急性主动脉夹层患者主动脉组织和血浆中微小核糖核酸的差异表达*
2015-12-15王晓建黄毕樊晓寒苏文君张良路天怡田力杨艳敏惠汝太张澍
王晓建 ,黄毕,樊晓寒,苏文君,张良,路天怡,田力,杨艳敏,惠汝太,张澍
急性主动脉夹层患者主动脉组织和血浆中微小核糖核酸的差异表达*
王晓建 ,黄毕,樊晓寒,苏文君,张良,路天怡,田力,杨艳敏,惠汝太,张澍
目的:本研究旨在寻找新的可作为急性主动脉夹层(AAD)诊断标志物的微小核糖核酸(miRNAs)。
方法:入选4例A型AAD患者的升主动脉病变组织标本(AAD1组)和4例无心血管疾病的器官捐赠者的升主动脉组织标本(NC1组);入选20例A型AAD患者(AAD2组)和20例无心血管疾病正常对照者(NC2组)并分别采集血浆,两组病例对照的年龄、性别严格匹配。利用miRNA芯片技术,检测四组受试者的升主动脉组织和血浆miRNAs表达谱,整合两组分析结果,确定AAD患者组织和血浆均差异表达的miRNAs。
结果:主动脉组织miRNA芯片结果显示,AAD患者中30个miRNAs表达显著改变,其中13个表达上调,17个表达下调。血浆miRNA芯片分析显示,AAD患者有93个miRNA差异表达,其中33个表达上调,60个表达下调。整合两个表达谱,共有4个miRNAs(miR-4313、-933、-1281和-1238)在血浆和组织均显著升高。其中,AAD1组较NC1组、AAD2组较NC2组miR-4313分别上调1.5倍和42.4倍, miR-933分别上调10.4倍和26.8倍,miR-1281分别上调1.7倍和17.8倍,miR-1238分别上调1.3倍和13.8倍。
结论:通过miRNA芯片分析,我们筛选到4个在AAD患者主动脉组织和血浆中均升高的miRNAs。这些miRNA可能是诊断AAD的生物标志物,但需大样本验证。
miRNA芯片;急性主动脉夹层;miRNA差异表达
Methods: Our work included 2 sets of groups.①AAD 1 tissue group, containing the aortic tissue of A type AAD patients and Control 1 group, containing the aortic tissue of the subjects without cardiovascular disease. n=4 in each group.②AAD 2 plasma group, containing the plasma of A type AAD patients and Control 2 group, containing the plasma of the subjects without cardiovascular disease. n=20 in each group. The age and gender were strictly matched between the patients and control subjects. The miRNAs expression in ascending aortic tissue and plasma were examined by microarray analysis in 4 groups, and the results in 2 sets of groups were integrated to identify the differential expression of miRNAs in AAD patients.
Results: The microarray analysis indicated that in AAD patients, the aortic tissue had 30 differentially expressed
miRNAs, 13 of them were up-regulated and 17 were down-regulated; plasma had 93 differentially expressed miRNAs, 33 of them were up-regulated and 60 were down-regulated. With integrated analysis, 4 miRNAs expressions were increased in both aortic tissue and plasma as miR-4313, miR-933, miR-1281 and miR-1238. Compared with Control 1 group and Control 2 group, AAD 1 group and AAD 2 group showed up-regulated miR-4313 at 1.5-fold and 42.4-fold, up-regulated miR-933 at 10.4-fold and 26.8-fold, up-regulated miR-1281 at 1.7-fold and 17.8-fold, up-regulated miR-1238 at 1.3-fold and 13.8-fold respectively.
Conclusion: There were 4 differentially expressed miRNAs in both aortic tissue and plasma in AAD patients, which might be the potential biomarkers of AAD, while large sample investigation is needed.
(Chinese Circulation Journal, 2015,30:154.)
