Larvicidal, ovicidal and repellent activities of marine sponge Cliona celata (Grant) extracts against Anopheles stephensi Liston (Diptera: Culicidae)
2015-12-08AppaduraiDanielReeganArokiaValanKinsalinMichaelGabrielPaulrajSavarimuthuIgnacimuthu
Appadurai Daniel Reegan, Arokia Valan Kinsalin, Michael Gabriel Paulraj, Savarimuthu Ignacimuthu
Entomology Research Institute, Loyola College, Chennai - 600 034, Tamil Nadu, India
Larvicidal, ovicidal and repellent activities of marine sponge Cliona celata (Grant) extracts against Anopheles stephensi Liston (Diptera: Culicidae)
Appadurai Daniel Reegan△, Arokia Valan Kinsalin△, Michael Gabriel Paulraj, Savarimuthu Ignacimuthu*
Entomology Research Institute, Loyola College, Chennai - 600 034, Tamil Nadu, India
ARTICLE INFO
Article history:
Received 26 October 2014
Received in revised form 10 November 2014
Accepted 22 December 2014
Available online 20 January 2015
Mosquito larvicide
Ovicide
Repellent
Cliona celata
Anopheles stephensi
Objective: To evaluate the larvicidal, ovicidal and repellent properties of solvent extracts of marine sponge Cliona celata (C. celata) (Grant) against the malarial vector Anopheles stephensi (An. stephensi) Liston. Methods: Marine sponge C. celata was thoroughly washed with distilled water and shade dried for 48 h. Then the sponges were homogenized and extracted sequentially with hexane, ethyl acetate and methanol. Larvicidal and ovicidal activities were tested at four different concentrations viz., 62.5, 125.0, 250.0 and 500.0 ppm. For repellent study extracts were taken in three different concentrations viz., 5.0, 2.5, 1.0 mg/cm2at. Results: Among the three solvent extracts of C. celata, methanol extract showed the highest larvicidal activity at 500 ppm against the fourth instar larvae of An. stephensi. The LC50and LC90values of C. celata methanol extract were recorded as 80.61 and 220.81 ppm against An. stephensi larvae respectively. High ovicidal activity of 91.2% was recorded at 500 ppm concentration of methanol extract. The haxane extract was found to be the most effective protectant against the adult female mosquitoes of An. stephensi. The mean protection time recorded in hexane extract was up to 245 min at 5 mg/cm2dosage against An. stephensi adults. Conclusions: The screening results suggest that the hexane and methanol extracts of C. celata are promising in mosquito control. Considering these bioactivities, C. celata could be probed further to obtain some novel pesticidal molecules.
1. Introduction
Mosquitoes are medically important arthropod vectors and most dangerous human health pests. Malaria remains the most important vector-borne parasitic disease in the world[1]. Anopheles stephensi (An. stephensi) Liston (Diptera) is widespread in tropical and subtropical regions; it is the primary vector of malaria in India and other West Asian countries[2]. Each year, more than 90% of malaria deaths occur in Africa, primarily among young children. The number of cases outside tropical Africa may be as high as 20 million, with about 80% being found in Asia where severe drug resistance has developed both in parasite and vector[3]. Therefore, improved and effective methods are urgently needed to control vector mosquitoes[4]. Natural insecticides are generally pest specific, biodegradable, usually non-allergic to human as well as non-target organisms[5], and may possess novel compounds with a wide range of activities[6,7]. In recent years, plant products and phytochemicals have been studied for the control of mosquitoes[8-13]. Likewise, some investigators have reported that the secondary metabolites of marine sponges possessed insecticidal activities[14-17].
