Synergistic effect of RhBMP-2 and bFGF on ectopic osteogenesis in mice
2015-12-08ShuYuanMaZhiQiangFengRenFaLaiZhiYingZhouZhongDaYin
Shu-Yuan Ma, Zhi-Qiang Feng, Ren-Fa Lai, Zhi-Ying Zhou, Zhong-Da Yin
First Affiliated Hospital of Ji'nan University, Guangzhou, Guangdong-510630, China
Synergistic effect of RhBMP-2 and bFGF on ectopic osteogenesis in mice
Shu-Yuan Ma#, Zhi-Qiang Feng#, Ren-Fa Lai*, Zhi-Ying Zhou*, Zhong-Da Yin
First Affiliated Hospital of Ji'nan University, Guangzhou, Guangdong-510630, China
ARTICLE INFO
Article history:
Received 26 October 2014
Received in revised form 10 November 2014
Accepted 22 December 2014
Available online 20 January 2015
BMP-2
bFGF
Ectopic osteogenesis
Micro-CT
Objective: To investigate the synergistic effect and mechanism of the combined application of recombinant human bone morphogenetic protein-2 (rhBMP-2) and basic fibroblast growth factor (bFGF). Methods: 24 KM male mice were randomly divided into 6 groups with 4 mice in each group, namely, Group A (control group), Group B (only treated with collagen), Group C (treated with 2 ng bFGF+collagen), Group D (treated with 4 μg rhBMP-2+collagen), Group E (treated with 4 μ g rhBMP-2+2 ng bFGF+collagen) and Group F (treated with 4 μg rhBMP-2+4 ng bFGF+collagen). The composites were implanted into the intermuscular septum of hind legs mice; whereas in control group, intermuscular septum of mice was separated and no implantation was performed. General observation, detection of concentration of calcium content, micro computed tomography (Micro-CT), three-dimensional reconstruction scan, measurement of bone mineral density (BMD), bone volume fraction (BVF) and trabecular thickness (Tb.Th), as well as histological observation with HE staining and ALP and CD34 immumohistochemical staining were performed. Results: Ectopic osteogenesis was found in Groups D, E and F mice. The difference in concentration of calcium contents was statistically significant between Groups D and E (P<0.05), but insignificant between Groups E and F (P>0.05). Micro-CT and three-dimensional reconstruction revealed continuous newborn bone substance in external surface of ectopic bone formation, and the center of bone formation did not show obvious substantial filling by bone substance. The differences in BMD, BVF and Tb.Th were statistically significant between Groups D and E or F (P<0.01 or <0.05). HE staining showed that in Groups D, E and F, newborn bone substance was mainly located at the edge of ectopic bone formation, and the bone formation in Groups E and F was better than that in Group D. ALP and CD34 immumohistochemical staining revealed the positive expression mainly at the edge of ectopic bone formation, and area of positive expression in Groups E and F was larger than that in Groups D. Conclusions: rhBMP-2 possesses the capacity to induce ectopic osteogenesis independently, but bFGF does not have this ability; the combined application of rhBMP-2 and bFGF can enhance the synergetic effect on inducing ectopic osteogenesis.
1. Introduction
Employing tissue engineering to repair various bone defects is the research focus of scholars. Growth factor is the key factor in tissue engineering technology; adding appropriate growth factor or rational collocation and use of growth factor can speed up the process of bone induction and osteogenesis, achieving the acceleration of bone defect repair. Bone morphogenetic protein-2 (BMP-2) and basic fibroblast growth factor (bFGF) are two important bone growth factors in bone matrix. BMP-2 possesses remarkable capacity to induce osteogenesis[1,2]; it can impel the directional differentiation of mesenchymal cell and precursor cell in bone marrow into chondroblast and osteoblast, expediting the regeneration and repair process of bone tissues. bFGF is a broad spectrum irritant for mitogen and blood capillary proliferation, effectively enhancing the proliferation of fibroblast, vascular endothelial cell, chondrocyte and osteoblast from mesoderm and neural ectoderm. bFGF is also applied in maintaining self-renewal, high proliferation, multiple-directional differentiation potential of stem cells[3]. However, there are few reports about the osteogenic activity and mechanism of combined application of BMP-2 and bFGF. The present study aims to evaluate the synergistic effect of BMP-2 and bFGF in mice
during the ectopic osteogenesis process, so as to investigate the mechanism of the combined application of these two substances, providing scientific basis for bone repair materials for tissue engineering.