急性主动脉夹层(AAD)是心血管疾病的危急重症,具有发病突然、进展迅速、死亡率高的特点。由于缺乏特异的临床表型,AAD初诊误诊率往往超过25%,甚至达到50%[1-6]。诊断延误与预后密切相关,未及时诊治的主动脉夹层患者3天和2周死亡率分别高达50%和80%[7,8]。因此,提高主动脉夹层诊断水平是改善患者预后的关键。血浆生物标志物具有操作便捷、价格便宜等特点,在主动脉夹层诊断中被日益重视。D-二聚体、平滑肌肌球蛋白重链(SM-MHC)、脑型肌酸激酶(CK-BB)、Calponin是已发现的AAD血浆标志物[9-12]。这些因子在AAD患者血浆中显著升高,具有一定的指示意义,但由于敏感性或特异性不高,尚不能成为诊断AAD理想的生物标志物。微小核糖核酸(miRNAs)是一类长约21~23个碱基的单链非编码小分子RNA,与疾病密切相关并在血浆中稳定存在[13,14]。目前已有多个miRNA被证实为心血管疾病的分子标示物,但miRNA与AAD的研究较少。本研究拟通过分析AAD患者和正常对照者主动脉组织和外周血miRNA的表达差异,筛选可能作为AAD诊断的生物标志物,为AAD的诊治提供新思路和新策略。
1 资料与方法
主动脉组织样本的选取及采集:2011-01至2011-05期间,采集我院4例A型AAD患者(AAD1组)的升主动脉病变组织标本。患者均为男性,平均年龄(49.1±4.9)岁。AAD患者均经过计算机断层扫描摄影术(CT)确诊,并排除马凡综合征、Loeys-Dietz综合征及家族性主动脉夹层瘤。患者从发病至手术时间不超过24小时。术中取出典型病变组织标本,立即分为1 cm3的小块,置于冰浴的无RNA酶冻存管中,液氮速冻,即刻转移至-80℃冰箱中保存备用。正常对照的组织标本来自4例无心血管疾病的器官捐赠者(NC1组),均为男性,平均年龄(47.9±6.7)岁。升主动脉组织标本的采集和保存方法同前。
血浆样本的选取及采集:2011-01至2011-09,选取我院入选急性A型AAD患者20例(AAD2组),平均年龄(53.3±13.5)岁,男性14例(70%);同时选取超声心动图和心电图检查确认无心血管病疾病者20例(NC2组)作为对照,男性14例(70%),平均年龄(51.9±11.2)岁。AAD患者诊断排除标准同前,且从发病至采血不超过24小时。采集两组受试者的4 ml静脉血,乙二胺四乙酸(EDTA)抗凝,4℃低温离心机离心(2 000 g,10分钟),分离血浆,-80℃储存备用。本项目全部研究内容均获得阜外心血管病医院伦理委员会同意,所有受试者均签署知情同意书。