Sponges (Phylum: Porifera) are the oldest metazoan organisms and have been recognized as rich source of biologically active compounds that are of potential interest to mankind[18,19] and are good alternatives for synthetic pesticides[20]. They are a potential source of novel antimicrobial agents[21]. Till date nearly 8 000 species of sponges have been described throughout the world and 108 species of sponges have been identified in Gulf of Mannar, India[22]. Till date, very few species of sponges have been
studied for their mosquito larvicidal activity[23-26]. However, studies on mosquitocidal properties of marine sponges from Indian waters are limited and the larvicidal, ovicidal and repellent efficacy of the marine sponge Cliona celata (C. celata) (Porifera: Hadromeridae: Clionidae) has not been studied previously against An. stephensi. Hence the present study was undertaken to screen the crude extracts of marine sponge C. celata from the Gulf of Mannar for their larvicidal, ovicidal and repellent activities against the malarial vector mosquito An. stephensi.
2. Materials and methods
2.1. Collection of sponges
Marine sponge C. celata was collected from the Gulf of Mannar (between 8°47’ to 9°05’ N Latitude and 78°12’ to 79°07’ E Longitude, India) during March 2012 at depths varying from 15-25 feet by scuba-diving. Sponges were gently removed from the substratum and transferred to laboratory within 48 h; then the specimen was identified and deposited (deposition number: MBRC/ZSI-S.225) in National Zoological Collection of Marine Bio Resource Centre, Zoological Survey of India (13°04’ N Latitude and 80°17’ E Longitude, India).
2.2. Preparation of crude extracts
The Marine sponge C. celata was thoroughly washed with distilled water to remove the sand particles and cut into small pieces before shade drying for 48 h. Then the sponges were homogenized and extracted sequentially with hexane, ethyl acetate and methanol. Initially the powder of the C. celata (500 g) was soaked in 1 L hexane for 72 h and filtered through filter paper. The residue was dried and then extracted sequentially with ethyl acetate and methanol solvents after 72 h of soaking in each solvent separately. The extracts were condensed separately under reduced pressure by using vacuum evaporator and the solvent free crude extracts were collected in glass vials and stored in 4 ℃ until use. Stock solutions (10% in acetone) of all the three solvent extracts were prepared and then subjected to bioassay screening.
2.3. Test insect
Eggs, larvae and adults of An. stephensi were obtained from the stock culture maintained at Entomology Research Institute, which were free of exposure to pathogens, insecticides or repellents. Laboratory rearing was done at a temperature of (27±2) ℃, 75%-85% relative humidity and a photoperiod of (11.0±0.5) h.
2.4. Larvicidal bioassay
Larvicidal activity was evaluated by following the methods of World Health Organization[27]. Twenty numbers of early fourth instar larvae of An. stephensi were introduced into the test containers. The extracts taken in four concentrations were 500.0, 250.0, 125.0 and 62.5 ppm. Acetone in water was used as solvent control. Mortality and survival rate were registered after 24 h exposure period. The moribund and dead larvae were collected, and larval mortality was calculated for each concentration. The bioassays were performed at a room temperature of (27±1) ℃ with five replicates for each concentration. Mortality was converted into percent mortality (a) and corrected mortality was calculated using Abbot’s formula (b)[28].
Where, n is the number of larvae, T-treated and C is the control.
The corrected percentage mortality value of each concentration was considered to estimate LC50and LC90values using SPSS Probit analysis statistical pack, version 11.5.
2.5. Ovicidal bioassay
Ovicidal activity was evaluated by following the method of Elango et al[29] with slight modification. Twenty five freshly laid eggs of An. stephensi were treated separately with C. celata extracts at 62.5, 125.0, 250.0 and 500.0 ppm concentrations. Acetone in water was served as control. Each treatment was replicated five times. The ovicidal activity was assessed up to 120 h post treatment and thereafter control and treated eggs were observed under the microscope and photographed using stereo zooming microscope (Wild M7S TYP 308700, Switzerland). The non-hatched eggs with unopened opercula were counted in each treatment and the percent mortality was calculated using the following formula and analysed in Graph Pad Prism version 3.0 for Windows, Graph Pad Software, San Diego, CA, U.S.A.