2. Materials and methods
2.1. Experimental animals and materials
KM male mice weighting 16-20 g were provided by experimental animal center of Sun Yat-sen University [License No.: SCXK (Yue) 2011-0029]. Recombinant human bone morphogenetic protein-2 (rhBMP-2), basic fibroblast growth factor (bFGF) and type Ⅰ collagen were obtained from Bioengineering Institute of Jinan University.
2.2. Experimental methods
2.2.1. Preparation of composite materials
The composite materials were prepared with 0.01 mol/L phosphate buffer solution (PBS), type Ⅰ collagen solution, 0.2 ng/μL bFGF solution and 0.4 mg/mL rhBMP-2 solution according to the following dosage.
Twelve samples of each above prepared solution were added into 96-well culture plate, and each well was added with 400μL solution. The 96-well plate was freeze-dried in a lyophilizer, and samples were sterilized at 60 ℃ for use. The rhBMP-2 and bFGF contents in each sample are as follows: Group B had no rhBMP-2 and bFGF; Group C had 2 ng bFGF; Group D had 4μg rhBMP-2; Group E had 4 μg rhBMP-2 and 2 ng bFGF; Group F had 4 μg rhBMP-2 and 4 ng bFGF (Table 1).
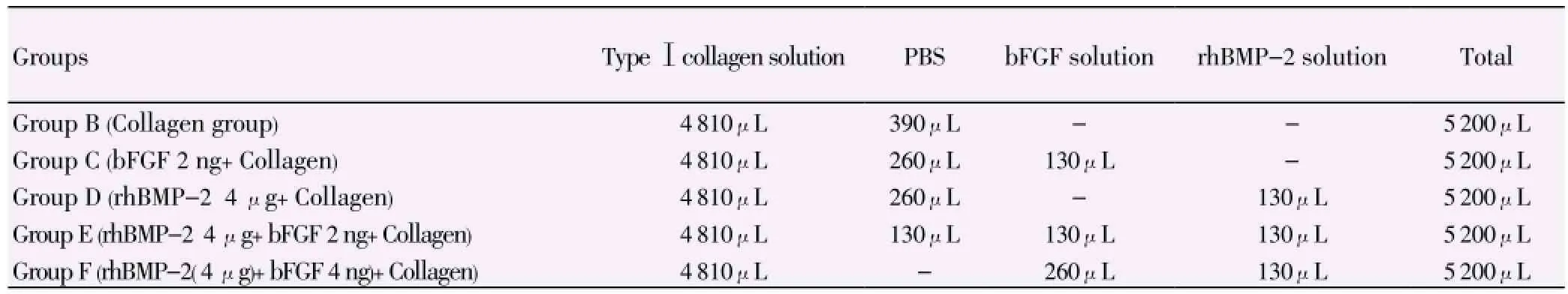
Table 1 Preparation of composite materials.
2.2.2. Establishment of animal model
Twenty four male KM mice were raised for one week for adaption to environment and randomly divided into 6 groups with 4 mice in each group, namely, Group A (control group), Group B (only treated with collagen), Group C (treated with 2 ng bFGF+collagen), Group D (treated with 4 μg rhBMP-2+collagen), Group E (treated with 4 μg rhBMP-2+2 ng bFGF+collagen) and Group F (treated with 4 μg rhBMP-2+4 ng bFGF+collagen). Mice were intraperitoneally injected with 2% pentobarbital sodium for anesthesia. The hair of mice hind legs was shaved, and skin was cleaned, sterilized and incised. Then the composite materials were implanted into the separated Intramuscular septum of mice. However, in control group, intermuscular septum of mice was separated and no implantation was performed. After surgery, each mouse was raised independently in cages.