总RNA提取及质检:①组织样本:使用mirVanaTMmiRNA Isolation Kit (AM1560, Ambion, Austin, TX, USA)提取总RNA。抽提所得总RNA经Agilent Bioanalyzer 2100 (Agilent technologies, Santa Clara, CA, USA) 检测浓度和纯度,变性琼脂糖凝胶电泳检测完整性。AAD1组4例患者样本和NC1组4例正常对照样本的总RNA的OD260/OD280均在2.0~2.1之间,28 s和18 s条带清晰,且28 s/18 s>2。 ② 血浆样本:从AAD2组和NC2组每个受试者的血浆中取10 μl,将各组的20位受试者血浆分别混合。使用mirVanaTMPARISTMKit(AM1556, Ambion, Austin, TX, USA) 提取血浆总RNA。Agilent Bioanalyzer 2100检测浓度和纯度。两组的总RNA的OD260/OD280均在1.9~2.1之间。
芯片实验:① 组织样本:采用Agilent human miRNA (8×60 K) V16.0芯片 (Agilent technologies, Santa Clara, CA, USA)检测AAD1组和CN1组组织样本miRNA表达谱,样本经荧光标记后,在滚动杂交炉中与芯片杂交,采用Agilent Microarray Scanner扫描芯片结果,用Feature Extraction software 10.7读取数据,采用Gene Spring Software 11.0进行归一化处理。②血浆样本:采用Agilent human miRNA(8×60 K)V19.0芯片检测AAD2组和NC2组血浆样本分别混合后miRNA表达谱。所有操作同前。组织和血浆样本的miRNA芯片实验由上海伯豪生物技术有限公司完成。
统计学分析:数据分析包括芯片数据的统计学分析、差异表达miRNA靶基因分析。以对照组主动脉组织样本中各miRNA表达量的数学均数为正常值,主动脉夹层患者组织样本分别与之比较。差异表达的miRNAs的筛选标准为:表达倍数变化量(foldchange)≥1.3倍(上调)或≤0.27倍(下调),且P<0.05。
2 结果
四组受试者的基线资料比较:AAD1组与NC1组、AAD2组与NC2组的年龄、性别完全匹配,差异无统计学意义(P>0.05)。AA2组较NC2组的高血压患者比例高,差异有统计学意义(P<0.05),四组的动脉粥样硬化患者比例差异无统计学意义(P>0.05)。表1
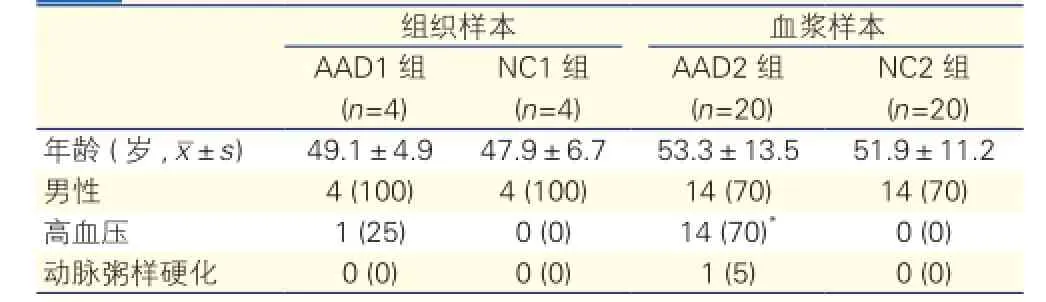
表1 四组受试者的基线资料比较 [例(%)]
