乳腺癌新辅助化疗后腋窝及内乳前哨淋巴结活检研究
2015-12-16曹晓珊丛斌斌邱鹏飞刘雁冰王春建王永胜
曹晓珊,丛斌斌,孙 晓,邱鹏飞,刘雁冰,赵 桐,陈 鹏,王春建,王永胜
1.山东省肿瘤医院乳腺病中心,山东 济南 250117;
2.济南大学山东省医学科学院医学与生命科学学院,山东 济南 250200
乳腺癌新辅助化疗后腋窝及内乳前哨淋巴结活检研究
曹晓珊1,2,丛斌斌1,2,孙 晓1,邱鹏飞1,刘雁冰1,赵 桐1,陈 鹏1,王春建1,王永胜1
1.山东省肿瘤医院乳腺病中心,山东 济南 250117;
2.济南大学山东省医学科学院医学与生命科学学院,山东 济南 250200
背景与目的:临床新辅助化疗(neoadjuvant chemotherapy,NAC)后腋窝淋巴结(axillary lymph node,ALN)转阴的患者腋窝前哨淋巴结活检(axillary sentinel lymph node biopsy, ASLNB)能否替代腋窝淋巴结清扫(axillary lymph node dissection,ALND)尚存在争议,且此前研究只评估ALN病理状况而未评估内乳淋巴结(internal mammary lymph node,IMLN)状况。本研究旨在评估NAC后乳腺癌患者接受ASLNB和内乳前哨淋巴结活检(internal mammary sentinel lymph node biopsy,IM-SLNB)的临床意义。方法:回顾性分析2012年1月—2014年12月山东省肿瘤医院乳腺病中心原发性乳腺癌(cT1-4N0-3M0)60例患者的临床资料,将患者分为3组:A组初始cN0且NAC后为ycN0,B组初始cN+且NAC后为ycN0,C组NAC后为ycN+。术前接受核素注射。术中A组和B组联合亚甲蓝行ASLNB。A组仅对腋窝前哨淋巴结(axillary sentinel lymph node,ASLN)阳性者行ALND;B组行ASLNB后转行ALND;C组直接行ALND。术前淋巴显像和(或)γ探测仪发现内乳前哨淋巴结(internal mammary sentinel lymph node,IM-SLN)的患者行IM-SLNB。结果:A组、B组和C组分别收集6例、45例和9例。A组ASLNB成功率为100%(6/6),仅1例ASLN阳性转行ALND。B组ASLNB成功率为100%(45/45),假阴性率为17.9%(5/28)。其中检出1枚、2枚和>2枚ASLN的假阴性率分别为27.3%(3/11)、20.0%(2/10)和0%(0/7)。C组所有患者ALN均有转移。IM-SLN总体显像率为63.3%(38/60)。IM-SLNB的总体成功率为97.4%(37/38),转移率为8.1%(3/37),并发症发生率为5.3%(2/38)。结论:对初始cN0且NAC后为ycN0者ASLN阴性时ASLNB可替代ALND;对初始cN+且NAC后为ycN0者,联合双示踪剂且检出>2枚ASLN可满足临床可接受的假阴性率(<10%);对NAC后仍为ycN+者应行ALND。NAC后IM-SLN显像者应行IM-SLNB,以获得完整分期、评估预后并指导术后放疗,有望完善病理完全缓解(pathological complete response,pCR)定义。
