump injury induced by hemoglobin and neurotoxicity. Oddo et al[14] also revealed that hematoma pathogenetic mechanism associates with neurotoxicity released by hemoglobin. As catalyst of lipid peroxidation, iron can produce oxygen radical which leads to Fe3+in hemoglobin restored to Fe2+, in turn, arouses damage to brain tissue lipid peroxidation, increases blood-brain barrier permeability to form hematoma.
Tf is a kind of glycoprotein which mainly synthesized by neurogliocyte. Many studies verified that Tf is the chief carrier in vivo iron transfer. TfR is distributed in brain tissue unequally, most of which can be seen in cerebral capillaries endothelial cells. Tf incorporates with TfR and participates in intra-tissue irontransfer. Steere[15] has affirmed that Tf/TfR is the major route for iron transmembrane transport through capillary endothelial cell, and peripheral hematoma Tf, TfR expression upregulation may be associated with brain tissue iron eliminating. Tf PCC in observation group of current study started to increase 24 h after surgery and peaked at 72 h to 7 d after surgery, then began to decrease. Microscope manifested that Tf was mainly expressed in hematoma peripheral neuron, gliocyte nucleus and cytoplasm. Liu[16] also confirmed that a great deal of apoptosis cells(principally neuron and gliocyte) can be witnessed around hematoma and center portion 24h after ICH. Since iron can lead to neuron cell apoptosis and overloaded iron is chiefly transferred by Tf, so we speculate that Tf may contribute to neuron cells protection. Additionally, expression patterns of Tf and TfR was basically consistency and correlation analysis revealed that Tf-positive cells were positively correlated with TfR-positive cells. We guess that it maybe the excessive iron after ICH that stimulates neuron and astrocyte secretes Tf and further induces upregulation of TfR expression.Finally, Tf/TfR jointly participates in the transfer of cerebral overburden iron.
To sum up, Tf and TfR mainly participate in post ICH brain tissue overloaded iron transfer.Detecting expression levels of the two indicators can provide reference for cerebral hemorrhage prognosis and effective treatment.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Aburto-Murrieta Y, Marquez-Romero JM, Bonifacio-Delgadillo D, López I,. Hernández-Curiel B. Endovascular treatment balloon angioplasty versus nimodipine intra-arterial for medically refractory cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Vasc Endovascular Surg 2012; 46(6): 460-465.
[2] Ishihara M, Yamanaka K, Nakajima S, Yamasaki M. Intracranial hemorrhage after intra-arterial administration of fasudil for treatment of cerebral vasospasm following subarachnoid hemorrhage: a serious adverse event. Neuroradiol 2012; 54(1): 73-75.
[3] Korfhagen J.J, Kandadai MA, Clark JF, Adeoye O, Shaw GJ. A prototype device for non-invasive continuous monitoring of intracerebral hemorrhage. J Neurosci Methods 2013; 213(1): 132-137.
[4] Yang S, Chen YZ, Deng XQ. Effect of post cerebral hemorrhage hemoglobin on blood brain barrier. Chin J Neuromed 2013; 12(4): 364-368.
[5] Lee JY, Keep RF, He Y, Sagher O, Hua Y, G. Xi G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab 2010; 30(11): 1793-1803.
[6] Yang J, Tang NH Tang ZP. Histopathological study of collagenaseinduced intracerebral hemorrhage in rats. Neural Injury Functional Reconstruction 2012; 7(4): 235-237.
[7] Caliaperumal J, Ma Y, Colbourne F. Intra-parenchymal ferrous iron infusion causes neuronal atrophy, cell death and progressive tissue loss: implications for intracerebral hemorrhage. Exp Neurol 2012; 237(2): 363-369.
[8] Yi YF, Wang G.Q, Chen YL. The effect of deferiprone on ferric Iron transporters and functional outcome in intracerebral hemorrhage rats. J Apoplexy Nervous Dis 2014; 31(2).
[9] Lekic, T, Manaenko A, Rolland W, Krafft PR, Peters R, Hartman, RE, et al. Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Exp Neurol 2012; 236(1): 69-78.
[10] Van Asch, Van der Schaaf CI, Rinkel G. Acute hydrocephalus and cerebral perfusion after aneurysmal subarachnoid hemorrhage. Am J Neuroradiol 2010; 31(1): 67-70.
[11] Harrigan MR, Rajneesh KF, Ardelt AA, Fisher III WS. Short-term antifibrinolytic therapy before early aneurysm treatment in subarachnoid hemorrhage: effects on rehemorrhage, cerebral ischemia, and hydrocephalus. Neurosurg 2010; 67(4): 935-940.
[12] Meng SS, Wang G.Q. Role of ferric iron transporters on iron accumulation in brain after rat intracerebral hemorrhage. J Apoplexy Nervous Dis 2013; 30(4).
[13] Starke RM, Kim GH, Fernandez A, Komotar RJ, Hickman ZL, Otten ML, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke 2008; 39(9): 2617-2621.
[14] Oddo M, Milby A, Chen I, Frangos S, MacMurtrie E, Maloney-Wilensky E, et al. Hemoglobin concentration and cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke 2009; 40(4): 1275-1281.
[15] Steere AN, Miller BF, Roberts SE, Byrne SL, Chasteen ND, Smith VC, et al. Ionic residues of human serum transferrin affect binding to the transferrin receptor and iron release. Biochem 2012; 51(2): 686-694.
[16] Sun Z, Zhang HY, Ke JF, Ma QR, Zhang LX, Qin Y, et al. Effect of astragalus injection for inhibiting neuron apoptosis following the focal intracerebral hemorrhage and reducing cerebral edema in rat. Chin J Anatomy 2012; 35(3): 336-340.
*Corresponding author: Wen-Hua Wang, M.M., Department of Neurosurgery, Kunshan Hospital, Nanjing University of Traditional Chinese Medicine, Suzhou 215300, China.
E-mail: chenlong1127a@163.com
Foundation project: It is supported by 2012 Kunshan Instruction Subject (KS1254).
Transferrin
Transferrin receptor
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Risk factors of polycystic ovarian syndrome among Li People
- Influence of overexpression of SOCS2 on cells of DN rat
- Effect of salinomycin on metastasis and invasion of bladder cancer cell line T24
- Effect of subarachnoid nerve block anesthesia on glutamate transporter GLAST and GLT-1 expressions in rabbits
- Relationship between gene polymorphisms and prostate cancer risk
- Anti-tumor effect of LTA combined with 5-FU on H22 tumor bearing mice
