Role of Aedes aegypti and Aedes albopictus during the 2011 dengue fever epidemics in Hanoi, Vietnam
2015-12-23PhamThiKimLienVuTrongDuocLaurentGavotteEmmanuelCornillotPhanThiNgaLaurenceBriantRogerFrutosTranNhuDuong
Pham Thi Kim Lien, Vu Trong Duoc, Laurent Gavotte, Emmanuel Cornillot, Phan Thi Nga, Laurence Briant, Roger Frutos,4, Tran Nhu Duong
1National Institute of Hygiene and Epidemiology, 1 Yersin Street, 10000 Hanoi, Vietnam
2CPBS, UMR 5236, CNRS-UM1-UM2, 1919, route de Mende 34293 Montpellier Cedex 5, France
3ISEM, UMR 5554, CNRS-UM2-IRD, Université Montpellier 2 CC065, Place E. Bataillon, 34095 Montpellier Cedex 5, France
4Intertryp, UMR 17, Cirad-IRD, Campus International de Baillarguet, 34398 Montpellier Cedex 5, France
Role of Aedes aegypti and Aedes albopictus during the 2011 dengue fever epidemics in Hanoi, Vietnam
Pham Thi Kim Lien1,2, Vu Trong Duoc1*, Laurent Gavotte3, Emmanuel Cornillot2, Phan Thi Nga1, Laurence Briant2, Roger Frutos2,4, Tran Nhu Duong1*
1National Institute of Hygiene and Epidemiology, 1 Yersin Street, 10000 Hanoi, Vietnam
2CPBS, UMR 5236, CNRS-UM1-UM2, 1919, route de Mende 34293 Montpellier Cedex 5, France
3ISEM, UMR 5554, CNRS-UM2-IRD, Université Montpellier 2 CC065, Place E. Bataillon, 34095 Montpellier Cedex 5, France
4Intertryp, UMR 17, Cirad-IRD, Campus International de Baillarguet, 34398 Montpellier Cedex 5, France
ARTICLE INFO
Article history:
Received 15 April 2015
Received in revised form 20 May 2015
Accepted 15 June 2015
Available online 20 July 2015
Aedes aegypti
Aedes albopictus
Dengue
Vector density
Objective: To record the human cases of dengue fever (DF) and investigate the Aedes mosquito species circulating during the Hanoi 2011 DF epidemics. Methods: 24 different outbreak points were recorded in 8 districts between August and December 2011. Results: 140 patients were hospitalized following dengue diagnostic with a predominance of males (59.3%) and the 15-34 age class. Only DENV-1 (11.27%) and DENV-2 (88.73%) serotypes were detected in human samples. Mosquito sampling performed in and around patients households revealed the predominance of Aedes aegypti (95.15%) versus Aedes albopictus (4.85%). There is a positive correlation between the population density of Aedes aegypti and the number of human cases and duration of outbreaks. Conclusions: This was not observed for Aedes albopictus. 3 pools of Aedes aegypti were positive with dengue virus, two with DENV-1 and one with DENV-2.
1. Introduction
Four distinct DENV serotypes are currently described which cause dengue fever in humans resulting in a range of clinical symptoms including fever, headache, muscle, joint pains, and a characteristic skin rash similar to measles[1-4]. Dengue fever (DF) can also evolve into severe forms such as dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS), which could result in death[5]. An estimated 390 million dengue infections occur every year, of which 96 million are aymptomatic[6], which could make the burden of dengue three times higher than considered[7,8]. More than 100 countries are affected by outbreaks of dengue and more than 60 have reported the occurrence of DHF[9]. Southeast Asia is among the regions most affected by dengue and Vietnam is one of the five countries in this region with the highest burden[10]. DF was first described in northern Vietnam in 1958 and expanded to the southern area in the 1960s[11-13]. DHF was first described in Hanoi during the rainy season of 1958[14,11] with a mortality rate of 7%[15]. During the 1998-2009 decade, two large outbreaks occurred in the central urban area of Hanoi, which resulted in a total of 25 983 cases mostly among young adults[16,17].