2.6. Repellent bioassay
For repellent experiment, 3 and 6 days old hundred laboratory reared blood-starved adult female mosquitoes were introduced into separate laboratory cages (45 cm×45 cm×40 cm). Experiments with An. stephensi were conducted in the night time between 19.00 pm to 24.00 pm. Before each test, the forearms of a human subject were washed with unscented neutral soap, thoroughly rinsed, and allowed to dry before the application of the extract at 5.0, 2.5, 1.0 mg/cm2concentration. The C. celata extracts being tested were applied on the right upper forearm and remaining regions were covered with gloves. The arm was left undisturbed. The left arm served as control. N-N Diethyl benzamide (12%, w/w) was used as negative control. The mosquito bites were observed for three full minute of every fifteen minutes. Protection time was recorded as the time elapsed between extract application and the observation period immediately preceding that in which a confirmed bite was obtained. The experiments were replicated five times in separate cages and in each replicate different volunteer were used to nullify any effect of skin differences on repellence. The protection time of each extract was calculated using previously established methods[30,31].
2.7. Statistical analysis
The results were presented as mean±SD. Statistical analyses of all the data obtained in larvicidal activity were evaluated using SPSS (version 11.5). The differences were considered as significant at P<0.05. Percent ovicidal activity was analysed in Graph Pad Prism version 3.0.
3. Results
3.1. Larvicidal activity and lethal doses
All the three extracts of the sponge C. celata showed larvicidal activity. The larvicidal activity varied between the solvent extracts. Table 1 shows the results on effective lethal concentration (LC50and LC90) values of hexane, ethyl acetate and methanol extracts of C. celata after 24 h treatment period. It was clear from the results that the methanol extract recorded the maximum larvicidal activity against An. stephensi. The LC50and LC90values of methanol extract were 80.61 and 220.81 ppm respectively. Significant chi-square values were recorded in all the extracts (Table 1). In control all the larvae were active and exhibited normal movement. But in the treated, restless movement was observed. After 1 h tremor and convulsion were observed in all the treated larvae and dead larvae settled down as reported earlier[12].
3.2. Ovicidal activity
The ovicidal activities of C. celata extracts on An. stephensi eggs are given in Figure 1. Methanol extract was found to be highly lethal to the eggs of An. stephensi. Methanol extract presented 91.2% ovicidal activity at 500 ppm concentration in 120 h post treatment period. The lowest concentration (62.5 ppm) of methanol extract caused 18.4% egg mortality against the eggs of An. stephensi. The ovicidal effect of all the three extracts was directly proportional to the concentration. In methanol extract treated An. stephensi eggs, most did not hatch and some hatched in an abnormal way at 500 ppm and the larvae died before completion of eclosion.
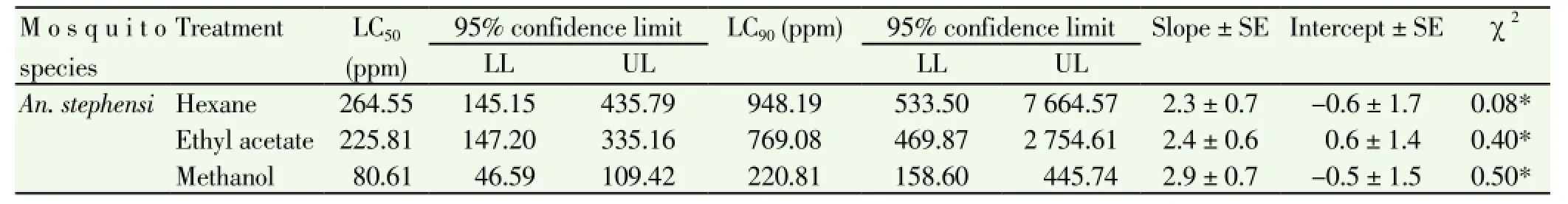
Table 1 Lethal concentrations (in ppm) of C. celata extracts against An. stephensi.