乳腺癌;新辅助化疗;腋窝前哨淋巴结活检;内乳前哨淋巴结活检
新辅助化疗(neoadjuvant chemotherapy,NAC)是局部晚期乳腺癌及部分有保乳意愿的早期乳腺癌患者的标准治疗模式。病理完全缓解(pathological complete response,pCR)是乳腺癌NAC后的独立预后指标[1]。达到pCR者的生存率能显著得到改善,尤其在侵袭性强的肿瘤亚型中更有预后价值[2]。pCR的定义为乳腺原发灶和腋窝淋巴结(axillary lymph node,ALN)手术标本病理检查无浸润性肿瘤细胞残余,且pCR后患者生存率显著提高[3],说明ALN病理状态在判断预后方面越来越受到重视。腋窝前哨淋巴结活检(axillary sentinel lymph node biopsy,ASLNB)替代腋窝淋巴结清扫(axillary lymph node dissection,ALND)已成为ALN阴性早期乳腺癌患者的标准治疗模式[4],但该模式用于NAC后ALN转阴患者尚存在争议。目前,pCR提升与患者生存改善的相关性仍待证实,可能与此前研究只评估ALN而未评估内乳淋巴结(internal mammary lymph node,IMLN)病理状况有关。本研究回顾性分析了NAC后ASLNB的成功率和假阴性率,以及内乳前哨淋巴结(internal mammary sentinel lymph node,IM-SLN)的显像率和内乳前哨淋巴结活检(internal mammary sentinel lymph node biopsy,IM-SLNB)的成功率、阳性率及并发症。
1 资料和方法
1.1 一般资料
通过回顾性分析2012年1月—2014年12月山东省肿瘤医院乳腺病中心连续收治的60例病理确诊为原发性浸润性乳腺癌(cT1-4N0-3M0)患者的资料,发现患者术前均接受完整疗程紫杉类联合蒽环类NAC(AC-T方案49例,AC-TH方案5例,TAC方案5例,FEC-T方案1例;人表皮生长因子受体-2阳性20例,仅5例接受曲妥珠单抗治疗),并接受手术治疗。排除标准为炎性乳腺癌、术前接受过腋窝手术或放疗的患者。
1.2 方法
1.2.1 研究分组
根据病例特点,将患者分为3组:A组为对NAC前超声及体格检查确定ALN阴性(cN0)且NAC后ALN仍为阴性(ycN0);B组为NAC前经细针穿
刺细胞学检查证实ALN阳性(cN+)且术前超声及体格检查未发现ALN异常(ycN0);C组为术前超声及体格检查发现ALN仍为阳性(ycN+)。
1.2.2 ASLNB
所有患者均术前3~18 h将99mTc-硫胶体(1.0~1.2 mL/9.25~18.5 MBq)在超声引导下注射于乳晕区外周6点和12点位的腺体层内,术前0.5 h行SPECT/CT淋巴显像(图1)。术中对A组和B组患者同时应用亚甲蓝[5]行ASLNB。A组腋窝前哨淋巴结(axillary sentinel lymph node,ASLN)术中行冷冻快速病理和印片细胞学检测[6],仅对ASLN阳性者行ALND;B组ASLNB后转行ALND;C组直接行ALND。