2.2.3. Observation after surgery
General observation was conducted for the activity, condition of taking food and wound healing of mice in each group and two weeks after surgery, mice were sacrificed. The sacrificed mice in each group were randomly divided into two subgroups. The composite and newborn bone tissues in the implantation area of one subgroup mice were collected and adhered muscle was removed. Then the collected parts were cut into pieces by ophthalmic scissors, and pieces were placed into tubes; each tube was added with 1 mL 0.6 M HCl, sealed, shaken up and stored at room temperature over 24 h. Then samples in tubes were centrifuged and supernatant was collected and constant volume was set for determination of concentration of calcium content. The corresponding implantation parts of hind legs of the other subgroup mice were obtained and fixed in 10% neutral formalin solution for Micro-CT scan and histological observation. The scanning parameters for Micro-CT are as follows: scanning resolution: 20μm; rotation angle: 360°; increment of rotation angle: 0.72°; voltage: 70 kV; power: 40 W; frame mean: 2; interlayer spacing: 20μm. The obtained CT image was established into three-dimensional reconstructed image using ZKKSMCT-Sharp corresponding software. Region of interest (ROI) (0.5 mm×0.5 mm×0.2 mm) was selected to evaluate the osteogenesis in each group mice, and bone parameters were analyzed. The parameters included bone mineral density (BMD, mg/cc), bone volume fraction (BVF), which refers to the ratio of bony structure volume and total volume of sample, namely, ratio of mineralized tissues, as well as trabecular thickness (Tb.Th, μm ), which is the mean thickness of trabecula and refers to hole wall thickness for porous materials. After Micro-CT scan, HE staining as well as ALP and CD34 immunohistochemical staining were performed for samples of each group mice, and histological findings were compared.
2.2.4. Statistical analysis
All the data obtained were expressed as mean±SD and analyzed for variance using software SPSS 13.0. Bonferroni
test was used for comparison between any two groups. If there is heterogeneity of variance in each group, Tamhane’s T2test will be employed to compare the sample mean between any two groups. Difference is considered to be statistically significant at P<0.05.
3. Results
3.1. General observation
All the mice in each group revived at the same day after surgery, but activity and intake of food were reduced. One to two days after surgery, activity and intake of food became normal. All the surgical wounds in mice were healed by first intention; no swelling and exudation were found, and the sutures were self-exfoliated. The body weight of each group mice increased slowly, and mice were active to move and take food. When mice were dissected for measurement of concentration of calcium content, ectopic osteogenesis was not found in implantation area of Groups A, B and C mice, and the implanted composites were generally degraded. However, Groups D, E and F mice showed ectopic osteogenesis.
3.2. Measurement of concentration of calcium content
The difference in calcium content between Groups E and D was statistically significant (P<0.05), and the concentration of calcium content in Group E was the highest (Table 2).

Table 2 Calcium content of each group mice (n=4, mg/dL).
3.3. Micro-CT scan and three- dimensional reconstruction
Micro-CT scan revealed that ectopic osteogenesis was not found in Groups A, B and C mice. However, Groups D, E and F mice showed ectopic osteogenesis; the shape of bone formation was irregular or long shuttle-like, and the gray level was comparatively lower than that of normal hindlimb bone cortex. The external surface of ectopic bone formation was surrounded by continuous newborn bone substance, and the newborn bone substance, with irregular or finger-like shape, extended disorderly to bone formation center. The center of bone formation did not show obvious substantial filling by bone substance. The three-dimensional reconstructed and axial images of Groups D, E and F mice were showed in Figure 1.
3.4. Micro-CT bone parameter analysis
Since ectopic osteogenesis was not found in Groups A, B and C mice through Micro-CT scan, the bone parameter analysis was only performed for ectopic osteogenesis in Groups D, E and F mice. The continuous newborn bone substance at the external surface of the longest axle of ectopic bone formation was selected as region of interest (about 0.5 mm×0.5 mm×0.2 mm). The relative bone parameters measured are showed in Table 3.