Dengue virus (DENV) is transmitted to humans by two mosquito species, Aedes aegypti (A. aegypti) and Aedes albopictus (A. albopictus). Aedes aegypti is considered the most important vector of DENV while Aedes albopictus is generally believed to be a less competent vector resulting in milder epidemics[18]. However, dengue outbreaks have been attributed to both A. aegypti and A. albopictus in different regions of the world including Asia[1,11,18,19]. Each species displays a specific ecology, behavior and geographical distribution. A. aegypti prefers urban habitats, whereas A. albopictus is primarily a forest species that has become adapted to rural, suburban andurban human environments[18,20,21]. Owing to the increasing presence of A. albopictus and the co-circulation of both mosquito species in Vietnam[22,23], this study was undertaken to investigate their respective potential role in DENV transmission in the recent epidemics and correlation between mosquito abundance and size and duration of outbreaks.
2. Materials and methods
2.1. Ethics and enrolment of patients
The study protocol was cleared and approved by the Scientific and Ethical Committee of the National Institute of Hygiene and Epidemiology, Vietnam. The study was conducted as part of the Vietnamese National Dengue Prevention and Control Program. All patients considered in the analysis gave a written informed consent of participation to the study.
2.2. Location of sampling
Mosquitoes and blood samples from hospitalized patients were collected during outbreaks from August 2011 to December 2011 in eight districts of Hanoi: Ba Dinh, Hai Ba Trung, Dong Da, Ha Dong, Thanh Xuan, ThanhOai, Thanh Tri, and TuLiem (Figure 1). The population of the Northern city of Hanoi was estimated in 2009 to be 2.6 million for urban districts, and 6.5 million for the metropolitan jurisdiction[24]. Climate is contrasted with hot and humid summers, a rainy season from May to September with temperatures from 38 ℃-40 ℃. Winters are relatively dry and cool from November to March with temperatures as low as 6 ℃. Spring is marked by light rain. The period from June to September is suitable for the development of the mosquitoes[25]. The DF outbreak areas were defined according to Ministry of Health guidelines as geographic areas(town/village/ hamlet, population groups or equivalent) where patients were tested positive for DENV and simultaneous detection of mosquitoes was confirmed[26]. Small outbreaks were defined as occurrences with less than 20 positive patients, medium outbreaks comprised 21 to 100 positive patients and large outbreaks were considered as involving more than 100 patients. Outbreaks were considered terminated when no case was reported for at least 14 days.
2.3. Case definition and sampling
Patients admitted to the National Hospital for Tropical Diseases in Hanoi between August 1, 2011 and December 21, 2011 were considered in the study when presenting dengue symptoms as defined both by WHO and Vietnamese Ministry of Health guidelines on surveillance, diagnosis, treatment of dengue. These symptoms were a continuous fever for 2 to 7 days in an individual from an endemic area and displaying two or more of the following clinical manifestations of DF: nausea, vomiting, rash, aches and pains; positive tourniquet test, leukopenia and any warning sign[7,27]. Blood samples were systematically collected from patients corresponding to the above-mentioned criteria. Acute phase serum sample was collected after fever onset from day 1 to 7 and a follow-up 3-5 Ml serum sample was taken, stored at 4 ℃ until being sent each day to arbovirus laboratory, NIHE for RNA extraction then stored at –80 ℃. Description of cases included the onset date and place, age and gender of patients with notified cases of DF infection. The serum obtained were subjected to serological and molecular testing to determine the presence of dengue virus and identify the serotype. With the patient’s consent, the following data were collected: full name, residence address, gender, time of onset and intensity, and location of symptoms. After data collection, contacts were taken with Preventive Medicine Centres in each district to implement captures of mosquitoes in patient’s house and households.
2.4. Mosquito collection and identification
Adult mosquitoes were collected from patient’s house, and 15 households around the patient’s household located within a radius of approximately 20 to 50 meters using a backpack aspirator. For each outbreak area, 50 to 100 households were randomly selected for daily collection. For each household, sampling was performed indoor and outdoor for approximately 15 minutes during the day. Mosquito collection were carried out by 4 groups volunteer with 2 sessions per day between 5-10 AM and 4-8 PM. Collected mosquitoes were stored in RNA later solution (Qiagen) and kept refrigerated at 2 ℃–8 ℃. Identification of A. aegypti and A. albopictus was performed according to morphological criteria following binocular examination. Mosquito samples were sorted according to species, gender, date of collection, geographical coordinates and number of mosquitoes foreach location, and then stored at -80 ℃ in RNA later solution until further use.