3.3. Repellent activity
The complete protection times for all the three extracts of C. celata against An. stephensi mosquitoes were recorded by standard skin repellence experiments and the results are given in Tables 2. The repellence was directly proportional to the dose and protection time (min) for each extract showed
variations against An. stephensi mosquitoes. In general hexane extract gave maximum protection time against An. stephensi compared to ethyl acetate and methanol extracts. Furthermore, it was noted that all the three extracts were found to be more effective against An. stephensi mosquitoes. Hexane extract gave a maximum protection time of 245 min against An. stephensi at a dose 5 mg/cm2(Table 2). These results were comparable with negative control (N-N Diethyl benzamide 12%, w/w), which showed maximum of 282 min protection at 5 mg/cm2dosage against An. stephensi mosquitoes.

Table 2 Complete protection time of three solvent extracts of C. celata against An. stephensi.
4. Discussion
Searching for eco-friendly pesticide molecules from natural sources has become an important research these days. Nature is providing innumerable bioactive molecules for the well-being of mankind. Plant kingdom and marine organisms provide majority of the beneficial biomolecules or products that are used by people in their daily life. Marine sponges are known to produce toxins and other compounds to repel and deter predators[32,33]. These compounds of marine sponges are reported to be bioactive compounds which can be used in the treatment of many diseases[34-38]. In recent years, researchers are concentrating on marine organisms to study their biological activities; especially marine sponges (Porifera) have attracted significant attention from various scientific disciplines[39]. The literature also indicates that the marine products possess maximum percentage of bioactive substances with novel biological properties than the molecules originating from terrestrial origin[40,25]. Recently, marine sponge extracts and their compounds have been screened for anti-microbial[41,42], anti-plasmodicidal[43], anti-filarial[44,45] and anti-helminthic activities[46]. Marine sponge extracts have also been screened against agricultural pests. Edrada et al[47] have reported an insecticidal and growth regulating compound from the philippine marine sponge Xestospongia ashmorica against Spodoptera littoralis, an important polyphagous pest. Supriyano et al[48] have reported that two guanidine alkaloids namely hymenialdisine and debromohymenialdisine from the tropical marine sponge Axinella carteri which exhibited insecticidal activity with LD50value of 88 and 125 ppm respectively, against the neonate larvae of S. littoralis in feeding bioassay experiments.
In this regard, very few researchers have studied the mosquito larvicidal potential of marine sponge extracts. Venkateswara Rao et al[24] have screened the methanoldichloromethane (1:1) extracts of 18 different sponges collected from Palk bay and Gulf of Mannar waters against the larvae of Aedes aegypti (Ae. aegypti) and houseflies (Musca domestica). They found that Psammaplysilla purpurea (LC50=25.9 ppm) and Haliclona cribricutis (LC50=31.46 ppm) were more effective against Ae. aegypti mosquito larvae. In our study we observed that the methanol extract of C. celata was the most effective as larvicide and the LC50of methanol extract against An. stephensi was found to be higher (80.61 ppm). An interesting finding in this study was that the low polar solvent extract, ie. hexane extract showed repellent activity and the high polar solvent (methanol) extract showed larvicidal and ovicidal activities. This result was comparable with the earlier report of Sonia and Lipton[26] who had screened the methanol extracts of five marine sponges namely Acanthella elongata, Echinodictyum gorgonoides, Axinella donnani, Callyspongia subarmigera and Callyspongia diffusa for larvicidal activity against Culex sp. They found that methanol extract of Acanthella elongata was the most effective with a LC50value of 0.066 mg/mL than other extracts.
In the present study all the three solvent extract treatments were not equally effective against An. stephensi larvae, eggs and adults. Aquatic life stages of eggs and larvae of An. stephensi were found to be more susceptible to methanol extract treatments and adults of An. stephensi were found to be more susceptible to hexane extract treatments. A similar result was reported by Martínez et al[23] who had screened the ethanol extracts of five marine sponges namely Amphimedon compressa, Topsentia ophiraphidites, Svenzea zeai, Ircinia campana and Agelas sventres, against fourth instar larvae of Ae. aegypti and Cx. quinquefasciatus. They found that Ircinia campana extract was the most effective against the larvae of two mosquitoes and the activity was higher in Ae. aegypti than in Cx. quinquefasciatus.