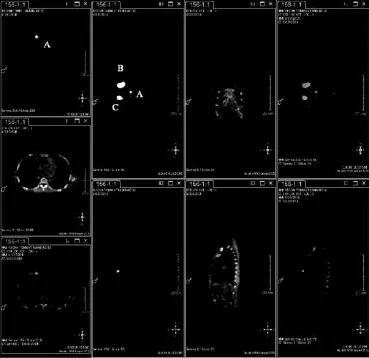
图 1 术前SPECT/CT显像Fig. 1 SPECT/CT image before operation
1.2.3 IM-SLNB
对术前淋巴显像和(或)术中γ探测仪发现内乳区有放射性活性聚集者均行经肋间IMSLNB,切开胸大肌并于胸骨旁相应肋间撑开胸大肌,暴露肋间肌,后于胸骨旁2~3 cm平行肋骨方向切开肋间肌,用γ探测仪定位IMSLN,将其完整取出,术中注意保护内乳血管及胸膜。接受保乳手术者,如原发肿瘤位置切口不适合行IM-SLNB,需另选合适的位置切口行IM-SLNB。
1.3 病理检查
所有检获的前哨淋巴结石蜡切片均行两层面苏木精-伊红染色病理检测。病理发现宏转移、微转移及孤立肿瘤细胞均认为前哨淋巴结阳性。
1.4 统计学处理
统计A组和B组ASLNB成功率、B组的假阴性率、C组的转移率,三组患者IM-SLN的显像率,IM-SLNB的成功率、阳性率及并发症。采用SPSS 17.0软件进行统计学分析。计量资料的组间比较采用独立样本t检验,计数资料的组间比较采用χ2检验或Fisher精确检验。P<0.05为差异有统计学意义。
2 结 果
入组60例患者的中位年龄为49岁(27~68岁),临床病理学特征见表1。
2.1 ASLNB结果
A组ASLNB成功率为100%(6/6),共检出19枚ASLN,中位数为3枚(2~6枚),1例ASLN阳性转行ALND。
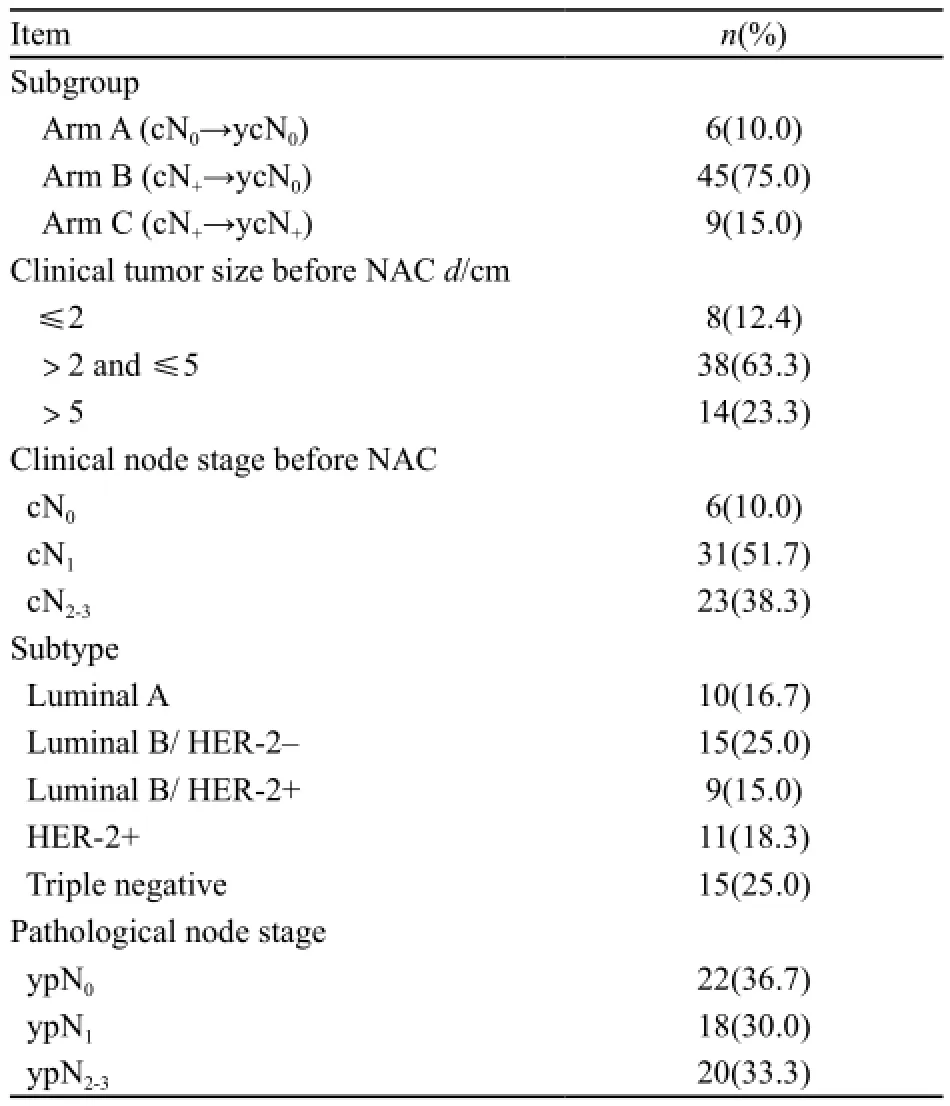
表 1 入组患者临床及病理学特征Tab. 1 Clinical and pathological characteristics of the enrolled patients
B组ASLNB成功率为100%(45/45),共检出99枚ASLN,中位数为2枚(1~6枚),1~6枚ASLN者分别有15、17、8、1、2和2例。B组ASLNB准确率为88.9%(40/45),假阴性率为17.9%(5/28),检出1枚、2枚和>2枚ASLN的假阴性率分别为27.3%(3/11)、20.0%(2/10)和0%(0/7)。B组cN1患者27例,总的假阴性率为21.1%,检出1枚、2枚和>2枚ASLN时假阴性率分别为33.3%(2/6)、33.3%(2/6)和0%(0/7);cN2-3患者18例,总的假阴性率为11.1%,检出1枚、2枚ASLN时假阴性率分别为20%(1/5)、0%(0/4),取出>2枚ASLN者仅3例,均达到ypN0,无法计算假阴性率。ALN转阴的患者17例,转阴率为37.8%(17/45)。
C组所有患者ALN均有转移。
2.2 IM-SLNB结果
IM-SLNB显像率为63.3%(38/60),γ探测仪均能发现IM-SLN(仅4例术前淋巴显像阴性)。38例患者的中位年龄为50岁(32~68岁),IM-SLN显像情况的临床资料见表2。
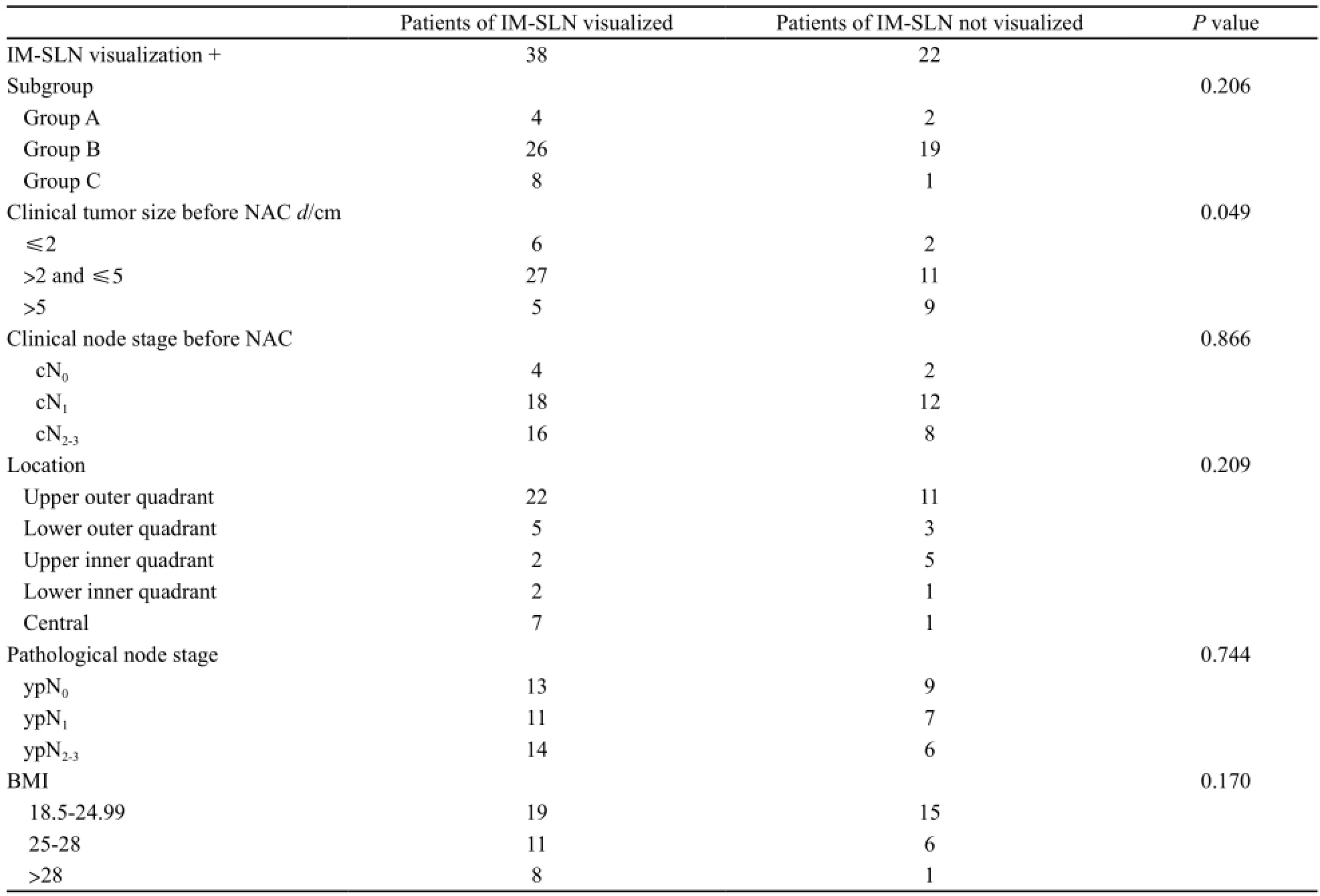