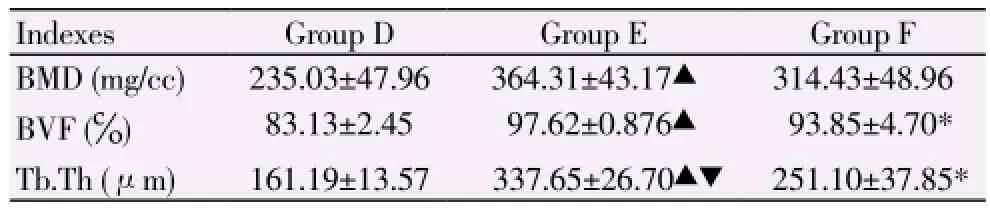
Table 3 Relative bone parameters measured in region of interest of Groups D, E and F mice (n=4, mean±SD).
3.5. Histological observation
3.5.1. HE staining
The muscle and bone tissues of Group A mice did not show inflammatory reaction caused by surgical trauma, and tissues of Groups B and C mice did not show ectopic bone formation or evident residue of composites. The ectopic bone formation was found in Group D mice, and the edge of bone formation was surrounded by a thin layer of continuous newborn bone substance. Cells in the bone substance were present individually, and bone lacuna was unconspicuous; discontinuous newborn bone substance was scattered in ectopic bone formation. The thin layer of continuous
newborn bone substance surrounding at the edge of ectopic bone formation in Group E mice was remarkably thicker than that in Group D mice; multiple cells in the bone substance aggregated, and bone lacuna was conspicuous. Also, in Group E, number of scattered single bone cell was larger than that of Group D, and visible scattered discontinuous newborn bone substance significantly increased compared with its counterpart in Group D. In Group F, the thin layer of continuous newborn bone substance surrounding at the edge of ectopic bone formation was thicker than that of Group D, but not remarkable than that of Group E. Most cells in the bone substance of Group F were present individually, but bone lacuna was more conspicuous than that of Group E. The visible scattered discontinuous newborn bone substance increased compared with its counterpart in Group D, but not remarkable than that of Group E (Figures 2).
3.5.2. Immunohistochemical staining
ALP is the specific marker of osteoblast differentiation at early stage, and ALP immunohistochemical positivity of osteoblast was mainly expressed by endochylema staining in cell. Muscle tissues in Groups A, B and C did not show ALP positive expression; whereas area of ALP positive expression was found in Groups D, E and F, and the expression was mainly located around the thin layer of continuous newborn bone substance on external surface of ectopic bone formation, but the degree of expression was inhomogenous. The area of ALP positive expression in Group E was the largest and the degree was also the highest among three groups (Figure 3).
CD 34 is the specific marker of vascular endothelial cell of capillaries, and positively expressed in membrane and endochylema of cells. Nearly no CD34 positive expression was found in muscle tissues of Groups A, B and C; whereas Groups D, E and F showed CD34 positive expression. The expression area was also mainly located around the thin layer of continuous newborn bone substance on external surface of ectopic bone formation; the highest expression level was found in Group E, followed by Groups F and D. Besides, in Groups E and F, the newborn bone substance was surrounded by brown band at the periphery (Figure 4).
4. Discussion
Adapting tissue engineering to repair bone defects has good prospect for clinical application. Searching for proper bone growth factor or reasonable collocation of bone growth factor is the focus of tissue engineering researchers now. BMP is the polypeptide growth factor in vivo, and major researches are related to BMP-2 at present. BMP-2 can induce irreversible differentiation of undifferentiated mesenchymal cell into chondroblast and osteoblast; it can also facilitate the proliferation and differentiation of various cells such as osteoblast and fibroblast, so as to induce the bone matrix synthesis and promote the new bone formation. BMP-2 has no species specificity, possessing ability of inducing new bone formation across species[4,5]. Many experiments and researches have proved that BMP-2 has highly effective activity to induce osteogenesis[4-6]. The present study also showed that evident ectopic osteogenesis can be found in mice muscle when using rhBMP-2 alone, revealing that rhBMP-2 possesses the capacity to induce ectopic osteogenesis independently.