AAD1组与NC1组主动脉组织miRNA差异表达的结果:30个miRNAs在AAD1组中差异表达(图1),13个miRNAs表达上调,17个miRNAs表达下调。miRNAs差异表达量增加或降低数倍至数百倍不等,上调量最大的miRNA是has-miR-31,较NC1组表达增加496倍,下调最大的miRNA是has-miR-936,较NC1组下调超过300(1/0.003)倍。表2
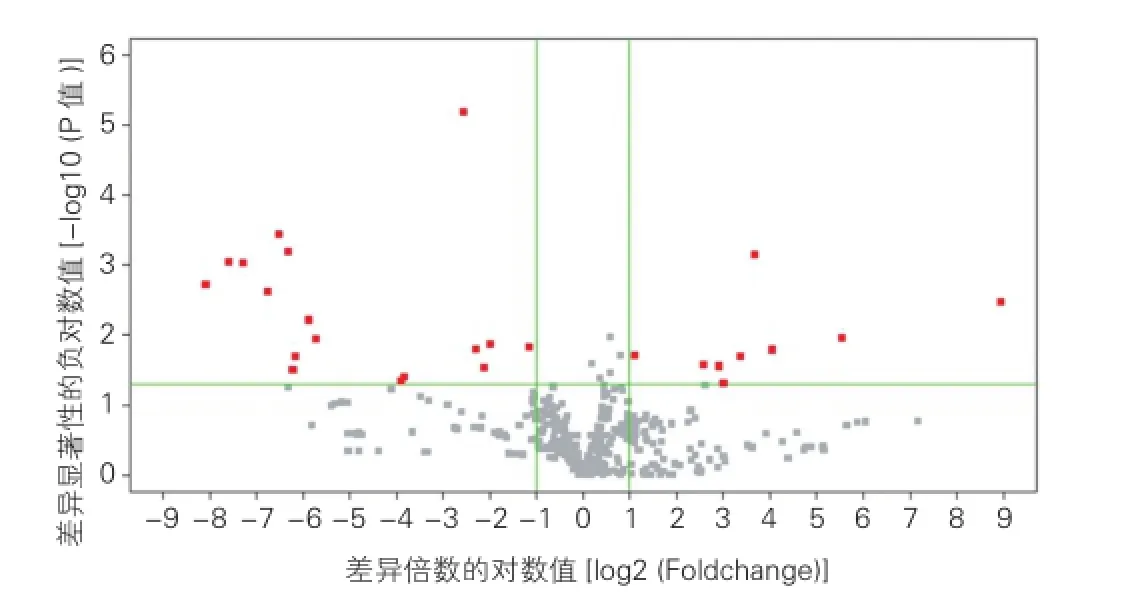
图1 AAD1组与NC1组的主动脉组织差异表达miRNA的火山图
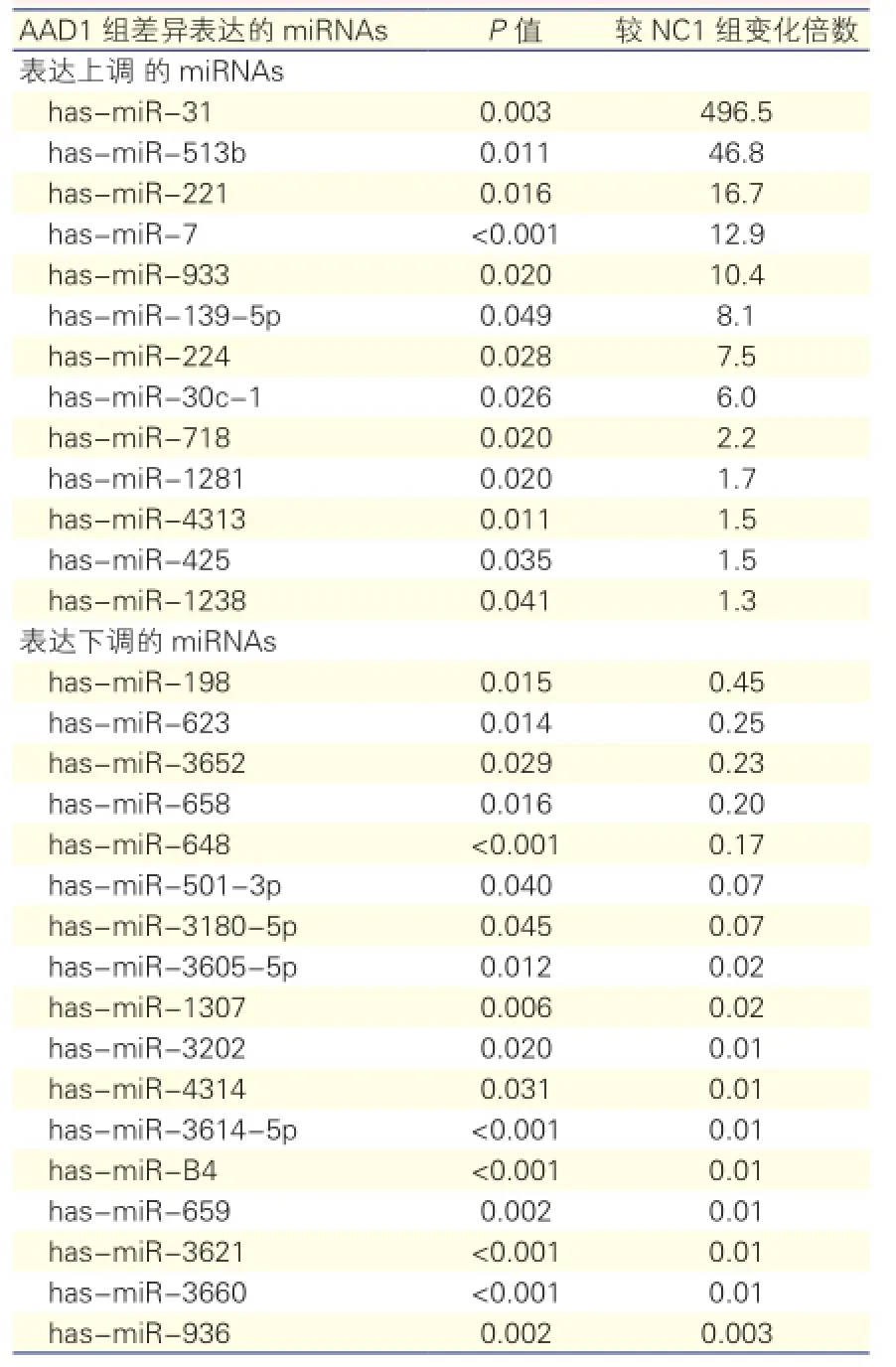
表2 AAD1组与NC1组主动脉夹层组织中miRNA差异表达的miRNAs
AAD2组与NC2组血浆miRNA表达的结果: 93个miRNAs在AAD2组中差异表达,33个表达上调,60个表达下调。表达差异最显著的有20个miRNAs(表3)。上调最显著的是has-miR-4313,AAD2较NC2组增加42.2倍;下调最显著的是hasmiR-4454,AAD2组仅为NC2组的1/100。
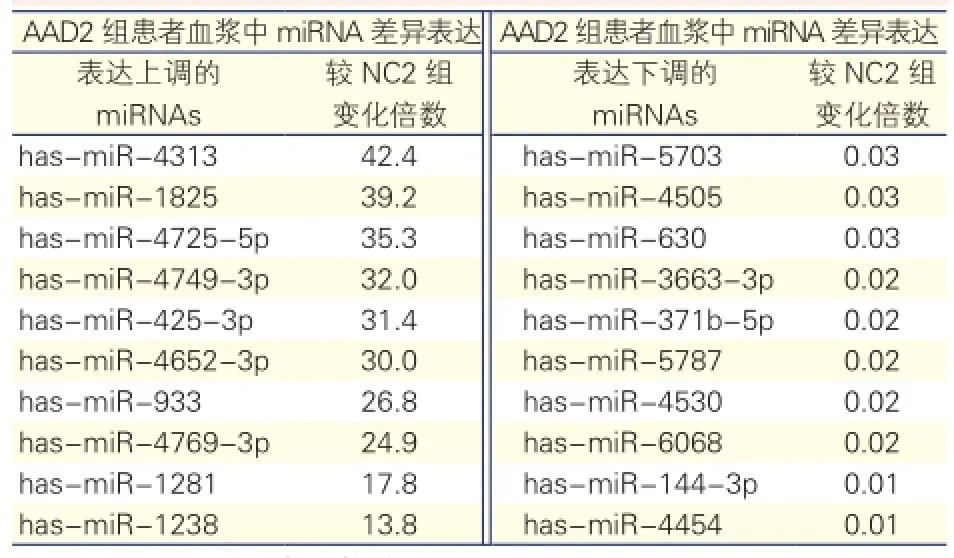
表3 AAD2组与NC2组患者血浆中miRNA差异表达最显著的20个miRNAs
AAD1组组织差异表达miRNA与AAD2组血浆差异表达miRNA整合分析结果:共发现了4个在组织和血浆中均差异表达的miRNAs,分别是hasmiR-4313、has-miR-933、has-miR-1281和hasmiR-1238(表4)。这4个miRNAs在AAD1组合AAD2组的组织和血浆中均表达上调,其中上调量较高的是has-miR-4313和has-miR-933。AAD1组较NC1组、AAD2组较NC2组has-miR-4313分别上调1.5倍和42.4倍,has-miR-933分别上调10.4倍和26.8倍。
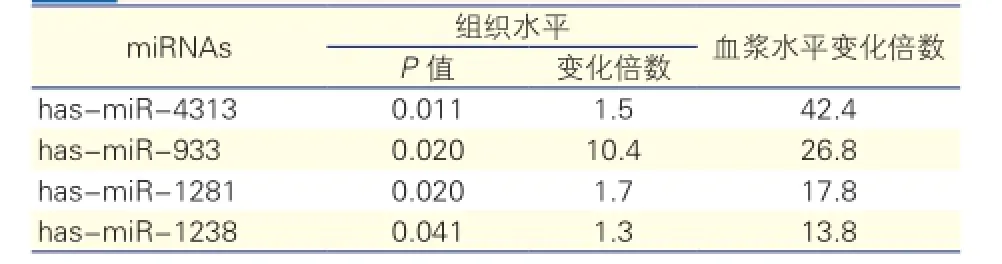
表4 AAD1组和AAD2组差异表达的miRNAs整合分析结果
3 讨论
本研究利用miRNA芯片分析了四组受试者的主动脉组织和血浆中miRNA表达曾找到了四个在组织和血浆中均差异表达的miRNAs。这四个miRNA既与主动脉夹层的病变密切相关,亦在外周血显著升高,很可能成为新的AAD诊断生物标志物。
近年来,多项研究表明miRNAs在血管性疾病的发生、发展中发挥重要的作用。全身敲除miRNAs成熟所必须的Dicer酶,小鼠在胚胎期即出现血管生成障碍[15],血管平滑肌特异性敲除Dicer基因,小鼠会出现腹腔出血和胚胎期死亡[16]。因此,miRNAs在血管发育过程中起重要调节作用。此外,miRNAs已被证实参与血管平滑肌细胞从收缩型向合成型的表型转化。过表达miR-143和mir-145可促进血管平滑肌细胞收缩表型的形成,而抑制miR-143和miR-145促进合成型表型的形成[17,18]。Leeper等[19]发现,过表达miR-21后可抑制腹主动脉瘤的进展,相反,抑制miR-21可促进腹主动脉瘤的进展[20]。