表 2 内乳前哨淋巴结显像者与未显像者临床资料Tab. 2 Clinical and pathological characteristics of the patients with and without IM-SLN visualization
IM-SLNB成功率为97.4%(37/38),仅1例因肋间隙太窄而未取出。共检出60枚IM-SLN,中位数为2枚(1~4枚),主要位于第2(44.7%,17/38,仅位于第2肋间6例)、3肋间(55.3%,21/38,仅位于第3肋间11例),中位耗时10 min(5~30 min)。
IM-SLN转移率为8.1%(3/37),1枚IM-SLN转移者2例,2枚IM-SLN转移者1例。3例IM-SLN阳性者均伴ALN转移(B组1例,C组2例)。
术中1例发生内乳动脉损伤,术中成功止血;1例出现点状胸膜损伤,术后行胸部X线检查未发现气胸。
2.3 NAC后pCR相关结果
在入组60例患者中,11例原发肿瘤和ALN均达到pCR,pCR率为18.3%(11/60),其中4例成功行IM-SLNB,IM-SLN病理均未转移。
3 讨 论
目前认为NAC后ALN的处理由患者NAC前ALN状况决定。《NCCN乳腺癌临床实践指南》[8]推荐,cN0患者在NAC前后均可接受ASLNB,但cN+患者的ASLNB存在较高假阴性率而尚存在争议。本研究采用蒽环类联合紫杉类方案行NAC,且蓝染料联合核素法行ASLNB,ASLN中位数为2,假阴性率为17.9%,仅检出1枚ASLN假阴性率为27.3%(SNFNAC 18.2%[9]、ACOSOGZ1071 31.5%[10]和SENTINA 24.3%[11]);检出2枚ASLN假阴性率为20.0%(ACOSOGZ1071 21.2%[10]、SENTINA 18.5%[11]);而检出>2枚ASLN假阴性率为0%(ACOSOGZ1071 9.1 %[10]、SENTINA 4.9%[11]),能满足临床可接受的假阴性率(<10%)。本研究示C组ALN均有转移,说明临床查体及超声检查有助于选择ASLNB的患者,对于ycN+患者不应行ASLNB。ACOSOGZ1071试验显示,NAC后超声可指导腋窝手术,超声检测ycN0且检出≥2枚ASLN有望使ASLNB的假阴性率低于10%[12]。此外,术前再次对ALN行细针穿刺活检、对苏木精-伊红染色阴性的ASLN再行免疫组织化学检测(对于微转移及孤立肿瘤细胞均认为ASLN阳性)也可帮助降低假阴性率[9]。NAC前在超声引导下对阳性ALN放置金属标记夹,并将此淋巴结作为ASLN,可以帮助降低假阴性率。
本研究显示,B组37.8%患者ALN达到ypN0,可能部分患者可免行ALND。在联合双示踪剂、检出>2枚ASLN等技术支持下(可使假阴性率<10%),可依据NAC的疗效,选择个体化手术治疗。对初始cN1且NAC后转阴者,在征得患者及家属同意后,可对ASLN阴性者选择ASLNB替代ALND。对于cN2-3患者,因临床资料较少,且肿瘤负荷较大,应谨慎处理,仍建议行ALND。这种模式转化,部分基于肿瘤负荷,部分基于全身治疗对局部区域控制的疗效,但其可行性还需等待前瞻性随机临床试验对总生存率和腋窝复发率结果的证实。而对NAC后ASLN阳性患者能否用腋窝放疗替代ALND还需等待A11202试验结果[12]。