2.5. RNA extraction and RT-PCR amplification
Viral RNA was extracted from 140 μL patient blood serum and from 970 mosquitoes (923 A. aegypti and 47 A. albopictus individuals) by pools of up to 10 mosquitoes depending upon sample size. Males and females were pooled separately. Viral RNA was extracted using QIAamp viral RNA Mini kit (Qiagen) according to the supplier and stored at -80 ℃ until further use. DENV RNA was detected and typed using a single tube multiplex RT-PCR according to an experimental protocol adapted from previously published procedures[28, 29]. Both reverse transcription and PCR were conducted using the Access Quick RT-PCR kit (Promega). Reverse transcription was conducted at 45 ℃ for 30 minutes using random primers (Invitrogen). PCR was then performed in a 50 μL reaction volume using a set of five primers (25 pmol each) comprising a dengue virus consensus reverse primer and four serotype-specific forward primers (Table 1). PCR was conducted for 35 cycles under the following conditions: denaturation at 94 ℃ for 2 minutes, annealing at 55 ℃ annealing for 45 seconds and extension at 72 ℃for 90 seconds followed by a final extension for 10 minutes at 72 ℃. PCR products were analysed in a 2% agarose gel electrophoresis using 10% SYBR safe DNA dye (Invitogen) in 1% TAE buffer. The expected size of the amplicons was 492 bp, 119 bp, 290 bp and 392 bp for DENV-1, DENV-2, DENV-3 and DENV-4, respectively.
2.6. Data analysis
Data were analyzed using STATA 10.0. Spearmen’s Rank correlation coefficient analysis was used to investigate the association between the density of Aedes mosquitoes, number of confirmed dengue cases and duration of outbreaks.
3. Results
3.1. Outbreaks location, size and duration
During the study period, a total of 24 infectious foci were detected within the eight districts in Hanoi, all of them being small or medium outbreaks (Figure 1). The mean duration of an individual outbreak was 69.3 days, ranging from 17 to 123 days (median duration 76 days). Samples were collected from a total of 140 hospitalized patients confirmed with dengue by serology. The number of confirmed cases in each district varied from 2 to 42 (mean = 16, and median = 23) (Table 2). No shock or haemorrhage characteristic ofsevere dengue was reported among cases included in the study. Men (59.3%) were more affected than women (40.7%) (Table 3). The youngest patient was 3 years old and the oldest was 88 (mean age = 33 years, medium = 29 years) (Table 3).

Table 1 Oligonucleotide primers used to amplify and type dengue viruses.
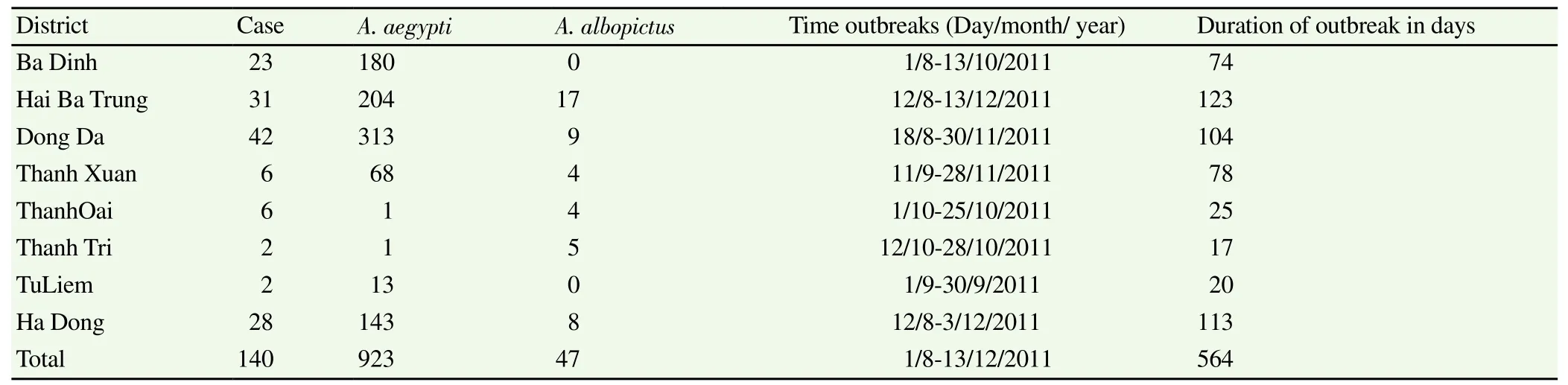
Table 2 Mosquito and human samples collected from outbreaks in Hanoi, 2011.

