Anti-hypercholesterolemic effect of kenaf (Hibiscus cannabinus L.) seed on high-fat diet Sprague dawley rats
2015-12-08NgShyKaiTeeAiNeeElaineLaiChiaLingTanChinPingLongKamariahNyamKarLin
Ng Shy Kai, Tee Ai Nee, Elaine Lai Chia Ling, Tan Chin Ping, Long Kamariah, Nyam Kar Lin*
1Department of Food Science and Nutrition, Faculty of Applied Sciences, UCSI University, 56000 Kuala Lumpur, Malaysia
2Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
3Malaysian Agricultural Research & Development Institute, PO Box 12301, 50774 Kuala Lumpur, Malaysia
Anti-hypercholesterolemic effect of kenaf (Hibiscus cannabinus L.) seed on high-fat diet Sprague dawley rats
Ng Shy Kai1, Tee Ai Nee1, Elaine Lai Chia Ling1, Tan Chin Ping2, Long Kamariah3, Nyam Kar Lin1*
1Department of Food Science and Nutrition, Faculty of Applied Sciences, UCSI University, 56000 Kuala Lumpur, Malaysia
2Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
3Malaysian Agricultural Research & Development Institute, PO Box 12301, 50774 Kuala Lumpur, Malaysia
ARTICLE INFO
Article history:
Received 26 October 2014
Received in revised form 10 November 2014
Accepted 22 December 2014
Available online 20 January 2015
Hibiscus cannabinus
Hypercholesterolemia
Phytochemical screening
Phenolic compounds
Sprague dawley
Oxidative stress
Objective: To determine the antihypercholesterolemic effects of kenaf seed samples and compare with the commercial hypocholesterolemic drug on serum lipids profiles and malondialdehyde (MDA) level in the rat. Methods: Kenaf seed oil (KSO), microencapsulated kenaf seed oil (MKSO), kenaf seed extract (KSE) and defatted kenaf seed meal (DKSM) were prepared and phytochemicals screening on these samples were done prior in vivo study. Phenolic compounds in KSE were quantified using high performance liquid chromatography. There were 40 (divided in eight diet groups of 5) male Sprague dawley rats adapted to normal standard diet or hypercholesterolemic diet (HD) with or without the treatment of these kenaf samples for 32 days. Results: All the kenaf samples exhibited to contain most of the major phytochemicals. KSE possessed gallic acid, tannic acid, catechin, benzaldehyde, benzoic acid, syringic acid, sinapic acid, ferulic acid, naringin acid, and protocatechuic acid. The significant higher (P<0.05) serum total cholesterol, low density lipoprotein cholesterol and MDA levels in HD group without treatment than the normal control group suggested the hypercholesterolemia was induced by the incorporation of cholesterol into diet. KSE exhibited higher cholesterol-lowering properties due to the significant lower (P<0.05) in serum triglycerides, total cholesterol and MDA levels. KSE showed the highest efficiency of cholesterol-lowering activity, followed by KSO, MKSO and DKSM. Conclusions: DKSM, MKSO, KSO and KSE appeared to have comparable anti-hypercholesterolemic effect with the commercial hypocholesterolemic drug. Hence, kenaf seed could be used as an alternative natural source to replace the synthetic hypocholesterolemic drugs.
1. Introduction
Hyperlipidemia is a condition, which characterized by elevated serum total cholesterol, triglyceride, and lipoproteins such as low density lipoprotein cholesterol (LDL-c), very low density lipoprotein cholesterol (VLDL-c) and decreased high density lipoprotein cholesterol (HDL-c)[1]. These are the biomarkers for elevated risk of cardiovascular diseases such as atherosclerosis, coronary artery diseases and cerebral vascular diseases[2]. Oxidative stress has been prescribed as the main mechanism responsible for cardiovascular diseases while hypercholesterolemia under oxidative stress could trigger the progression of atherosclerosis and abnormal lipid metabolism[3]. The generation of reactive oxygen species (ROS) including superoxide anions, hydrogen peroxide (H2O2) and hydroxyl radicals would react with unsaturated fatty acid chain in membrane lipids and cause lipid peroxidation. Certain primary and secondary lipid peroxidation products would decompose into malondialdehyde (MDA)[4]. Hence, elevated of malondialdehyde in serum is a biological marker of lipid peroxidation. The literature studies in both animals and humans have demonstrated that prolonged high
cholesterol concentration in the circulating blood positively correlates with developing atherosclerosis[5,6].
Kenaf (Hibiscus cannabinus L.) has been well-known especially to its seed which was disposed as industrial waste or by-product in the past. The relatively high oil level and the reasonable amounts of phytosterols and phospholipids suggest that kenaf oil can be used as a source of edible oil[7]. Kenaf seed oil has a relatively high amount of monounsaturated and polyunsaturated fatty acids (PUFAs), which are nutritionally beneficial for human health[8]. The PUFAs and phytosterols content in kenaf seed oil have cholesterol lowering ability, which are beneficial to human health[9]. In the most recent study, microencapsulation has been applied to overcome the problem associated with lipid oxidation in the kenaf seed oil due to the high PUFA levels, which are susceptible to oxidation. The microencapsulated kenaf seed oil (MKSO) was reported to possess abundant of bioactive compounds and unsaturated fatty acids even after spray drying process[10]. Recently, numerous attempts to replace solvent extraction have been made, of which ultrasonic-assisted extraction (UAE) is one of the most promising methods[11-13]. This could be related to its improvements in efficiency and speed, which can be performed at low operation temperatures. Hence, thermal damage to the extracts and preserves the structural and molecular properties of bioactive compounds could be minimized and avoided[11,14,15]. Therefore, the UAE was used to obtain the kenaf seed extract. The mass extraction of kenaf seed oil resulted in large amounts of defatted kenaf seed meal (DKSM) as kenaf seed contains only approximately 20% of oil[16]. In fact, DKSM may be declared and commercialized as highly antioxidative and nutritive edible flour, which can be prospectively used in the development of natural food preservative, nutraceuticals and functional foods.