In conclusion, the present study reports for the first time
the repellent, larvicidal and ovicidal activity of marine sponge C. celata against An. stephensi. The screening results suggest that the hexane and methanol extracts of C. celata are promising in mosquito control. The isolation and identification of active principles present in this marine sponge species will be useful for developing an ecofriendly mosquito control product.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are thankful to Entomology Research Institute, Loyola College for financial assistance. The authors are grateful to Dr. G. Sivaleela, Scientist, MBRC, Zoological Survey of India for the cooperation and contribution in identifying the marine sponge.
[1] World Health Organization. World malaria report. Geneva: WHO; 2010.
[2] Burfield T, Reekie SL. Mosquitoes, malaria and essential oils. Int J Aroma 2005; 15: 30-41.
[3] Garcia LS. Malaria. Clin Lab Med 2010; 30(1): 93-129.
[4] Senthil Nathan S. The use of Eucalyptus tereticornis Sm. (Myrtaceae) oil (leaf extract) as a natural larvicidal agent against the malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Bioresour Technol 2009; 98: 1856-1860.
[5] Bowers WS. Biorational approaches for insect control. Korean J Appl Entomol 1992; 31: 289-303.
[6] Schmutterer H. The neem tree Azadirachta indica A. Juss and other meliaceous plants. Germany: VCH publishers, Weinneim; 1995.
[7] Ghosh A, Chowdhury N, Chandra G. Laboratory evaluation of a phytosteroid compound of mature leaves of Day Jasmine (Solanaceae: Solanales) against larvae of Culex quinquefasciatus (Diptera: Culicidae) and nontarget organisms. Parasitol Res 2008; 103: 271-277.
[8] Mehlhorn H, Schmahl G, Schmidt J. Extract of the seeds of the plant Vitex agnus castus proven to be highly efficacious as a repellent against ticks, fleas, mosquitoes and biting flies. Parasitol Res 2005; 95: 363-365.
[9] Amer A, Mehlhorn H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res 2006; 99(4): 478-490.
[10] Gleiser RM, Zygadlo JA. Insecticidal properties of essential oils from Lippia turbinata and Lippia polystachya (Verbenaceae) against Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 2007; 101: 1349-1354.
[11] Govindarajan M, Jebanesan A, Pushpanathan T. Larvicidal and ovicidal activity of Cassia fistula Linn. leaf extract against filarial and malarial vector mosquitoes. Parasitol Res 2008; 102: 289-292.
[12] Maheswaran R, Ignacimuthu S. A novel herbal formulation against dengue vector mosquitoes Aedes aegypti and Aedes albopictus. Parasitol Res 2012; 110: 1801-1813.
[13] Chellaiah Muthu, Appadurai Daniel Reegan, Selvadurai Kingsley, Savarimuthu Ignacimuthu. Larvicidal activity of pectolinaringenin from Clerodendrum phlomidis L. against Culex quinquefasciatus Say and Aedes aegypti L. (Diptera: Culicidae). Parasitol Res 2012; 111: 1059-1065.
[14] Weiss B, Ebel R, Elbrächter M, Kirchner M, Proksch P. Defence metabolites from marine sponge Verongia aerophoba. Biochem Syst Ecol 1996; 24(1): 1-12.
[15] Balbin-Oliveros M, Edrada RA, Proksch P, Wray V, Witte Nad L, Van Soest RW. A new meroditerpenoid dimer from an undescribed Philippine marine sponge of the genus Strongylophora. J Nat prod 1998; 61: 948-952.