目前pCR的定义仅包含ALN,IMLN同ALN一样是乳腺癌重要的淋巴转移途径,区域淋巴结的转移和降期不仅包含ALN,也需要考虑IMLN。本研究显示,在NAC后仍有8.1%的患者IM-SLN阳性,因此乳腺癌NAC后仍有必要行IM-SLNB,明确IMLN的病理状况,获得淋巴结的完整分期,从而预测预后。
IMLN转移的高危因素(IMLN转移率>20%)包括:≥4枚ALN转移、内侧肿瘤并ALN阳性、T3肿瘤并年龄<35岁、T2肿瘤并ALN阳性或内侧象限[13]。行NAC的大部分患者为局部晚期,是IMLN转移的高危患者,因此有必要明确这部分患者IMLN的状况。而由于IMLN解剖位置深在,且通常体积较小(长径1~5 mm),目前临床上应用的影像学检查只能检测出>5 mm的肿瘤,敏感性不能满足临床要求[14],术前很难准确评估IMLN是否存在转移,因此对NAC后患者行IM-SLNB,可明确IM-SLN的病理状况。目前,《NCCN乳腺癌临床实践指南》[8]推荐对NAC后IMLN阳性者行内乳区放疗,对于NAC后降期且行全乳切除+Ⅰ/Ⅱ级腋窝清扫+胸壁和锁骨上/下淋巴引流区域放疗者,即使临床未发现IMLN转移也强烈建议行内乳区放疗。在未行NAC的患者中IM-SLNB可以为内乳区提供准确的放疗指征,对病理诊断IM-SLN阳性者应行内乳区放疗,对阴性者无需行内乳区放疗[14]。但在
NAC后IM-SLN阴性者是否需要放疗仍需进一步研究。
本研究应用“新型示踪技术”(乳晕周边两点位、大体积和超声引导下)[15]IM-SLN的显像率达到63.3%,术前淋巴显像联合术中γ探测仪可有效定位IM-SLN。IM-SLNB的成功率为96.9%,中位耗时10 min,并发症发生率为5.3%,均在可接受的范围内,且术中内乳血管损伤及胸膜破损者术后无出血及气胸发生,IMSLNB作为一项微创分期、诊断技术,在NAC后也应得到认可和实践。
本研究中,3例IM-SLN阳性者ALN均有转移,随着接受IM-SLNB的患者样本量增大,将会出现原发肿瘤和ALN达到pCR而IM-SLN阳性者。ALN手术标本病理检查无浸润性肿瘤细胞残余已成为pCR定义的重要组成部分,可能在不久的将来,IM-SLN手术标本病理检查无浸润性肿瘤细胞残余也会成为pCR定义的组成部分,包括IMLN的pCR才是更精确的区域淋巴结pCR。
综上所述,初始cN0且NAC后为ycN0者ASLN阴性时ASLNB可替代ALND;对初始cN+且NAC后ALN转阴的患者,双示踪剂且检出>2枚ASLN可以满足临床可接受的假阴性率(<10%);对术前临床查体及超声检查发现ALN有异常者应行ALND;对NAC后IM-SLN有显像的患者可行IM-SLNB,明确乳腺癌患者的完整分期、评估预后并指导术后放疗,避免乳腺癌分期不准确、治疗不足或过度,并完善pCR的定义。
[1] BONNEFOI H, LITIERE S, PICCART M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial[J]. Ann Oncol, 2014, 25(6): 1128-1136.