Table 3 Distribution of gender and age in dengue case.
3.2. Collection of mosquitoes in and around patients’households
A total of 1.200 households were sampled during the study and 970 mosquitoes were collected (Table 2). All mosquitoes collected belonged to the genus Aedes. 923 (95%) belonged to the A. aegypti species whereas 47 (5%) were A. albopictus. For each district, the total number of Aedes collected ranged from 5 to 322. A. aegypti largely predominated in each district with the exception of ThanhOai and Thanh Tri where the number of A. albopictus was higher. Hai Ba Trung district recorded the highest number of captured A. albopictus (17/47 or 36%). The observed density of Aedes mosquitoes was higher during outbreaks. However, due to the small size of mosquito samples collected in Thanh Tri, ThanhOai and TuLiem districts, we cannot exclude that this result was biased as mosquitoes may have been collected under different meteorological conditions or time periods.
3.3. Detection and identification of dengue virus
Out of the 140 dengue serology-positive blood samples, 71 were tested by PCR. Only DENV-1 and DENV-2 serotypes were detected. Only 8 patients (11.27%) distributed over four districts, ie. Ba Dinh, Hai Ba Trung, Dong Da, and Thanh Tri, tested positive for DENV-1 (Table 3). 63 patients (88.73%) tested positive for DENV-2 which was present in all the districts investigated with the exception of Thanh Tri. In three districts, ie. Dong Da, Hai Ba Trungand Ba Dinh, the presence of both DENV-1 and DENV-2 was recorded.
3.4. Correlation between Aedes population size and outbreak intensity
A positive correlation was observed between the population density of A. aegypti and the number of human cases recorded during an outbreak (R=0.57, P=0.003). A similarly positive association was observed between the number of A. aegypti mosquitoes collected at outbreak sites and the duration of outbreaks. A. aegypti population density was found higher when outbreak was longer (R=0.57, P= 0.003). The number of A. aegypti individuals collected in the early period of outbreaks was higher than the number collected towards the end of the outbreaks. Conversely, no correlation was found between the number A. albopictus collected in outbreak areas and the number of confirmed dengue cases in the same areas (R=- 0.62) or with the duration of outbreaks (R=-0.67). All A. albopictus samples were negative for dengue virus (Table 4). Only A. aegypti female mosquitoes collected in the districts of Ba Dinh, Hai Ba Trung and Dong Da were positive for DENV-1 and DENV-2 (Table 5).
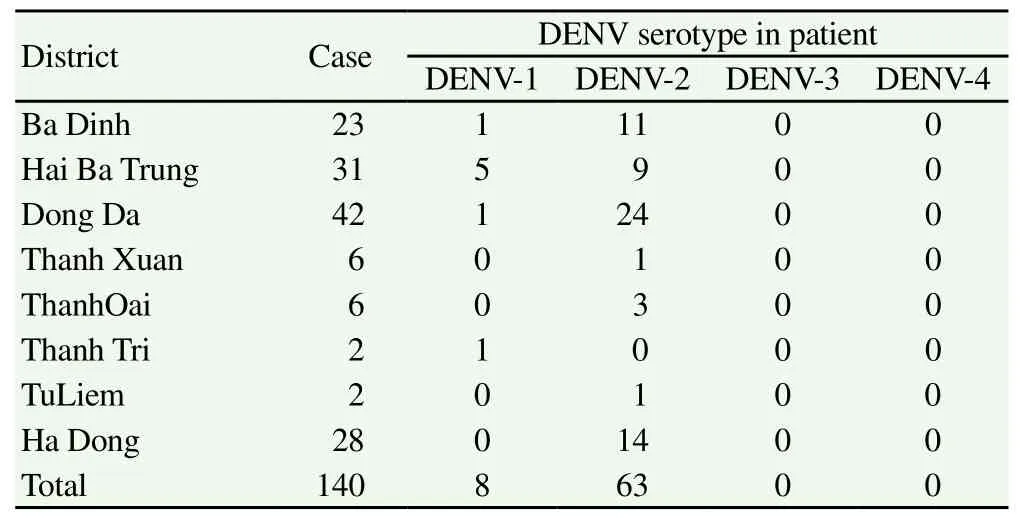
Table 4 Distribution of serotypes among confirmed dengue positive patients.