miRNAs在病灶部位的表达与疾病进程密切相关,由于其在血浆中稳定存,病灶部位的miRNA表达改变会直接反应到外周血miRNA水平,进而可作为诊断疾病、判断治疗反应或预测预后的血清标志物[21-25]。多个miRNAs已被证实对心血管疾病如心肌梗死、心力衰竭及心律失常等的诊断具有重要价值[14,26],但miRNAs在主动脉疾病方面的研究还很少。Liao等[27]对6例主动脉夹层患者和6例正常对照通过芯片分析差异表达的miRNAs,结果发现主动脉夹层患者18个miRNAs上调,56个miRNAs下调。胡孜阳等[28]对5例主动脉夹层患者和4例正常对照进行主动脉组织的miRNAs差异表达分析,发现主动脉夹层患者有3个miRNA表达上调,2个miRNAs表达下调。这些研究均表明在AAD的病变血管中miRNA表达发生显著改变,但是AAD患者血浆中miRNA是否有改变,miRNA在AAD的临床诊断及预后方面是否具有价值,目前尚不明确。
本研究首次同时分析AAD患者病变血管组织和外周血血浆中miRNA表达差异,筛选出了4个患者主动脉组织和血浆均表达上调的miRNAs。生物信息学分析提示,这些miRNAs预测的靶蛋白广泛参与胶原、蛋白聚糖代谢、细胞信号转导及炎症等多个与主动脉夹层的发病机制密切相关的分子通路。因此,这些差异表达的miRNAs不仅是潜在的AAD的血清标志物,也很可能参与了AAD的发病过程。
我们的研究虽然有了明确的提示,但也存在一些局限性,例如样本数较少、只关注了AAD发病急性期的血浆水平等。既往的研究提示,miRNA芯片技术虽然可用来高通量筛选差异miRNAs,但存在一定的假阳性,需经大样本的验证以减少假阳性。将来,我们课题组将扩大样本量,进一步验证这些miRNAs鉴别诊断AAD的特异性和敏感性。在AAD发病的不同时间点上收集血浆,确定miRNA表达变化的时间窗。另外,值得注意的是,虽然本研究发
现AAD患者和正常对照miRNAs表达有差异,但距临床应用尚有较长距离。由于影像学技术对AAD的诊断具有较高的敏感性和特异性,影像学方法诊断和排除AAD仍是首选。本研究发现AAD患者主动脉组织和血浆均表达上调的4个miRNAs,可能对鉴别诊断急性AAD有一定帮助,但需要更大样本量的研究予以证实。