To the best of our knowledge, the consequence of the consumption of diets containing these kenaf seed oil, defatted seed meal and extracts on circulating lipid has not been examined. The current study was conducted to determine the effect of kenaf seed on high-fat diet Sprague dawley rats in altering the serum lipid profile and MDA level. The study hypothesis was that the unsaturated fatty acids and bioactive compounds in kenaf seed would have anti-hypercholesterolemic effect and also able to reduce oxidative stress on the studied rats. To test this hypothesis, hypercholesterolemic diet (HD) was introduced to the studied rats. The rats were divided into several groups and treated with different kenaf seed samples, respectively. The effects of consumption of these samples were compared among each others as well as compared with the commercial hypocholesterolemic drug on serum lipids profiles and MDA level in the rats. Therefore, new alternative natural plant source can be produced to be the replacement for synthetic hypocholesterolemic drugs.
2. Materials and methods
2.1. Samples
Kenaf (Hibiscus cannabinus L.) seeds were obtained from Malaysian Agricultural Research and Development Institute. Wall materials, such as sodium caseinate (China), maltodextrin DE10 (China) and soy lecithin (China; used as an emulsifier) were purchased from a local food ingredient supplier (VIS Food Tech Ingredient Supplies, Malaysia). All chemicals used were analytical grade (Merck, Darmstadt, Germany).
2.1.1. Kenaf seed oil (KSO)
Extraction of oil was done according to the method of previous study[10]. The kenaf seeds were ground into fine powder using a grinder (National, Osaka, Japan). The oils were extracted from the seeds with Soxhlet extractor using hexane at 60 ℃ for 8 h. The oil was then recovered by evaporating off the solvent using rotary evaporator Model N-1 (Eyela, Tokyo Rikakikal Co., Ltd., Japan) and residual solvent was removed by flushing with 99.9% nitrogen.
2.1.2. DKSM
The defatted seeds were obtained after the oil extraction, which were subsequently placed under a stream of hot air in a ventilated oven (Memmert, Germany) at 50 ℃ to remove the residual solvent.
2.1.3. Kenaf seed extract (KSE)
A total of 50 g of ground kenaf seed was added to a 500 mL Schott bottle containing 500 mL of 80% ethanol. Ultrasonic extraction (Ultrasonic Homogenizer Labsonic P, 400 W, Sartorius, AG) was done for 30 min with a 5-min pulse duration period and a 5-min pulse interval period. The extraction was repeated for 3 cycles. The kenaf seed extract collected was centrifuged at 3 500 rpm for 10 min. The supernatant of the kenaf seed extract was collected and filtered; the pellet was discarded. The filtered supernatant was subjected to rotary evaporation.
2.1.4. Microencapsulated kenaf seed oil (MKSO)
Microencapsulated of kenaf seed oil was prepared according to previously described method[10]. Briefly, kenaf seed oil (core) was added to pre-dissolved wall materials (sodium caseinate/maltodextrin ratio of 1:9) at the ratio of 1:3 to obtain a 40% of total solids level of
emulsion. Subsequently, 2 g of soy lecithin was added as an emulsifier (emulsifier/protein ratio of 0.1:1). The mixture was then homogenised in a shear homogeniser (Silverson L4R, Buckinghamshire, UK) for five min at 3 000 rpm until complete dispersion was observed. The resultant emulsions were further homogenised in a high-pressure homogeniser (APV, Crawley, UK) at pressure of 500 bars for four cycles. For spray drying, the emulsion was spray-dried in a Buchi B-290 model mini spray dryer (Buchi Labortechnik AG, Switzerland) equipped with a 0.7 mm standard diameter nozzle at the inlet temperature of 160 ℃ and outlet temperature of (85±2)℃.
2.2. Phytochemical screening
Prior to analyses, the total oil of MKSO was extracted out from the wall material[10]. In the preparation of deemulsifier, 10 g of sodium salicylate and 10 g of sodium citrate were dissolved separately in deionised water, followed by mixing these solutions together with 18 mL of n-butanol, and top up to 90 mL with deionised water. Every 10 g of MKSO was mixed with 20 mL water at 50 ℃ in an Erlenmeyer flask with a stopper. 15 mL of the pre-prepared de-emulsifier was added in, and the mixture was shaken vigorously and left to stand in a 70 ℃ water bath for 6 min. The resulting mixture was then centrifuged (Centrifuge machine X-22R, Beckman Coulter, United State) at 3 000× g for 10 min, and the total oil (supernatant) was collected. The KSE, KSO and DKSM and total oil extracted from MKSO were evaluated for the presence of different phytochemicals to ascertain the presence of metabolites such as terpenoids, tannis, cardiac glycosides, alkaloids, saponins, and flavonoids by using the procedures described by Ayoola et al[17], Parekh et al[18] and Usman et al[19].