4. Discussion
This study conducted in Hanoi, Vietnam, reported 140 confirmed cases of DF in 24 outbreaks over eight districts. The patients were mainly males over 15 with the highest number of dengue cases found in the 15-34 years age class. Only 10% of patients were under 15. The age distribution reported in this study differs from other works which reported dengue mainly in children[30-37]. However, increasing dengue incidence in older age groups was also reported in Latin America and Southeast Asia[38-41]. During the study period, we also found that more males were affected than females. Others have reported similar findings in Vietnam, China and Nepal[30,42,43]. There is no clear explanation for this bias which might be associated to an occupational factor.
The detection of only DENV-1 and DENV-2 in mosquitoes along with the predominance of DENV-2 reported in this work is in agreement with previous reports on from Hanoi in recent years[16,42]. This suggests that DENV-1 and DENV-2 were already co-circulatingduring previous outbreaks in the same area and probably maintained over time through vertical transmission in mosquito populations. The overwhelming presence of A. aegypti in the captured mosquitoes, its widespread distribution and the positive correlation of higher local density with the number of human cases reported in outbreaks, altogether strongly suggest that A. aegypti is involved in dengue virus transmission in Hanoi, with a pivotal role during the 2011 dengue outbreaks and before as a maintenance host.
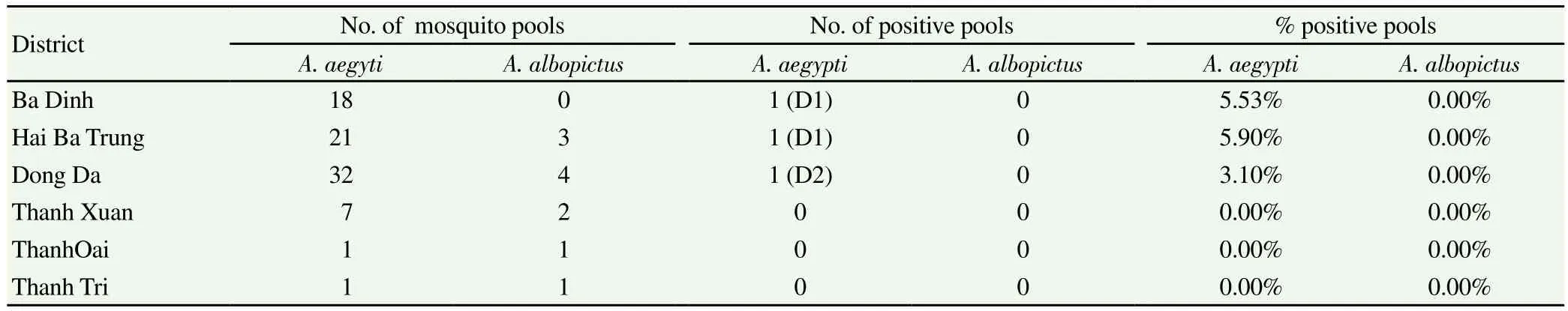
Table 5 Dengue virus serotypes detected in Aedes mosquitoes collected in outbreaks areas.
Despite the fact that it is considered to be a less efficient vector, A. albopictus is adapted to urban domestic environments and was described as a vector in DENV outbreaks in different regions including China, Gabon or Madagascar[44-46]. However, in this work, DENV were not detected in A. albopictus and no correlation was found between the number of A. albopictus captured and the number of human cases. These data corroborate reports suggesting that A. albopictus is not an important vector in urban environment when compared to A. aegypti[21,47-50]. Despite its growing importance and presence it remains a secondary dengue vector, indicating that control actions must remain directed towards A. aegypti habitats.