bFGF is a broad spectrum mitogen, and it acts by binding to membrane specific receptor. FGF receptor is present on the surface of osteoblast, therefore bFGF binding to receptor on the surface of osteoblast can accelerate the mitosis of osteoblast. Among various factors for cell growth regulation, bFGF possesses the strongest proliferative effect on BMSCs; it can not only increase the proliferation rate and life span, but also maintain the multi-differentiation potential during the proliferation process[7-10]. Nevertheless, the present study found that different from the fact that ectopic osteogenesis can be induced by using rhBMP-2 alone, mice muscle did not show ectopic osteogenesis when used bFGF alone, indicating that bFGF does not possess the capacity to induce ectopic osteogenesis independently. Therefore, it can be presumed that bFGF, when used solely, does not have the ability to induce differentiation of mesenchymal cell into chondroblast and osteoblast , thus cannot initiate the synthetic process of newborn bone substance. However, newborn bone formation can be found with the combined application of rhBMP-2 and bFGF. Concentration of calcium content measurement, analysis of bone structure parameters and histological observation revealed that the efficacy of osteogenesis induced by combined application is superior to sole application of rhBMP-2 or bFGF, and the combined application has synergistic effect on inducing ectopic osteogenesis. Based on the findings of the present study, it can be presumed that the synergistic effect firstly started with differentiation of mesenchymal cell in muscle tissue induced by rhBMP-2, namely, partial release of rhBMP-2 stimulates mesenchymal cell to proliferate and move to osteogenesis region, and meanwhile the mesenchymal cell differentiates into chondroblast and osteoblast, initiating the osteogenesis process. During this process, bFGF is provided with massive target cells (chondroblast and osteoblast), and by binding to receptors on the surface of target cells, bFGF promotes further proliferation and differentiation of differentiated chondroblast and osteoblast, so as to increase the amount of cells which can form original osteoid. At the same time, bFGF also stimulates continuous fission and proliferation of mesenchymal cells, further enlarge the number of mesenchymal cell and keep its characteristic as stem cell, maintaining its potential to differentiate into chondroblast and osteoblast induced by rhBMP-2 after proliferation, and forming a virtuous circle of interaction between rhBMP-2 and bFGF. Hence the combined application of rhBMP-2 and bFGF speeds up the formation of newborn bone substance, enhancing the synergic effect on ectopic osteogenesis. However, as aforesaid, bFGF does not possess the capacity to induce ectopic osteogenesis independently, in other words, bFGF cannot induce the differentiation of mesenchymal cell into chondroblast and osteoblast when applied solely. Only after differentiation of mesenchymal cell into chondroblast and osteoblast induced by rhBMP-2 and beginning of osteogenesis process, bFGF can be provided with massive target cells and thus stimulates the target cell fission and proliferation, and accelerates the new bone formation process. A research indicated that rhBMP-2 and bFGF exert regulating effect on cells at different differentiation stage by different signal pathways respectively.
At present, consistent viewpoint about the effect of bFGF concentration during osteogenesis process has not been achieved. In an experiment about the BMSCs differentiation induced by bFGF, it was observed that the bFGF does not positively promote the proliferation of BMSCs in a dose-dependent manner. For example, Varkey et al[11] believed that bFGF can stimulate the formation of calcium nodule at low concentration, but inhibit the osteoblast differentiation capacity at high concentration. Wang et al[12] found that when BMP-2 was combined with bFGF at a ratio of 2:1, their impact of promoting proliferation on BMSCs was superior than that at ratios of 1:1, 4:1 and 8:1. However, BMP-2 and bFGF, when combined at ratios of 1:1 and 2:1, showed better effect on BMSCs differentiating into osteoblast than the effect at ratios of 4:1 and 8:1, and the effects at ratios of 1:1 and 2:1 did not show significant difference. This means that when BMP-2 is combined with bFGF at a ratio of 2:1 (comparatively high concentration of bFGF), bFGF has better effect on BMSCs proliferation and differentiation. In the present study, the osteogenesis efficacy in Group E was better than its counterpart in Group F. The difference of trabecular thickness between Groups E and F showed statistical significance (P<0.05); whereas the mean value of calcium content, BMD and BVF of Group E was insignificantly higher than that of Group F (P>0.05), indicating that the synergic effect of rhBMP-2 and low concentration or less content of bFGF (2 ng) is slightly superior than that of high concentration or more content of bFGF (4 ng). This finding is similar to Varkey’s, hence it can be presumed that high concentration or more content of bFGF (4 ng) may inhibit the differentiated osteoblast or differentiation of BMSCs into osteoblast. However, this inhibition cannot be clearly proved in the present study because the difference of calcium content, BMD and BVF between Groups E and F did not show statistical significance, but this finding may be attributed to insufficient samples and region of interest selected. Since the combined application of rhBMP-2 and bFGF has complex biological effect on BMSCs, and the effect is related to multiple processes, and also the present study took short period of time (2 weeks), the effect of bFGF concentration or content on osteogenesis efficacy, when bFGF is combined with rhBMP-2, needs further investigation.