[1] Spittell PC, Spittell JJ, Joyce JW, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc, 1993, 68: 642-651.
[2] Klompas M. Does this patient have an acute thoracic aortic dissection? J Am Med Assoc, 2002, 287: 2262-2272.
[3] Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol, 2007, 99: 852-856.
[4] 滕玥, 高燕, 冯树行, 等. 主动脉夹层131例急诊诊断及误诊分析.中国误诊学杂志, 2012, 12: 116.
[5] 明广华, 张宇辉, 吴海英, 等. 179例主动脉夹层患者的临床资料分析. 中国循环杂志, 2004, 19: 363-366.
[6] 王水云, 马润芬, 黄志军, 等. 主动脉夹层急诊诊断与误诊分析.中华急诊医学杂志, 2003, 12: 619-621.
[7] Coady MA, Rizzo JA, Goldstein LJ, et al. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin, 1999, 17: 615-635.
[8] Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease. Circulation, 2010, 121: e266-e369.
[9] Shimony A, Filion KB, Mottillo S, et al. Meta-analysis of usefulness of d-dimer to diagnose acute aortic dissection. Am J Cardiol, 2011, 107: 1227-1234.
[10] Katoh H, Suzuki T, Hiroi Y, et al. Diagnosis of aortic dissection by immunoassay for circulating smooth muscle myosin. Lancet, 1995, 345: 191-192.
[11] Suzuki T, Katoh H, Kurabayashi M, et al. Biochemical diagnosis of aortic dissection by raised concentrations of creatine kinase BB-isozyme. Lancet, 1997, 350: 784-785.