The importance of mosquito population densities in the dynamic of dengue fever highlighted by this work might, however, be underestimated. Indeed, a limitation to this study is that it only covered the latter part of the rainy season, considered an ideal time for dengue transmission. Additionally, no information was available for artificial containers that might have acted as breeding habitats for Aedes mosquitoes. There may also have been case ascertainment biases as we relied on patients presenting themselves to health services, thus milder and asymptomatic cases may not have been identified. Nevertheless, this work strongly supports the need of further investigation in other geographic areas and environment, ie. suburban and rural, to determine the distribution and relative role of A. aegypti and A. albopictus in the transmission of DF and DHF in Vietnam and a potential evolution linked to the spread of A. albopictus. This work also underlines the need of a better understanding of the dynamics of mosquito populations to implement efficient and adapted mosquito population monitoring and mosquito control strategies, which is at the moment the best way of controlling dengue and other mosquito-borne diseases.
Conflict of interest statement
We declar that we have no conflicts of interest.
Acknowledgements
PTKL was supported in part by the Erasmus Mundus project MAHEVA and by the CNRS-UM1-UM2 PEPS project MoDyCA. The study was supported by the Entomology department, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
The authors are grateful to leaders and medical staff of the National Hospital for Tropical Diseases, the Center for Preventive Medicine in Hanoi, the District Health Centre and commune healthcentre/ wards in Hanoi city for their enthusiastic support, active participation and close collaboration. The authors are very grateful to Dr Babatunde Olowokure, WHO Vietnam, for his support and fruitful comments on the manuscript.
[1] Kyle JL, Harris E. Global Spread and Persistence of Dengue. Annu Rev Microbiol 2008; 62(1): 71–92.
[2] Dietz VJ, Nieburg P, Gubler DJ, Gomez I. Diagnosis of measles by clinical case definition in dengue-endemic areas: implications for measles surveillance and control. Bull World Health Organ 1992; 70(6): 745–750.
[3] López-Vélez R, Pérez-Casas C, Vorndam AV, Rigau J. Dengue in Spanish travelers returning from the tropics. Eur J Clin Microbiol Infect Dis 1996; 15(10): 823–826.
[4] Pull L, Brichler S, Bouchaud O, Siriez J-Y. Differential Diagnosis of Dengue Fever: Beware of Measles! J Travel Med 2012; 19(4): 268–271.
[5] Martina BEE, Koraka P, Osterhaus ADME. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 2009; 22(4): 564–581.
[6] Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature 2013; 496(7446): 504–507.
[7] WHO. Dengue guidelines for diagnosis, treatment, prevention and control: new edition. Available from: http://www.who.int/rpc/ guidelines/9789241547871/en/
[8] WHO. Dengue guidelines for diagnosis, treatment, prevention and control. 2009;
[9] Kyle, J.L, Hariss, E. Dengue Fever in a Warming World. Global spread and persistence of dengue. Annu Rev Microbiol 2008; 62: 71–92.
[10] Shepard DS, Undurraga EA, Halasa YA. Economic and Disease Burden of Dengue in Southeast Asia. PLoS Negl Trop Dis 2013; 7(2): e2055.
[11] Karine, Luu Le Loan, Tran Huu Hoang, Tran Khanh Tien, Francois, Rodhain, Anna-Bella Failloux. Aedes aegpti in South Vietnam: Ecology, genetic structure, vectorial competence and resistance to insecticides. Southeast Asian J Trop Med Public Health 2003; 34(1): 81–86.
[12] Tran KT, Vazeille-Falcoz M, Mousson L, Tran HH, Rodhain F, Ngugen TH, et al. Aedes aegypti in Ho Chi Minh City (Viet Nam): susceptibility to dengue 2 virus and genetic differentiation. Trans R Soc Trop Med Hyg 1999; 93(6): 581–586.
[13] Do QH, Nguyen T KT, Vu TQH,Huynh TKL, Cao MT. Dengue Epidemic in Southern Vietnam, 1998. Emerg Infectious Dis 2000; 6(4): 422-425.
[14] Mihov C, Tuong CV, Tuong HP. A propos d’une épidémie du type des fièvres hémorragiques à. Hanoi. Folia Med 1959; 1: 169–173.