Angiogenesis is still the key factor for tissue engineered bone, and it has been proposed to use recombinant protein or gene transduction of angiogenesis growth factor to stimulate the angiogenesis for construction of larger-scale bone tissue structure[13,14]. A study showed that while stimulating the proliferation and migration of capillary endothelial cell, bFGF promotes the secretion of
plasminogen activator and collagenase, so as to give rise to collagen synthesis and formation of small blood vessels; bFGF is a powerful irritant for angiogenic factor and blood capillary proliferation[8,15-17]. In this present study, through histological observation, it was found that after employing bFGF, the amount of capillaries increased remarkably in the ectopic bone formation of Groups D, E and F mice. By ALP and CD34 immumohistochemical staining, it was observed that around the continuous newborn bone substance on external surface of ectopic bone formation, the positive expression area of angiogenesis is basically consistent with the positive expression area of osteogenesis activity; the expression quantity of angiogenesis was also consistent with the final bone formation quantity. The endothelium of newborn capillaries of Groups E and F mice was complete, the lumen was typical, and turgor level of red blood cell was high. In Groups E and F, the newborn bone substance around the ectopic bone formation was surrounded by brown band at the periphery, but this brown band was not found in Group D and each group with ALP immumohistochemical staining. However, typical vascular lumen was not found in this brown band, its effect needs further discussion. Hence, when combine the application of rhBMP-2 and bFGF, the effect of promotingangiogenesis exerted by bFGF can enhance the osteogenesis efficacy, speeding up the generation of capillaries as well as cooperating with rhBMP-2 to increase the bone formation quantity, and advance maturity of newborn bone substance and cartilage. Nevertheless, this effect was non-concentration-dependent in the present study because the newborn capillaries quantity and newborn bone substance quantity did not increase with the enhancement of concentration and content of rhBMP-2 and bFGF, but the quantity was greater than that of Group E.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
[1] Kim IS, Song YM, Cho TH, Park YD, Lee KB, Noh I, et al. In vitro response of primary human bone marrow stromal cells to recombinant human bone morphogenic protein-2 in the early and late stages of osteoblast differentiation. Dev Growth Differ 2008; 50(7): 553-564.
[2] Einhorn TA, Majeska RJ, Mohaideen A, Kagel EM, Bouxsein ML, Turek TJ, et al. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am 2003; 85-A(8): 1425-1435.
[3] Zhang X, Wang Y, Gao Y, Liu X, Bai T, Li M, et al. Maintenance of high proliferation and multipotent potential of human hair follicle-derived mesenchymal stem cells by growth factors. Int J Mol Med 2013; 21(8): 9139-9121.
[4] Fujioka-Kobayashi M, Ota MS, Shimoda A, Nakahama K, Akiyoshi K, Miyamoto Y, et al. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials 2012; 33(30): 7613-7620.
[5] Ercolin ACM, Mkrtschjan M, Bionaz M, Jensen T, Wheeler MB. Osteogenic activity of in house-produced porcine Bmp2 on adipose-derived stem cells. Reprod Fertility Dev 2013; 25(1): 288.
[6] Shi Q, Li Y, Sun J, Zhang H, Chen L, Chen B, et al. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 2012; 33(28): 6644-6649.