2.3. Phenolic compounds quantification
Phenolic compounds in KSE were quantified using high performance liquid chromatography (HPLC) as previously described method[20] with slightly modification. The HPLC (Agilent Technologies 1200 Series, United Stated) system consisted of diode array detector. Separation of phenolic compounds was carried out using a reversed-phase HPLC column (Purospher star 5 μm×250 mm×4.6 mm). The column temperature and detection wavelength were set at 30℃ and 210 nm, respectively. A gradient elution system of solvent A (Water with 0.1% phosphoric acid) and solvent B (methanol with 0.1% phosphoric acid) was used [5% B (0 min); 50% B (5 min); 55% B (65 min); 5% B (70 min)]. The flow rate was 1 mL/ min. The volume injected was 20 μL. The chromatographic peaks of phenolic compounds were confirmed by comparing their retention times with those of the reference standards. Gallic acid, tannic acid, catechin hydrate, 4-hydroxybenzaldehyde, 4-hydroxybenzoic acid, syringic acid, sinapsic acid, ferullic acid, naringin, and protocatechuic acid were used as phenolic compound standards.
2.4. Experimental animals
Male Sprague dawley rats (250-300 g) that used for this study were obtained from the institutional animal house (Universiti Kebangsaan Malaysia, Bangi, Malaysia). The animals were housed two or three in each cage under standard laboratory conditions (temperature at (22±2)℃, and humidity at (75±5)% with a 12 h dark-light cycle. The animals were fed with meat free rat and mouse diet (as normal standard diet) that purchased from Speciality Feeds (Glen Forrest, Western Australia) and were allowed to drink water ad libitum. The animals were allowed to acclimatize for 2 weeks before the treatments started. The research on animal models was approved by the Faculty’s adhoc ethics committee of UCSI University (Cheras, Malaysia) with project code Proj-FAS-EC-12-019. The experimental procedures were in strict accordance with animal ethical committee guidelines for the care and use of laboratory animals.
2.5. Diet preparation
HD was used to induce the hyperlipidemia among the experimental animals. The composition of HD was 10% cholesterol (Sigma-Aldrich, Germany) and 90% normal diet. In the preparation of 100 g of HD, briefly, every 90 g of normal standard diet were ground into powder using grinder (Sharp, United State) and was mixed with 10 g of cholesterol. Adequate amount of distilled water was added into the mixture and was regimented by hand. Similar pellet-shape with the normal standard diet was shaped by the hand. The resulting pellets were dried overnight in a conventional oven (Memmert, Germany) at 50 ℃ until a constant weight was obtained. Similar procedures were repeated in the preparation of diet for Group IV, which consisted of 10% of DKSM with of 90% HD. Group VII, which consisted of 10% MKSO. Group Ⅷ, which consisted of 10% wall materials, MDSC (maltodextrin/sodium caseinate ratio of 9:1) was acted as negative control group to eliminate the hyper/ hypolipidemic effect that caused by the wall materials in Group Ⅶ.
2.6. Experimental protocol
The rats were divided into 8 groups of 5 rats in each
group such that the weight difference within and between groups does not exceed±20% of the average body weight of the sample population. Treatments of all groups were shown in Table 1. The rats were fed with HD for 32 days to induce hyperlipidemia among the animals except normal control group (Group I), which fed with normal standard diet. A standard amount of 20 g/ rat/ day was fixed to all groups to eliminate the effect of unequal diets among the groups. Simvastatin (Teva Simvastatin®, Teva UK Limited, Eastbourne, UK), as positive control, KSE and KSO were administered by oral gavage. Body weights of rats were taken with Mettler weighing balance (Mettler Toledo Type BD6000, Mettler-Toledo GmbH, Greifensee, Switzerland) and were recorded weekly. All treatment was done as a single daily oral treatment for 32 days.
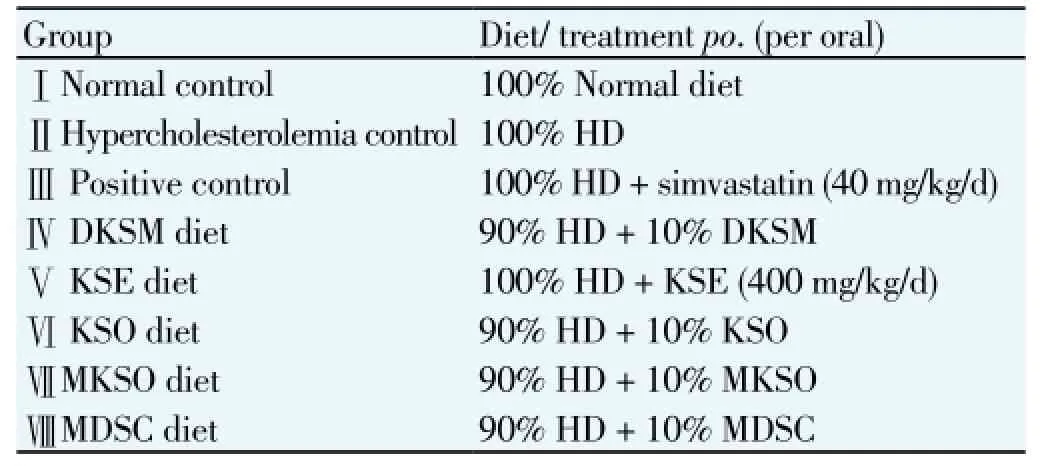
Table 1 Experimental treatments of the respective groups.