[16] Okada A, Watanabe K, Umeda K, Miyakado M. Calyculin E and F, novel insecticidal metabolites from the marine sponge, Discoderma sp. Agric Biol Chem 2006; 55: 2765-2771.
[17] Funda NY. Biological activities of the marine sponge auxinella. J Facul Pharm 2007; 14: 47-60.
[18] Faulkner DJ. Marine pharmacology. Antonie Van Leeuwen 2000; 77: 135-145.
[19] Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep 2005; 22: 15-61.
[20] Fatope MO, Ibrahim H, Takeda Y. Screening of higher plants reputed as pesticides using the brine shrimp lethality assay. Int J Pharmacog 1993; 31: 250-254.
[21] Selvin J, Lipton AP. Biopotentials of secondary metabolites isolated from marine sponges. Hydrobiologia 2004; 513: 231-238.
[22] Limna Mol VP, Raveendran TV, Abhilash KR, Parameswaran PS. Inhibitory effect of Indian sponge extracts on bacterial strains and larval settlement of the barnacle, Balanus Amphitrite. Int Biodet and Biodeg 2010; 64: 506-510.
[23] Martínez AM, Galeano EJ, Cadavid J, Miranda YR, Llano JL, Montalvo YKM. Acción insecticida de extractos etanólicos de esponjas del golfo de urabá sobre larvas de Aedes aegypti Y Culex quinquefasciatus. Vitae, Revista De La Facultad De Química Farmacéutica 2007; 14(2): 90-94.
[24] Venkateswara Rao J, Usman PK, Bharat Kumar J. larvicidal and insecticidal properties of some marine sponges collected in Palk bay and Gulf of Mannar Waters. Afr J Biotech 2008; 7(2): 109-113.
[25] Sujatha S and Joseph B. Effect of few marine sponges and its biological activity against Aedes aegypti Linn. Musca domestica
(Linnaeus, 1758) (Diptera: Culicidae). J Fish Aqua Sci 2011; 6(2): 170-177.
[26] Sonia ASG, Lipton AP. Mosquito Larvicidal activity of marine sponge metabolites. Global J Pharmacology 2012; 6(1): 01-03.
[27] World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/ 2005.13. Geneva: WHO; 2005.
[28] Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol 1925; 18: 265-267.
[29] Elango G, Bagavan A, Kamaraj C, Abduz Zahir A, Rahuman AA. Oviposition deterrent, ovicidal, and repellent activities of indigenous plant extracts against Anopheles subpictus Grassi (Diptera: Culicidae). Parasitol Res 2009; 105: 1567-1576.
[30] Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med 2002; 347(1): 13-18.
[31] Venkatachalam MR, Jebanesan A. Repellent activity of Ferronia elephantum Corr. (Rutaceae) leaf extract against Aedes aegypti (L.). Bioresource Technol 2001; 76: 287-288.
[32] Uriz MJ, Turon X, Becerro MA, Galera J. Feeding deterrence in sponges: The role of toxicity, physical defenses, energetic contents and life-history stage. J Exp Mar Biol Ecol 1996; 205: 187-204.
[33] Pawlik JR, McFall G, Zea S. Does the odor from sponges of the genus Ircinia protect them from fish predators? J Chem Ecol 2002; 28: 1103-1115.
[34] Haefner B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov Today 2003; 8: 536-544.
[35] Rajeevkumar J Xuzirong S. Biomedical compounds from marine organisms. Marine Drugs 2004; 2: 123-146.
[36] Mayer AM and Hamann MT. Marine pharmacology in 2001-2002: Marine compounds with antihelminthic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis and antiviral activities: affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol Part C: Pharmacol Toxicol 2002; 140: 265-286.
[37] Mayer AM, Rodriguez AD, Berlinck RG, Hamann MT. Marine pharmacology in 2003-2004: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C: Pharmacol Toxicol 2007; 145: 553-581.