[7] Yang C, Liu Y, Li C, Zhang B. Repair of mandibular defects by bone marrow stromal cells expressing the basic fibroblast growth factor transgene combined with multi-pore mineralized Bio-Oss. Mol Med Rep 2013; 7(1): 99-104.
[8] Qu D, Li JH, Li YB, Gao Y, Zuo Y, Hsu Y, et al. Angiogenesis and osteogenesis enhanced by bFGF ex vivo gene therapy for bone tissue engineering in reconstruction of calvarial defects. J Biomed Mater Res A 2011; 96(3): 5435-5451.
[9] Wodewotzky TI, Lima-Neto JF, Pereira-Júnior OC, Sudano MJ, Lima SA, Bersano PR, et al. In vitro cultivation of canine multipotent mesenchymal stromal cells on collagen membranes treated with hyaluronic acid for cell therapy and tissue regeneration. Braz J Med Biol Res 2012; 45(12): 1157-1162.
[10] Li Q, Liu T, Zhang L, Liu Y, Zhang W, Liu W, et al. The role of bFGF in down-regulating alpha-SMA expression of chondrogenically induced BMSCs and preventing the shrinkage of BMSC engineered cartilage. Biomaterials 2011; 32(21): 4773-4781.
[11] Varkey M, Kucharski C, Haque T, Sebald W, Uludağ H. In vitro osteogenic response of rat bone marrow cells to bFGF and BMP-2 treatments. Clin Orthop Relat Res 2006; 443: 113-123.
[12] Wang L, Huang Y, Pan K, Jiang X, Liu C. Osteogenic responses to different concentrations/ratios of BMP-2 and bFGF in bone formation. Ann Biomed Eng 2010; 38(1): 77-87.
[13] Li HY, Chang J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic. Acta Biomater 2013; 9(2): 5379-5389.
[14] Liu F, Zhang X, Yu X, Xu Y, Feng T, Ren D. In vitro study in stimulating the secretion of angiogenic growth factors of strontium-doped calcium polyphosphate for bone tissue engineering. J Mater Sci-Mater Med 2011; 22(3): 683-692.
[15] Pu F, Wang E, Jiang H, Ren J. Identification of polyoxometalates as inhibitors of basic fibroblast growth factor. Mol Biosys 2013; 9(1): 113-120.
[16] Zong A, Zhao T, Zhang Y, Song X, Shi Y, Cao H, et al. Anti-metastatic and anti-angiogenic activities of sulfated polysaccharide of Sepiella maindroni ink. Carbohydr Polym 2013; 91(1): 403-409.
[17] Farhat FS, Tfayli A, Fakhruddin N, Mahfouz R, Otrock ZK, Alameddine RS, et al. Expression, prognostic and predictive impact of VEGF and bFGF in non-small cell lung cancer. Crit Rev Oncol Hematol 2012; 84(2): 149-160.
ment heading
10.1016/S1995-7645(14)60187-5
# These authors contributed equally to this study and share first authorship.
*Corresponding author: Ren-Fa Lai and Zhi-Ying Zhou, First Affiliated Hospital of Jinan University, Guangzhou, Guangdong-510630, China.
Tel: 020-38688109.
E-mail: tlrf@jnu.edu.cn
Foundation project: This research are supported by Guangdong Provincial Technology and Development Foundation: (No: 2012B0617000911 & No: 2011B080701053)
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Inhibition of advanced glycation endproducts formation by Korean thistle, Cirsium maackii
- Anti-hypercholesterolemic effect of kenaf (Hibiscus cannabinus L.) seed on high-fat diet Sprague dawley rats
- Susceptibility to temephos, permethrin and deltamethrin of Aedes aegypti (Diptera: Culicidae) from Muang district, Phitsanulok Province, Thailand
- Cloning, expression, purification and bioinformatic analysis of 2-methylcitrate synthase from Mycobacterium tuberculosis
- Prevalence of shiga toxins (stx1, stx2), eaeA and hly genes of Escherichia coli O157:H7 strains among children with acute gastroenteritis in southern of Iran
- Larvicidal, ovicidal and repellent activities of marine sponge Cliona celata (Grant) extracts against Anopheles stephensi Liston (Diptera: Culicidae)