2.6.1. Measurement of body weight changes and relative liver weight
The measurement of body weight changes (Δwt) and relative liver weight were done according to the previous described method[21]. The Δwt of rats in each group were calculated and were expressed in percentage (%) as shown in Equation (1):
(body weight on day 33-body weight on day 1)/(body weight on day 1)×100 (1)
The absolute liver weight of each rat group was measured using Mettler weighing balance, from which the relative liver weight per 100 g body weight of rat was calculated according to Equation (2):
[Weight of rat liver (g)/body weight on day 33 (g)]×100 (2)
2.6.2. Collection of serum
At the end of treatment period, the animals were fasted for 18 h to 24 h after the last administration. The animals were then anaesthetized with Ketamine (Sigma-Aldrich, Germany) at dosage of 500 mg/ kg with intraperitoneal method. Blood samples were collected from posterior vena cava and were transferred into ethylenediaminetetraacetic acid tubes immediately to prevent coagulation of bloods. The collected blood samples were centrifuged using centrifuge machine (X-22R, Beckman Coulter, United State) at 3 000×g for 15 min at 4 ℃ to remove red blood cells and recover serum[22]. The serum samples (supernatant) were collected and were used for the following analyses.
2.6.3. Analysis of serum lipid profile
The serum lipid profile was determined including total cholesterol (TC), triglycerides (TG), HDL-c and LDL-c were analysed using Cobas C 311 analyzer (Roche, Germany)[23].
2.6.4. Determination of AI and CRI
Atherogenic index (AI) and coronary risk index (CRI) were calculated according to the prescribed method[21] as shown in Equation (3) and (4), respectively:
2.6.5. Determination of serum MDA level
Serum MDA was measured by the thiobarbituric acid reactive substances (TBARS) assay[22]. Briefly, 20% trichloroacetic acid (1.0 mL) was added to 100 μL serum, then 1% TBARS reagent (1.0 mL) was added, mixed and incubated at 100 ℃ for 30 min. After cooling on ice, samples were centrifuged using centrifuge machine at 1 000×g for 20 min and absorbance of the supernatant was read at 532 nm by UV-Vis spectrophotometer (Secoman, France). Blanks for each sample were prepared and assessed in the same way to correct for the contribution of A532 to the sample. TBARS results were expressed as micromole MDA production per liter (μmol/L) serum, using tetramethoxypropane as standard.
2.7. Statistical analysis
All the results were analysed using Minitab software (Version 15.1.1.0) and SPSS software (Version 10.0). Data were expressed as mean±standard deviation (n=5). Oneway analysis of variance (ANOVA) was used to determine significant difference (P<0.05) between the means.
3. Results
3.1. Phytochemicals screening
Table 2 shows that the presences of phytochemicals in KSO, DKSM, KSE and MKSO. Terpenoids, tannis, cardiac
glycosides, alkaloids, saponins and flavonoids were present in all samples. Cardiac glycosides was absent in DKSM and KSE, respectively.
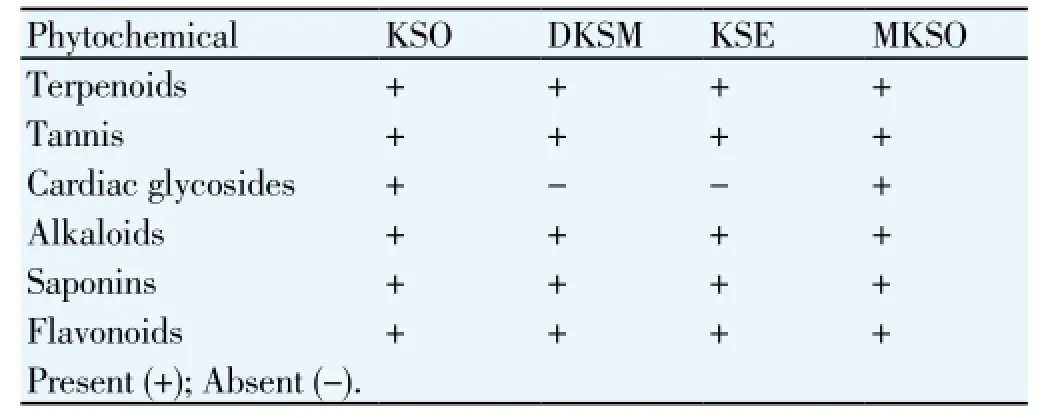
Table 2 Phytochemicals in KSO, DKSM, KSE and MKSO.
3.2. Phenolic compounds
Phenolic compounds in KSE (Table 3), revealing the presence of gallic acid, tannic acid, catechin, benzaldehyde, benzoic acid, syringic acid, sinapic acid, ferulic acid, naringin acid, and protocatechuic acid. Tannic acid (2302.20 mg/100g extract) and sinapic acid (1 198.22 mg/100g extract) were the major compounds in KSE.
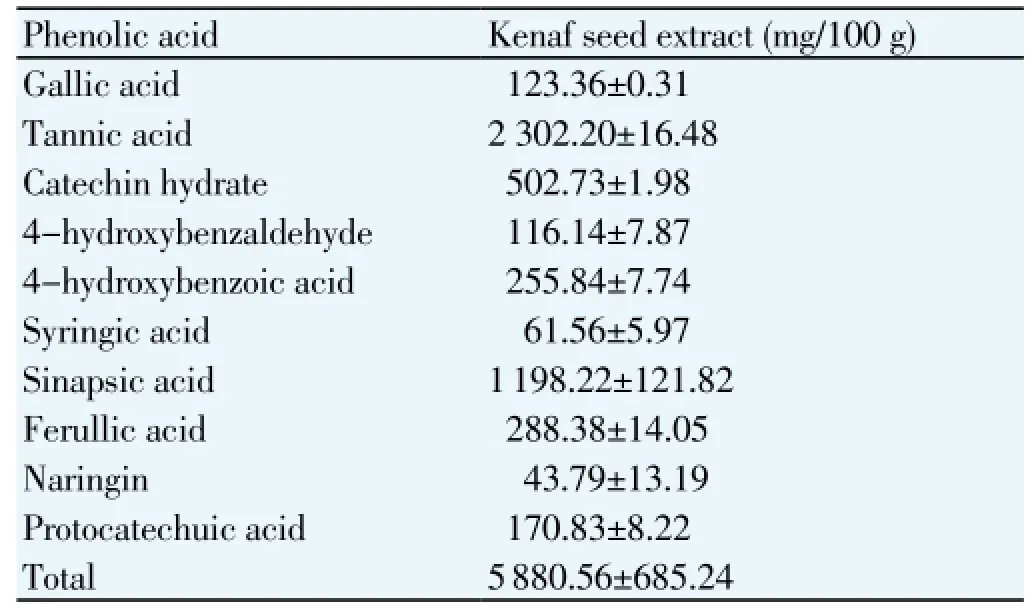
Table 3 Quantitative of phenolic acid content in ultrasound-assisted KSE.