[12] Suzuki T, Distante A, Zizza A, et al. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur Heart J, 2008, 29: 1439-1445.
[13] Hata A. Functions of miRNAs in cardiovascular biology and disease. Annu Rev Physiol, 2013, 75: 69-93.
[14] Huang W, Yu Q, Wang Q, et al. Roles of miRNA in cardiovascular development and dysfunction. Curr Med Chem, 2013, 20: 3613-3622.
[15] Yang WJ, Yang DD, Na S, et al. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem, 2005, 280: 9330-9335.
[16] Albinsson S, Suarez Y, Skoura A, et al. MiRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol, 2010, 30: 1118-1126.
[17] Cordes KR, Sheehy NT, White M P, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature, 2009, 460: 705-710.
[18] Xin M, Small EM, Sutherland LB, et al. miRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev, 2009, 23: 2166-2178.
[19] Leeper NJ, Raiesdana A, Kojima Y, et al. miRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol, 2011, 226: 1035-1043.
[20] Maegdefessel L, Azuma J, Toh R, et al. miRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med, 2012, 4: 122ra22.
[21] Turchinovich A, Weiz L, Langheinz A, et al. Characterization of extracellular circulating microRNA. Nucleic Acids Res, 2011, 39: 7223-7233.
[22] Zhu X, Lv M, Wang H, et al. Identification of Circulating miRNAs as Novel Potential Biomarkers for Gastric Cancer Detection: A Systematic Review and Meta-Analysis. Dig Dis Sci, 2014, 59: 911-919.
[23] Murakami Y, Tamori A, Itami S, et al. The expression level of miR-18b in hepatocellular carcinoma is associated with the grade of malignancy and prognosis. BMC Cancer, 2013, 13: 99.
[24] Lu J, Xu X, Liu X, et al. Predictive value of miR-9 as a potential biomarker for nasopharyngeal carcinoma metastasis. Br J Cancer, 2014, 110: 392-398.
[25] To KK. MicroRNA: a prognostic biomarker and a possible druggable target for circumventing multidrug resistance in cancer chemotherapy. J Biomed Sci, 2013, 20: 99.
[26] Kinet V, Halkein J, Dirkx E, et al. Cardiovascular extracellular microRNAs: emerging diagnostic markers and mechanisms of cell-tocell RNA communication. Front Genet, 2013, 4: 214.
[27] Liao M, Zou S, Weng J, et al. A microRNA profile comparison between thoracic aortic dissection and normal thoracic aorta indicates the potential role of microRNAs in contributing to thoracic aortic dissection pathogenesis. J Vasc Surg, 2011, 53: 1341-1349.
[28] 胡孜阳, 罗建方, 钟诗龙, 等. 应用基因芯片初步分析主动脉夹层与正常主动脉微小RNA的差异表达. 中华心血管病杂志, 2012, 40: 406-410.
Differential Expression of microRNAs in Aortic Tissue and Plasma in Patients With Acute Aortic Dissection
WANG Xiao-jian, HUANG Bi, FAN Xiao-han, SU Wen-jun, ZHANG Liang, LU Tian-yi, TIAN Li, YANG Yan-min, HUI Ru-tai, ZHANG Shu.
State Key Laboratory of Cardiovascular Disease, Cardiovascular Institute and Fu Wai Hospital, CAMS and PUMC, Beijing (100037), China
Objective: We want to identify the new microRNAs (miRNAs) biomarker for diagnosing the patients with acute aortic dissection (AAD).
miRNA chips; Acute aortic dissection; miRNA differential expression
2014-06-04)
(编辑:曹洪红)
国家自然科学基金(81170286,81300184)
100037 北京市,中国医学科学院 北京协和医学院 国家心血管病中心 阜外心血管病医院 心血管疾病国家重点实验室 (王晓建、惠汝太), 心律失常中心(樊晓寒、张澍) ,急重症中心(黄毕、田力、杨艳敏) ,心外科(张良、苏文君、路天怡)
王晓建 副研究员 博士 主要从事心血管疾病遗传学及转化医学研究 Email:wang_xiaojian@vip.163.com 通讯作者:樊晓寒
Email:ehan4348ff@gmail.com
R541
A
1000-3614(2015)02-0154-05
10.3969/j.issn.1000-3614.2015.02.015