[2] CORTAZAR P, ZHANG L, UNTCH M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis[J]. Lancet, 2014, 384(9938): 164-172.
[3] BERRUTI A, AMOROSO V, GALLO F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies[J]. J Clin Oncol, 2014, 32(34): 3883-3891.
[4] KUMAR A, PURI R, GADGIL P V, et al. Sentinel lymph node biopsy in primary breast cancer: window to management of the axilla [J]. World J Surg, 2012, 36(7): 1453-1459.
[5] 刘 广, 邱鹏飞, 王永胜, 等. 新辅助化疗后腋窝淋巴结转阴乳腺癌患者前哨淋巴结活检研究[J]. 中华内分泌外科杂志, 2013, 7(2): 111-114.
[6] SILVERSTEIN M J, LAGIOS M D, RECHT A, et al. Imagedetected breast cancer: state of the art diagnosis and treatment[J]. J Am Coll Surg, 2005, 201(4): 586-597.
[7] EDGE S B, BYRD D R, COMPTON C C, et al. AJCC cancer staging manual[M]. 7th ed. New York: Springer, 2010: 347-376.
[8] GRADISHAR W J, ANDERSON B O, BALASSANIAN R, et al. Breast Cancer Version 2.2015[J]. J Natl Compr Canc Netw, 2015, 13(4): 448-475.
[9] BOILEAU J F, POIRIER B, BASIK M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study[J]. J Clin Oncol, 2015, 33(3): 258-264.
[10] BOUGHEY J C, SUMAN V J, MITTENDORF E A, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial[J]. JAMA, 2013, 310(14): 1455-1461.
[11] KUEHN T, BAUERFEIND I, FEHM T, et al. Sentinellymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study[J]. Lancet Oncol, 2013, 14(7): 609-618.