The body weight changes and relative liver weight of the studied rats after 32 days were shown in Table 4. HD caused increased of body weight in the studied rats, which could be seen from the relatively higher weight gained among all the rats fed with HD either with or without treatment (GroupⅡ- Group Ⅷ). However, no significant difference (P>0.05) in weight gained among all the HD fed rat groups with the normal standard diet control rats was observed except Group Ⅲ. Treatment of 40 mg/ kg/ day of simvastatin caused significant weight gained in the studied rats, which was significantly higher (P<0.05) than the normal standard diet control rats. The rats fed with HD without treatment revealed the significant higher (P<0.05) relative liver weight than the control rats and rats fed with KSO treatment rats.
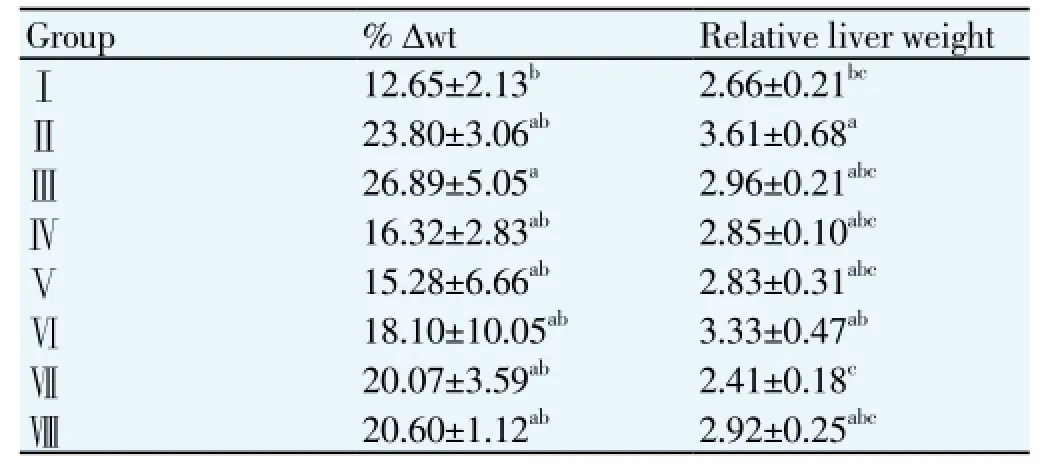
Table 4 Effect of different treatments on the percentage body weight changes (% Δwt) and relative liver weight in the studied rats.
3.4. Serum lipid profile
Table 5 shows the serum lipid profile of all studied rats. HD feeding without treatment rats revealed the significant highest (P<0.05) amount of serum TC levels and LDL-c. All the rats fed with HD and treated with the different kenaf samples (Group Ⅲ- Ⅶ) were shown to have significant lower (P<0.05) amount of TC levels than the untreated rats (Group Ⅱ). No significant difference (P>0.05) of HDL-c was observed among all the studied rats. The TC and LDL-c of the rats treated with MKSO were shown to be lower than the Group Ⅷ (negative control group), which the rats that were fed with wall materials (MDSC).

Table 5 Effect of different treatments on serum TC, TG, HDL-c, LDL-c, CRI, AI, and MDA levels in the studied rats.
3.5. Atherogenic index (AI) and coronary risk index (CRI)
Rats treated with simvastatin, KSE and MKSO showed to
have significant lower (P<0.05) of AI than the untreated rats. Normal diet rats exhibited the lowest AI, which was significantly lower than the untreated HD rats, rats treated with DKSM and KSO but insignificant different (P>0.05) with the rest of groups. Rats treated with KSE and MKSO showed to have significant lower (P<0.05) of CRI than the untreated HD rats. Similar to AI, normal diet rats revealed the lowest CRI, but was significantly lower (P<0.05) than the untreated HD rats and rats treated with KSO only (Table 5).
3.6. Serum MDA level
MDA level has always been used as a biological marker of lipid peroxidation. The serum MDA level of the untreated HD rats was significantly higher (P<0.05) than all other groups. Significant lower (P<0.05) of serum MDA level of rats treated with simvastatin and KSE than the normal diet rats was observed (Table 5).
4. Discussion
Phytochemicals are biologically active plant chemicals other than traditional nutrients that have a beneficial effect on human health[24]. The presences of phytochemicals including glycosides, flavonoids and terpenoids in the kenaf seed could possess serum lipid lowering properties that help in preventing or slowing the progress of atheroma related diseases[25]. Therefore, phytochemical screening was done prior to in vivo study mainly due to their antioxidant activity and other potential health benefits.