[15] Halstead SB. Mosquito-borne haemorrhagic fevers of South and South-East Asia. Bull World Health Organ 1966; 35(1): 3–15.
[16] Toan DTT, Hu W, Thai PQ, Hoat LN, Wright P, Martens P. Hot spot detection and spatio-temporal dispersion of dengue fever in Hanoi, Vietnam. Glob Health Action. [Online] Available from: http://www.ncbi. nlm.nih.gov/pmc/articles/PMC3556563/
[17] Cuong HQ, Hien NT, Duong TN, Phong TV, Cam NN, Farrar J, et al. Quantifying the emergence of dengue in Hanoi, Vietnam: 1998–2009.PLoS Negl Trop Dis 2011; 5(9): e1322.
[18] Giovanni Rezza. Aedes albopictus and the reemergence of Dengue. BMC Public Health 2012; 12(1): 72.
[19] Brathwaite Dick O, San Martín JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 2012; 87(4): 584–593.
[20] Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne Zoonotic Dis Larchmt N 2010; 10(3): 259–266.
[21] WHO. The mosquito [Online] Available from: http://www.who.int/ denguecontrol/mosquito/en/ [Accessed on 2013 Oct 30]
[22] Kay BH, Tuyet Hanh TT, Le NH, Quy TM, Nam VS, Hang PVD, et al. Sustainability and cost of a community-based strategy against Aedes aegypti in northern and central Vietnam. Am J Trop Med Hyg 2010; 82(5): 822–830.
[23] Higa Y, Thi Yen N, Kawada H, Hai Son T, Thuy Hoa N, Takagi M. Geographic distribution of Aedes aegypti and Aedes albopictus collected from used tires in Vietnam. J Am Mosq Control Assoc 2010; 26(1): 1–9.
[24] The Vietnam Population and Housing census 2009. Ministry of planning and investment. General Statistics Office.
[25] Toan D, Hu W, Thai P, Hoat L, Wright P, Martens P. Hot spot detection and spatio-temporal dispersion of dengue fever in Hanoi, Vietnam. Glob Health ACTION 2013; 6: 7–15.
[26] MOH, Vietnam. Decided of MOH, no 1499/ QD-BYT, date 17/5/2011.
[27] MOH, Vietnam. Guidelines on the surveillance, diagnosis, and treatment of dengue hemorrhagic fever. Medical Publishing House.
[28] Saxena P, Dash PK, Santhosh SR, Shrivastava A, Parida M, Rao PL. Development and evaluation of one step single tube multiplex RT-PCR for rapid detection and typing of dengue viruses. Virol J. 2008; 5(1): 20. [29] Harris E, Roberts TG, Smith L, Selle J, Kramer LD, Valle S, et al. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J Clin Microbiol 1998; 36(9): 2634–2639.
[30] Luo L, Liang H, Hu Y, Liu W, Wang Y, Jing Q, et al. Epidemiological, virological, and entomological characteristics of dengue from 1978 to 2009 in Guangzhou, China. J Vector Ecol J Soc Vector Ecol 2012; 37(1): 230–240.
[31] Halstead SB. More dengue, more questions. Emerg Infect Dis 2005; 11(5): 740–741.
[32] Halstead SB, Scanlon JE, Umpaivit P, Udomsakdi S. Dengue and chikungunya virus infection in man in Thailand, 1962-1964. IV. Epidemiologic studies in the Bangkok metropolitan area. Am J Trop Med Hyg 1969; 18(6): 997–1021.
[33] Huy R, Buchy P, Conan A, Ngan C, Ong S, Ali R, et al. National dengue surveillance in Cambodia 1980-2008: epidemiological and virological trends and the impact of vector control. Bull World Health Organ 2010; 88(9): 650–657.
[34] Kongsomboon K, Singhasivanon P, Kaewkungwal J, Nimmannitya S, Mammen MP Jr, Nisalak A, et al. Temporal trends of dengue fever/ dengue hemorrhagic fever in Bangkok, Thailand from 1981 to 2000: an age-period-cohort analysis. Southeast Asian J Trop Med Public Health 2004; 35(4): 913–917.
[35] Thai KTD, Nagelkerke N, Phuong HL, Nga TTT, Giao PT, Hung LQ, et al. Geographical heterogeneity of dengue transmission in two villages in southern Vietnam. Epidemiol Infect 2010; 138(4): 585–591.