[12] BOUGHEY J C, BALLMAN K V, HUNT K K, et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American College of Surgeons Oncology Group Z1071 trial (Alliance)[J]. J Clin Oncol, 2015. [Epub ahead of print]
[13] HUANG O, WANG L, SHEN K, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy[J]. Breast Cancer Res Treat, 2008, 107(3): 379-387.
[14] CONG B B, QIU P F, WANG Y S. Internal mammary sentinel lymph node biopsy: minimally invasive staging and tailored internal mammary radiotherapy[J]. Ann Surg Oncol, 2014, 21(7): 2119-2121.
[15] QIU P F, LIU J J, LIU Y B, et al. A modified technology could significantly improve the visualization rate of the internal mammary sentinel lymph nodes in breast cancer patients[J]. Breast Cancer Res Treat, 2012, 136(1): 319-321.
A retrospective study of axillary and internal mammary sentinel lymph node biopsy in breast cancer
patients after neoadjuvant chemotherapy
CAO Xiaoshan1,2, CONG Binbin1,2, SUN Xiao1, QIU Pengfei1,
LIU Yanbing1, ZHAO Tong1, CHEN Peng1, WANG Chunjian1, WANG Yongsheng1(1.Breast Cancer Center, Shandong Cancer Hospital & Institute, Jinan Shandong 250117, China; 2.School of Medicine and Life Sciences, Jinan University-Shandong Academy of Medical Sciences, Jinan Shandong 250200, China)
WANG Yongsheng E-mail: wangysh2008@ aliyun.com
Background and purpose: Whether axillary sentinel lymph node biopsy (ASLNB) could replace axillary lymph node dissection (ALND) in patients who converted after neoadjuvant chemotherapy (NAC) from cN+to ycN0is still contentious, and the previous study only evaluated the pathological status of ALN without internal mammary lymph node (IMLN) condition. This study is to evaluate roles of ASLNB and internal mammary sentinel
Breast neoplasm; Neoadjuvant chemotherapy; Axillary sentinel lymph node biopsy; Internal mammary sentinel lymph node biopsy
10.3969/j.issn.1007-3969.2015.08.008
R737.9
A
1007-3639(2015)08-0608-06
2015-02-06
2015-04-15)
山东省自然科学基金重点项目(ZR2014HZ2003)。
王永胜 E-mail:wangysh2008@ aliyun.com
lymph node biopsy (IM-SLNB) in breast cancer patients after NAC. Methods: From Jan. 2012 to Dec. 2014, 60 breast cancer cT1-4N0-3M0patients who were scheduled for neoadjuvant chemotherapy (NAC) and agreed to accept surgery after NAC from our department were enrolled into the retrospective study. Patients with cN0 before NAC and ycN0 after NAC underwent ASLNB (group A). Patients with cN+received NAC and ycN0after NAC (group B) were treated with ASLNB and ALND. Only patients whose clinical nodal status remained positive (ycN+) after NAC underwent ALND without ASLNB (group C). All the patients received radiotracer injection and patients in group A and group B received blue dye injection additionally. Meanwhile, IM-SLNB would be performed for all patients with IMSLN visualization. Results: The number of patients enrolled in group A, group B and group C was 6, 45 and 9 cases respectively. The accuracy rate of ASLNB in group A was 100% (6/6). Only one patient was axillary sentinel lymph node (ASLN) positive performed ALND. With combination of blue dye and radiolabeled colloid, the accuracy rate of ASLNB in group B was 100% (48/48) and the false negative rate (FNR) was 17.9% (5/28). The FNR in patients with 1, 2 and >2 SLNs examined was 27.3% (3/11), 20.0% (2/10) and 0% (0/7). All of the ALNs were positive in group C. The visualization rate of IM-SLN was 63.3% (38/60). The detection rate of IM-SLNB was 97.4% (37/38) and the metastasis rate was 8.1% (3/37). The incidence of complications was 5.3% (2/38). Conclusion: ASLNB can be performed either before or after preoperative chemotherapy for patients with cN0disease. Among women with cN+converted to ycN0who had 3 or more SLNs examined, the FNR could return to be less than 10%. Those patients whose nodes are still ycN+should perform ALND. IM-SLNB should be performed routinely in all breast cancer patients after NAC, for it might help to make clear of the nodal staging and the pathological status of IM-SLN and provide the accurate indication of radiation to the internal mammary area in case of under-stage and under-/over-treatment, expecting to develop the definition of pathological complete response (pCR).