Cholesterol feeding has often been used to elevate serum cholesterol levels to study the etiology of hypercholesterolemia-related metabolic disturbances[26]. Feeding of HD to the studied animals caused significant increase of serum TC and LDL-c compared to control rats. This is associated with the excessive load of cholesterol to the liver, which exceed the normal physiological limits, causes the inability of liver to metabolize the lipids, thus resulting in high cholesterol return in the circulating blood[27]. Consequently, the accumulation of fatty acid in the hepatocyte caused an increased of the relative liver weight[28]. This can be seen from the significant higher relative liver weight on the HD induced groups (both untreated and treated) compared to the control group. Similarly, the body weights gained in the HD groups were shown to be higher than the control diet group. HD fed to the studied rats induced greater body fat deposition because of increased in adipocyte number and size[29]. The HD groups, which treated with kenaf seed samples, were shown to have relatively lower relative liver weight and lower body weight gained. These indicated that the kenaf seed samples were able to inhibit the accumulation of fats in the body and liver.
Linoleic acid plays an important role in reduction of total cholesterol level[30]. In addition, the bioactive compounds such that phytosterols and tocopherols play their role in lowering serum cholesterol level. Phytosterols (β-sitosterol) and saponins could increase cholesterol excretion from the body. Besides, phytosterols also help in inhibition of cholesterol absorption[31], lower the TG and increase HDL[32]. The ability of vitamin E (α-tocopherol) was reported to be able to reduce the serum MDA level[33] and the risk of cardiovascular diseases[34]. From the previous study done on the effect of microencapsulation on KSO, linoliec acid (C18:2n6c) that presence in the KSO and MKSO was reported as (33.6±0.2)% and (25.7±0.2)%, respectively. The phytosterols content in KSO and MKSO were (6 510.3±54.2) mg/100 g and (4 680.4±171.9) mg/100 g, respectively. Total tocopherols of KSO and MKSO were (186.83±2.70) mg/100 g and (154.89±3.15) mg/100 g, repectively. Therefore, the anti-hypercholesterolemic properties of KSO and MKSO could be explained due to the presence of these bioactive compounds. DKSM was reported to exhibit high reducing power and effective radicals scavenging activity, which revealed that DKSM could potentially break the free radicals chain reactions that initiate and propagate the process of lipid peroxidation in food products[16]. Therefore, the free radicals such as ROS produced from lipid metabolism had been scavenged and reduce the lipid peroxidation, thus, lowers the serum MDA level.
The highest level of serum MDA level and TC in the HD rats without treatment suggested that the lipid peroxidation caused hypercholesterolemia[3]. This also can be observed from the low serum MDA level and low TC in the normal diet control rats. Despite the absence of cardiac glycosides, KSE showed to be the most effective among other samples in reducing the cardiovascular risk. In fact, cardiac glycosides are steroidal glycosides, which exert a slowing and strengthen effect on the failing heart. They act by affecting the availability of intracellular Ca2+for myocardial contraction or increasing the sensitivity of myocardial contractile proteins[35]. Hence, they increase the force of heart contraction. Treatment of HD fed rats with KSE effectively reduced the serum MDA level, which was significant difference (P>0.05) with the treatment of HD fed rats with simvastatin. KSE showed its ability to reduce TC and LDL-c in the HD fed rats. This can be explained by the lower levels of serum TC, LDL-c and MDA but high level of HDL-c in the KSE diet-treated rats. Generally, LDL-c is often known as “bad cholesterol” as opposed to HDL-c, which is known as “good cholesterol”. Elevated level of LDL-c promotes health problems and cardiovascular disease[36] while HDL-c involves in the removal cholesterol from within the artery and transports it back to the liver for excretion or re-utilization[31].
UAE involves the solid-liquid extraction by ultrasound,
which improved the penetration of the solvent into the plant tissue and capillary that intensify the release of organic compounds such as polyphenols within the plant body at enhanced mass transfer rate and produced higher yields[37,38]. These characteristics make this extraction alternative very useful for isolating natural compounds from plants with pharmacological properties[39]. The significant high amount of total phenolic compounds [(5 880.56±685.24) mg/100 g of extract] detected in the KSE compared to DKSM (199.98 mg/100 g of DKSM)[16], KSO (12.23 mg/100 g of oil) and MKSO possessed (11.07 mg/100 g of oil)[10] suggested the phenolic compounds play more significant role in reducing oxidative stress. Tannic acid has been shown to possess antioxidant, antimutagenic and anticarcinogenic properties[40] while sinapic acid has already been pharmacologically evaluated for its potent antioxidant, anxiolytic, anti-inflammatory, peroxynitrite scavenging effects and neuroprotective effects[41]. In fact, these two major phenolic compounds in KSE were not detected in the KSO and MKSO[10]. Higher content of antioxidants were found in KSE to reduce the lipid peroxidation, which would prevent the occurrence of hypercholesterolemia. This study has demonstrated the close relationship between lipid peroxidation and hypercholesterolemia. In the literature, the investigation in vivo concluded that the sample with higher antioxidant capacity is biologically more active than sample with lower antioxidant capacity[42]. The higher antioxidant capacity in the KSE significantly reduced the lipid peroxidation, hence, reduced the serum TC and TG levels.
The effects of different samples treatment on the AI and CRI were also notable. These two indices are known as reliable indicators of the deposition of the cholesterols into tissues are being metabolized or excreted[43]. In humans, the normal reference values for atherogenic index should not be higher than 4 while the coronary artery index should not be higher than 2.5[44]. Thus, patients with both indices higher than these reference values are more prone to develop ischaemic heart disease and thrombotic cardiovascular disease[45]. In this present study, results showed that treatment with KSE caused the significant lower of both indices than the untreated HD rats, which suggested that KSE possessed cardioprotective potential.