[36] Teixeira MG, Costa MCN, Coelho G, Barreto ML. Recent Shift in Age Pattern of dengue hemorrhagic fever, Brazil. Emerg Infect Dis 2008; 14(10): 1663–1663.
[37] Phuong HL, Vries PJ de, Nga TT, Giao PT, Hung LQ, Binh TQ, et al. Dengue as a cause of acute undifferentiated fever in Vietnam. BMC Infect Dis 2006; 6(1): 123.
[38] Teng AK, Singh S. Epidemiology and New initiatives in the prevention and control of dengue in Malaysia. 2001 Dec [Online]; Available from: http://repository.searo.who.int/handle/123456789/15837[Accessed on 2013 Nov 2]
[39] Guzmán MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg 1990; 42(2): 179–184.
[40] Ooi E-E, Goh K-T, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis 2006; 12(6): 887–893.
[41] Rigau-Pérez JG, Vorndam AV, Clark GG. The dengue and dengue hemorrhagic fever epidemic in Puerto Rico, 1994-1995. Am J Trop Med Hyg 2001; 64(1-2): 67–74.
[42] Fox A, Hoa LNM, Simmons CP, Wolbers M, Wertheim HFL, Khuong PT, et al. Immunological and viral determinants of dengue severity in hospitalized adults in Ha Noi, Viet Nam. Rico-Hesse R, editor. PLoS Negl Trop Dis 2011; 5(3): e967.
[43] Gupta G, Shah Y, Poudel A, Pun R, Pant K, Kshetri R, et al. Serological and molecular study of dengue viruses in different hospitals of Nepal. Nepal J Med Sci 2013; 2(1): 20-25.
[44] Guozhang Xu HD. An outbreak of dengue virus serotype 1 infection in Cixi, Ningbo, People’s Republic of China, 2004, associated with a traveler from Thailand and high density of Aedes albopictus. Am J Trop Med Hyg 2007; 76(6): 1182–1188.
[45] Leroy EM, Nkoghe D, Ollomo B, Nze-Nkogue C, Becquart P, Grard G, et al. Concurrent Chikungunya and Dengue Virus Infections during Simultaneous Outbreaks, Gabon, 2007. Emerg Infect Dis 2009; 15(4): 591–593.
[46] Ratsitorahina M, Harisoa J, Ratovonjato J, Biacabe S, Reynes J-M, Zeller H, et al. Outbreak of Dengue and Chikungunya Fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis 2008; 14(7): 1135–1137.
[47] Lozano-Fuentes S, Hayden MH, Welsh-Rodriguez C, Ochoa-Martinez C, Tapia-Santos B, Kobylinski KC, et al. The dengue virus mosquito vector Aedes aegypti at high elevation in Mexico. Am J Trop Med Hyg 2012; 87(5): 902–909.
[48] Romero-Vivas, Leake, Falconar. Determination of dengue virus serotypes in individual Aedes aegypti mosquitoes in Colombia. Med Vet Entomol 1998; 12(3): 284–248.
[49] Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol 2004; 18(3): 215–227.
[50] Richards SL, Anderson SL, Alto BW. Vector competence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) for dengue virus in the Florida Keys. J Med Entomol 2012; 49(4): 942–946.
*Corresponding author: Vu Van Duoc, National Institute of Hygiene and Epidemiology, 1 Yersin Street, 10000 Hanoi, Vietnam.
Tran Nhu Duong, Intertryp, UMR 17, Cirad-IRD, Campus International de Baillarguet, 34398 Montpellier Cedex 5, France.
E-mail: roger.frutos@univ-montp2.fr; frutossmt@gmail.com
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Risk factors of polycystic ovarian syndrome among Li People
- Influence of overexpression of SOCS2 on cells of DN rat
- Effect of salinomycin on metastasis and invasion of bladder cancer cell line T24
- Expression of transferrin in hematoma brain tissue at different stages after intra cerebral hemorrhage in rats
- Effect of subarachnoid nerve block anesthesia on glutamate transporter GLAST and GLT-1 expressions in rabbits
- Relationship between gene polymorphisms and prostate cancer risk