[38] Anake K, Rawiwan W, Nair C, Maria N, Madalena P, Werner H. Anticancer activity evaluation of Kuanoniamines A and C isolated from the marine sponge Oceanapia sagittaria collected from the gluf of Thailand. Marine Drugs 2007; 5: 6-22.
[39] Narsinh LT, Roopesh J, Filipe N, Bojan H, Archana NT, Werner EGM. Marine molecular biology: An emerging field of biological sciences. Biotechnol Advances 2008; 26: 233-245.
[40] Volk CA, Lippert, Lichte E, Köch M. Two new haliclamines from the arctic sponge Haliclona viscose. Eur J Org Chem 2004; 14: 3154-3158.
[41] Moura RM, Queiroz AFS, Fook JMSLL, Dias ASF, Monterio NKV, Ribeiro JKC, et al. CvL a lectin from the marine sponge Cliona varians: isolation, characterization and its effects on pathogenic bacteria and Leishmania promastigotes. Comp Biochem Physiol 2006; 145: 517-523.
[42] Anthony DW, Adam M, Mark JR, Kylie AM, Christopher PG, Jana G. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpene isonitriles from the tropical marine sponge Cymbastela hooperi. Org Biomol Chem 2011; 9: 400-407.
[43] Elkin G, Olivier PT, Sara R, Diana M, Alejandro M. Antiparasitic bromotyrosine derivatives from the marine sponge Verongula rigida. Mar Drugs 2011; 9: 1902-1913.
[44] Lakshmi V, Srivastava S, Mishra SK, Misra S, Verma M, Misra-Bhattacharya S. In vitro and in vivo antifilarial potential of marine sponge, Haliclona exigua (Kirkpatrick), against human lymphatic filarial parasite Brugia malayi. Parasitol Res 2009; 105: 1295-1301.
[45] Gupta J, Misra S, Mishra SK, Srivastava S, Srivastava MN, Lakshmi V, et al. Antifilarial activity of marine sponge Haliclona oculata against experimental Brugia malayi infection. Exp Parasitol 2012; 130: 449-455.
[46] Mayer AM, Rodriguez AD, Berlinck RG, Hamann MT. Marine pharmacology in 2005-2006: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, antiinflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Biochim Biophys Acta 2009; 1790: 283-308.
[47] Edrada RA, Proksch P, Wray V, Witte L, Müller WEG, Van Soest RWM. Four new bioactive manzaminetype alkaloids from the Philippine marine sponge Xestospongia ashmorica. J Nat Prod 1996; 59: 1056-1060.
[48] Supriyano A, Schwarz B, Wray V, Wittle L, Muller WE, Van Soest R, et al. Bioactive alkaloids from the tropical marine sponge Axinella carteri. Z Naturforsch C 1995; 50: 669.
ment heading
10.1016/S1995-7645(14)60183-8
*Corresponding author: Savarimuthu Ignacimuthu, Entomology Research Institute, Loyola College, Chennai - 600 034, Tamil Nadu, India.
△These authors contributed equally to this work.
Tel: +9144-28178348
Fax: +9144-28175566
E-mail: entolc@hotmail.com
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Inhibition of advanced glycation endproducts formation by Korean thistle, Cirsium maackii
- Anti-hypercholesterolemic effect of kenaf (Hibiscus cannabinus L.) seed on high-fat diet Sprague dawley rats
- Susceptibility to temephos, permethrin and deltamethrin of Aedes aegypti (Diptera: Culicidae) from Muang district, Phitsanulok Province, Thailand
- Cloning, expression, purification and bioinformatic analysis of 2-methylcitrate synthase from Mycobacterium tuberculosis
- Prevalence of shiga toxins (stx1, stx2), eaeA and hly genes of Escherichia coli O157:H7 strains among children with acute gastroenteritis in southern of Iran
- Characterization of mycobacterium isolates from pulmomary tuberculosis suspected cases visiting Tuberculosis Reference Laboratory at Ethiopian Health and Nutrition Research Institute, Addis Ababa Ethiopia: a cross sectional study