In conclusion, this study suggested that the cholesterollowering activity of KSE, DKSM, KSO and MKSO appeared to have comparable effect with the commercial hypocholesterolemic drug, simvastatin. Experimented diets did not affect the TG levels of serum. The absence of cardiac glycosides in KSE did not influence its effectiveness in lowering serum TG and LDL-c levels. Moreover, KSE had the significant effect in preventing lipid peroxidation in the rats, which was similar to simvastatin. The efficiency of different kenaf samples in anti-hypercholesterolemia were in the ascending order of DKSM < MKSO < KSO < KSE. Hence, this study suggested the kenaf seed samples to be used as an alternative natural source to replace the synthetic hypocholesterolemic drugs.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgements
Financial support of this work by the Ministry of Higher Education through the Fundamental Research Grant Scheme (FRGS/2/2010/SG/UCSI/03/1) is gratefully acknowledged.
[1] Tilak KS, Veeraiah K, Koteswara Rao DK. Restoration on tissue antioxidants by fenugreek seeds (Trigonella foenum Graecum) in alloxan-diabetic rats. Indian J Physiol Pharmacol 2001; 45: 408-420.
[2] Villanueva MJ, Yokoyama WH, Hong, YJ, Barttley GE, Rupérez P. Effect of high-fat diets supplemented with okara soybean byproduct on lipid profiles of plasma, liver and faeces in Syrian hamsters. Food Chem 2011; 124: 72-79.
[3] Zulkhairi HA, Khairunnuur AF, Hafipah MRN, Azrina A, Rasadah MA, Kamilah KAK, et al. An aqueous extract of Citrus mitis posessess antioxidative properties and improves plasma lipid profiles in rat induced with high cholesterol diet. J Med Plant Res 2010; 4: 49-57.
[4] Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biol Med 1990; 9: 515-540.
[5] Pratico D. Lipid peroxidation in mouse models of atherosclerosis. Trends Cardiovas Med 2001; 11: 112-116.
[6] Tohru K, Fumi I, Aiko N, Yoshihisa N, Shin-Ichiro K, Naomi O. Suppressive effects of cacao liquor polyphenols (CLP) on LDL oxidation and the development of atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. Atherosclerosis 2005; 179: 237-246.
[7] Mohamed A, Bhardwaj H, Hamama A, Webber C. Chemical composition of kenaf (Hibiscus cannabinus L.) seed oil. Ind Crop Prod 1995; 4: 157-165.
[8] Coetzee R, Labuschagne MT, Hugo A. Fatty acid and oil variation in seed from kenaf (Hibiscus cannabinus L.). Ind Crop Prod 2008; 27: 104-109.
[9] Nyam KL, Tan CP, Lai OM, Long K, Yaakob CM. Physicochemical properties and bioactive compounds of selected seed oils. LWTFood Sci Technol 2009; 42: 1396-1403.
[10] Ng SK, Lau Jessie LY, Tan CP, Long K, Nyam KL. Effect of accelerated storage on microencapsulated kenaf seed oil. J Am Oil Chem Soc 2013; 90: 1023-1029.
[11] Zhang ZS, Wang LJ, Li D, Jiao SS, Chen XD, Mao ZH. Ultrasound-assisted extraction of oil from flaxseed. Sep Purif Technol 2008; 62: 192-198.
[12] Shalmashi A. Ultrasound-assisted extraction of oil from tea seeds. J Food Lipids 2009; 16: 465-474.
[13] Sun Y, Liu Z, Wang J. Ultrasound-assisted extraction of five isoflavones from Iris tectorum Maxim. Sep Purif Technol 2011; 78: 49-54.
[14] Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason Sonochem 2001; 8: 303-313.
[15] Vilkhu K, Mawson R, Simons L, Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry - A review. Innov Food Sci Emerg 2008; 9: 161-169.
[16] Chan KW, Khong NMH, Iqbal S, Mansor SM, Ismail M. Defatted kenaf seed meal (DKSM): Prospective edible flour from agricultural waste with high antioxidant activity. LWT- Fd Sci Technol 2013; 53: 308-313.
[17] Ayoola GA, Coker HAB, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, et al. Phytochemical screening and antioxidant activities of some selected medical plants used for malaria therapy in southwestern Nigeria. Trop J Pharm Res 2008; 7: 1019-1024.
[18] Parekh J, Chanda SV. In vitro antimicrobial activity and phytochemical analysis of some Indian medicine plants. Turk J Biol 2007; 31: 53-58.
[19] Usman H, Abdulrahman FI, Usman A. Quantitative phytochemical screening and in vitro antimicrobial effects of methanol stem bark extract of Ficus thonningii (Moraceae). Afr J Tradit Complem 2009; 6: 289-295.
[20] Baydar NG, Sagdic O, Ozkan G, Cetin ES. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L.) seed extracts. Int J Food Sci Technol 2006; 41, 799-804.
[21] Adeneye AA, Adeyemi OO, Agbaje EO. Anti-obesity and antihyperlipidaemic effect of Hunteria umbellate seed extract in experimental hyperlipidaemia. J Ethnopharmacol 2010; 130: 307-314.
[22] Ma FX, Liu LY, Xiong XM. Protective effects of lovastatin on vascular endothelium injured by low density lipoprotein. Acta Pharm Sinic 2003; 24: 1027-1032.
[23] Hamed EO, Zaky NA, Din AKN. Serum levels of adiponectin and ghrelin in patients with acute myocardial infarction. Life Sci J 2012; 9, 523-526.
[24] Hasler CM. Functional foods: Their role in disease prevention and health promotion. Food Technol 1998; 52: 63-70.
[25] Githa EM, Bijo M, Sajeeth CI. Phytochemical evaluation and lipid lowering property of leaves of Vitex negundo linn. in hypercholestremic rats. IJPSRR 2011; 2: 18-22.
[26] Ahmad S, Beg ZH. Alleviation of plasma, erythrocyte and liver lipidemic-oxidative stress by thymoquinone and limonene in atherogenic suspension fed rats. J Funct Fd 2013; 5: 251-259.
[27] Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wuv-Bustick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal woman. New Eng J Med 1996; 334: 1156-1162.
[28] Matos SL, de Paula H, Pedrosa ML, Santos RC, Oliveira EL, Silva ME. Dietary models for inducing hypercholesterolemia in rats. Braz Arch Biol Technol 2005; 48: 203-209.
[29] Chilliard V. Dietary fat and adipose tissue metabolism in ruminants, pigs, and rodents: A review. J Dairy Sci 1993; 76: 3897-3931.
[30] Melgarejo P, Artes F. Total lipid level and fatty acid composition of oil seed from lesser known sweet pomegranate clones. J Sci Fd Agric 2000; 80: 1452-1454.
[31] Lin M, Hoke C, Ettinger B. Evaluation of homogeneous highdensity lipoprotein cholesterol assay on a BM/Hitachi 747-200 analyzer. Clin Chem 1998; 5: 1050-1054.
[32] Khandelwal S, Shidhaye R, Demonty I, Lakshmy R, Gupta R, Prabhakaran D, et al. Impact of omega-3 fatty acids and/or plant sterol supplementation on non-HDL cholesterol levels of dyslipidemic Indian adults. J Funct Foods 2013; 5: 36-43.
[33] Tchankou Leudeu BC, Tchiegang C, Barbe F, Nicolas B, Gueant JL. Ricinodendron heutelotii (Bail.) or Tetracarpidium conophorum Mull. oils fed to male rats lower blood lipids. Nutr Res 2009; 29: 503-509.
[34] Burton GW. Vitamin E: molecular and biological function. Proc Nutr Soc 1994; 53: 251-262.
[35] Walker AF, Marakis G, Morris AP, Robinson PA. Promising hypotensive effect of hawthorn extract: A randomized doubleblind pilot study of mild, essential hypertension. Phytother Res 2002; 16: 48-54.
[36] Superko HR, Nejedly M, Garrett B. Small LDL and its clinical importance as a new CAD risk factor: A female case study. Prog Cardiovasc Nurs 2002; 4: 167-173.
[37] Toma M, Vinatoru M, Paniwnyk L, Mason TJ. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason Sonochem 2001; 8: 137-142.
[38] Chemat S, Lagha A, AitAmar H, Bartels PV, Chemat F. Comparison of conventional and ultrasound-assisted extraction of carvone and limonen from caraway seeds. J Flavour Fragr 2004; 19: 188-195.
[39] Priego-Capote F, Luque de Castro MD. Analytical uses of ultrasound: Sample preparation. Trends Anal Chem 2004; 23: 644-653.
[40] Gulcin I, Huyut Z, Elmastas M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arabian J Chem 2010; 3: 43-53.
[41] Pari L, Jalaludeen AM. Protective role of sinapic acid against arsenic: induced toxicity in rats. Chem-Biol Interact 2011; 194: 40-47.
[42] Gorinstein S, Leontowicz H, Leontowicz M, Lojek A, Ciz M, Krzeminski R, et al. Seed oils improve lipid metabolism and increase antioxidant potential in rats fed diets containing cholesterol. Nutr Res 2003; 23: 317-330.
[43] Hartog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhouy D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 1993; 342: 1007-1011.
[44] Murray MT, Pizzorno J. Cholesterol. In: Encyclopedia of natural medicine. 2nd edition. 1998, p. 347-400.
[45] Kanekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. Brit J Med 1996; 312: 478-481.
ment heading
10.1016/S1995-7645(14)60179-6
*Corresponding author: Nyam Kar Lin, Department of Food Science and Nutrition, Faculty of Applied Sciences, UCSI University, 56000 Kuala Lumpur, Malaysia.
Tel: +603-91018880
Fax: +603-91023606
E-mail address: nyamkl@ucsiuniversity.edu.my
Foundation project: It is supported by the Ministry of Higher Education through the Fundamental Research Grant Scheme (FRGS/2/2010/SG/UCSI/03/1) is gratefully acknowledged.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Inhibition of advanced glycation endproducts formation by Korean thistle, Cirsium maackii
- Susceptibility to temephos, permethrin and deltamethrin of Aedes aegypti (Diptera: Culicidae) from Muang district, Phitsanulok Province, Thailand
- Cloning, expression, purification and bioinformatic analysis of 2-methylcitrate synthase from Mycobacterium tuberculosis
- Prevalence of shiga toxins (stx1, stx2), eaeA and hly genes of Escherichia coli O157:H7 strains among children with acute gastroenteritis in southern of Iran
- Larvicidal, ovicidal and repellent activities of marine sponge Cliona celata (Grant) extracts against Anopheles stephensi Liston (Diptera: Culicidae)
- Characterization of mycobacterium isolates from pulmomary tuberculosis suspected cases visiting Tuberculosis Reference Laboratory at Ethiopian Health and Nutrition Research Institute, Addis Ababa Ethiopia: a cross sectional study
